Académique Documents
Professionnel Documents
Culture Documents