Académique Documents
Professionnel Documents
Culture Documents
Buffers Notes
Transféré par
vinaybharadwajbsCopyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Buffers Notes
Transféré par
vinaybharadwajbsDroits d'auteur :
Formats disponibles
Buffers
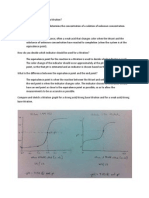
If the [H+] (or pH) of a solution is not appreciably affected by the addition of small amounts of acids and bases, the solution is said to be buffered. A solution will have this property if it contains relatively large amounts of both a weak acid and a weak base. If a small amount of a strong acid is added to this solution, most of the added H+ will combine with an equivalent amount of the weak base of the buffer to form the conjugate acid of that weak base; thus the [H+] of the solution remains almost constant. If a small amount of a strong base is added to the buffer solution, most of the OH- will combine with an equivalent amount of the weak acid of the buffer to form the conjugate base of that weak acid. In this way, the [H+] of the buffer solution is not appreciably affected by the addition of small amounts of acid or base. The buffer capacity is the amount of acid or base that may be added to a buffer solution before a significant change in pH occurs. When too much acid or base is added, the buffer capacity of a solution is exceeded and a significant change in pH is observed.
Buffers page 1
Any pair of weak acid, weak base can be used to form a buffer solution, as long as each can form its conjugate base or conjugate acid in water solution. All pH curves have at least one region where buffering action occurs. The curves in these relatively constant pH regions are most nearly horizontal at a volume of titrant which is one-half the first equivalence point, or halfway between successive equivalence point for polyprotic substances. Write Brnsted-Lowry equations to show what happens when acid or base is added to the following buffers. 1. ethanoic acid/ethanoate buffer 2. ammonium ion/ammonia buffer 3. phosphoric acid/dihydrogen phosphate ion buffer 4. methanoic acid/methanoate buffer (formic acid/formate) (acetic acid/acetate)
Buffers page 2
Vous aimerez peut-être aussi
- Biochemistry : A Practical ManualD'EverandBiochemistry : A Practical ManualÉvaluation : 5 sur 5 étoiles5/5 (1)
- Buffer AssignmentDocument4 pagesBuffer AssignmentHannahnel Anasco QuidatoPas encore d'évaluation
- The chemical review of buffering systemsDocument6 pagesThe chemical review of buffering systemsRezaul Karim TutulPas encore d'évaluation
- Buffers: Analytical TechniquesDocument17 pagesBuffers: Analytical TechniquesAbdul FarooqPas encore d'évaluation
- Buffer ChemistryDocument9 pagesBuffer ChemistrySidra chaudharyPas encore d'évaluation
- Buffer PreparationDocument3 pagesBuffer PreparationEzzati Aziz0% (2)
- Buffers Experiment 1: Rüveyda AKÇİN, Gebze Technical University, TurkeyDocument6 pagesBuffers Experiment 1: Rüveyda AKÇİN, Gebze Technical University, TurkeyRüveyda Akçin100% (1)
- Analytical Techniques: Topic: BuffersDocument18 pagesAnalytical Techniques: Topic: BuffersAbdul FarooqPas encore d'évaluation
- BUFFERDocument10 pagesBUFFERakinolaboluwatife83Pas encore d'évaluation
- ALEVEL Chemistry notes (1)Document2 pagesALEVEL Chemistry notes (1)jacksmithers98Pas encore d'évaluation
- 2023 BCH 211 Masondo - BL 202126087 Prac5 WriteupDocument6 pages2023 BCH 211 Masondo - BL 202126087 Prac5 WriteupBenson MasondoPas encore d'évaluation
- How Do We Choose A BufferDocument2 pagesHow Do We Choose A BufferShahir AshuPas encore d'évaluation
- Assignment 2Document3 pagesAssignment 2edelyn telewikPas encore d'évaluation
- CHP 16, BuffersDocument5 pagesCHP 16, BuffersTiurma Debora SimatupangPas encore d'évaluation
- BuffersDocument10 pagesBuffersbruno de jesus fontesPas encore d'évaluation
- PH Buffers & Isotonic Solutions STTDocument35 pagesPH Buffers & Isotonic Solutions STTAdiksha LendePas encore d'évaluation
- 07 Preparation of A Buffer KatyDocument1 page07 Preparation of A Buffer Katyapi-235913605Pas encore d'évaluation
- General Chemistry 2: Buffer SolutionsDocument15 pagesGeneral Chemistry 2: Buffer SolutionsSteiner100% (1)
- 2.pH, Buffers and IsotonicDocument48 pages2.pH, Buffers and Isotonicrajender91% (11)
- Buffer SolutionsDocument3 pagesBuffer Solutionsemad_abdelaal57Pas encore d'évaluation
- Buffer Solutions FinalDocument62 pagesBuffer Solutions Finalshripathyd1100% (1)
- Buffering AgentDocument3 pagesBuffering Agents.sabapathyPas encore d'évaluation
- 09 Exp 11 Buffer SolutionsDocument8 pages09 Exp 11 Buffer SolutionsShainmaugne AdvientoPas encore d'évaluation
- CHEM 40.1 BUFFER REVIEWDocument5 pagesCHEM 40.1 BUFFER REVIEWSteffi GatdulaPas encore d'évaluation
- Buffer BasicsDocument5 pagesBuffer BasicsKuldipsinh ZalaPas encore d'évaluation
- BufferDocument34 pagesBufferYendro Try SaturaPas encore d'évaluation
- 19 Reactions of Acids and BasesDocument19 pages19 Reactions of Acids and BasesrachelelizabethPas encore d'évaluation
- Lec 7 Analytical ( Buffer Solution)Document13 pagesLec 7 Analytical ( Buffer Solution)alhyaly857Pas encore d'évaluation
- Summary of Buffer Solution: A Buffer Solution Is An Aqueous Solution That Can Maintain The PH of A SystemDocument2 pagesSummary of Buffer Solution: A Buffer Solution Is An Aqueous Solution That Can Maintain The PH of A SystemWiji Tri UtariPas encore d'évaluation
- Buffers: Maintaining pH EquilibriumDocument62 pagesBuffers: Maintaining pH EquilibriumSara FatimaPas encore d'évaluation
- Acid-Base Equilibria in Aqueous SolutionsDocument48 pagesAcid-Base Equilibria in Aqueous SolutionsAdrian ChombaPas encore d'évaluation
- BufferDocument5 pagesBufferabdulghaffarsp8Pas encore d'évaluation
- Bronsted-Lowry Acid Base TheoryDocument21 pagesBronsted-Lowry Acid Base TheoryGrace Ann ArimadoPas encore d'évaluation
- BuffersDocument20 pagesBuffersgopimpharmPas encore d'évaluation
- Pharmaceutical Chemistry NotesDocument8 pagesPharmaceutical Chemistry NotesZaheer Uddin100% (1)
- Understanding PH and BufferDocument2 pagesUnderstanding PH and BufferRyan Carlo Conde100% (1)
- Experiment 11 - Buffor SolutionsDocument7 pagesExperiment 11 - Buffor SolutionsBridget BurnsPas encore d'évaluation
- What Is A Buffer Solution?: Analticaltechniques Assignment COURSE#509Document4 pagesWhat Is A Buffer Solution?: Analticaltechniques Assignment COURSE#509Altaf Ur RehmanPas encore d'évaluation
- 6.0 Conceitos Sobre PH e Soluções TampãoDocument13 pages6.0 Conceitos Sobre PH e Soluções TampãoFernando SperandioPas encore d'évaluation
- CH 15Document58 pagesCH 15Chala1989Pas encore d'évaluation
- Buffer Solution pHDocument16 pagesBuffer Solution pHroxan clabria100% (1)
- BCH Report 1Document3 pagesBCH Report 1Nosibusiso KhaliphaPas encore d'évaluation
- QUIZ Compressed-1.pdf - BIOCHEMISTRY LAB MODULE 1 PH OF...Document91 pagesQUIZ Compressed-1.pdf - BIOCHEMISTRY LAB MODULE 1 PH OF...B-Panganiban, Cyrus SalvadorPas encore d'évaluation
- PH Acids and BasesDocument3 pagesPH Acids and BasesboultonbaileyPas encore d'évaluation
- Buffer SolutionsDocument19 pagesBuffer SolutionsMuskaan BindalPas encore d'évaluation
- QuestionsDocument3 pagesQuestionsapi-203606735Pas encore d'évaluation
- Unit-V - PH, Buffer, Buffer Equation, Isotonicity-1Document27 pagesUnit-V - PH, Buffer, Buffer Equation, Isotonicity-1IKHLASH MOHDPas encore d'évaluation
- Buffered and Isotonic Solutions: A (Salt) (Acid) A ADocument2 pagesBuffered and Isotonic Solutions: A (Salt) (Acid) A AMary Loise LimaPas encore d'évaluation
- PH IndicatorDocument25 pagesPH IndicatorAdil AminPas encore d'évaluation
- Buffers CompleteDocument46 pagesBuffers CompleteSunshine_Bacla_4275100% (2)
- UNIT II Acid Base TitrationDocument48 pagesUNIT II Acid Base TitrationDr Priti JainPas encore d'évaluation
- Pharmaceutical Buffers and Buffer CapacityDocument6 pagesPharmaceutical Buffers and Buffer Capacityharoon41Pas encore d'évaluation
- Buffer Solutions: September 2020Document22 pagesBuffer Solutions: September 2020Nitin DapkePas encore d'évaluation
- Buffers Solution & Its Applications: Abuzar KhalidDocument8 pagesBuffers Solution & Its Applications: Abuzar Khalidabuzar khalidPas encore d'évaluation
- Buffer Solution - Acidic and Basic Buffers, Preparations, Examples PDFDocument5 pagesBuffer Solution - Acidic and Basic Buffers, Preparations, Examples PDFSakshi DevalekarPas encore d'évaluation
- Chem 41 Lab Formal Report 01 - Preparation of Buffers & Amino Acids As AmpholytesDocument13 pagesChem 41 Lab Formal Report 01 - Preparation of Buffers & Amino Acids As AmpholytesFaith VillahermosaPas encore d'évaluation
- Acid-Base Equilibria and Solubility EquilibriaDocument24 pagesAcid-Base Equilibria and Solubility EquilibriaAndrew John CellonaPas encore d'évaluation
- Biochemistry LN03Document16 pagesBiochemistry LN03Rahaf Al-muhtasebPas encore d'évaluation
- Vdocument - in Buffer Solutions 568b0b28b560dDocument14 pagesVdocument - in Buffer Solutions 568b0b28b560dAmiraPas encore d'évaluation
- IP 4. Protocol - Chemical Principles II LaboratoryDocument9 pagesIP 4. Protocol - Chemical Principles II LaboratoryJavier PratdesabaPas encore d'évaluation
- AbstractDocument1 pageAbstractvinaybharadwajbsPas encore d'évaluation
- ExperimentalDocument7 pagesExperimentalvinaybharadwajbsPas encore d'évaluation
- Photostability Of: Was Photostable After 18 Hours IrradiationDocument1 pagePhotostability Of: Was Photostable After 18 Hours IrradiationvinaybharadwajbsPas encore d'évaluation
- IUPAC Technical Report on Chemical ActinometryDocument47 pagesIUPAC Technical Report on Chemical ActinometryvinaybharadwajbsPas encore d'évaluation
- A, A, A, A,: The Spectrum and Structure of The Hno MoleculeeDocument45 pagesA, A, A, A,: The Spectrum and Structure of The Hno MoleculeevinaybharadwajbsPas encore d'évaluation
- LC-MS Findings ReportDocument1 pageLC-MS Findings ReportvinaybharadwajbsPas encore d'évaluation
- AbstractDocument1 pageAbstractvinaybharadwajbsPas encore d'évaluation
- IUPAC Technical Report on Chemical ActinometryDocument47 pagesIUPAC Technical Report on Chemical ActinometryvinaybharadwajbsPas encore d'évaluation
- IntroductionDocument2 pagesIntroductionvinaybharadwajbsPas encore d'évaluation
- Presentation 2Document1 pagePresentation 2vinaybharadwajbsPas encore d'évaluation
- Brief Experiment DetailsDocument11 pagesBrief Experiment DetailsvinaybharadwajbsPas encore d'évaluation
- Presentation 151Document1 pagePresentation 151vinaybharadwajbsPas encore d'évaluation
- Determination of The Hardness of WaterDocument3 pagesDetermination of The Hardness of WatervinaybharadwajbsPas encore d'évaluation
- Presentation 151Document1 pagePresentation 151vinaybharadwajbsPas encore d'évaluation
- Wittig Reaction SibiDocument4 pagesWittig Reaction SibivinaybharadwajbsPas encore d'évaluation
- Wittig ReactionDocument15 pagesWittig ReactionvinaybharadwajbsPas encore d'évaluation
- Reaction Rate NotesDocument10 pagesReaction Rate NotesvinaybharadwajbsPas encore d'évaluation
- NoteDocument1 pageNoteSuryanarayana RaoPas encore d'évaluation
- Acid-Ba Se Calculations: - (O.r5o N) Tzo - o "R'-AxDocument3 pagesAcid-Ba Se Calculations: - (O.r5o N) Tzo - o "R'-AxvinaybharadwajbsPas encore d'évaluation
- Chemistry Common Equations..............Document6 pagesChemistry Common Equations..............devagyna_p_pandyaPas encore d'évaluation
- Chem Kinetics PracticeDocument4 pagesChem Kinetics PracticevinaybharadwajbsPas encore d'évaluation
- Acid Base Theory AnswersDocument3 pagesAcid Base Theory Answersvinaybharadwajbs0% (1)
- Chem Kinetics PracticeDocument4 pagesChem Kinetics PracticevinaybharadwajbsPas encore d'évaluation
- 13 10 PhenolDocument13 pages13 10 PhenolvinaybharadwajbsPas encore d'évaluation
- Acid Base TheoryDocument1 pageAcid Base TheoryvinaybharadwajbsPas encore d'évaluation
- Acid Base Theory NotesDocument1 pageAcid Base Theory NotesvinaybharadwajbsPas encore d'évaluation
- Chapter 8Document12 pagesChapter 8vinaybharadwajbsPas encore d'évaluation
- 13 10 PhenolDocument13 pages13 10 PhenolvinaybharadwajbsPas encore d'évaluation
- Acid Bases Intro Asn AnswersDocument3 pagesAcid Bases Intro Asn AnswersvinaybharadwajbsPas encore d'évaluation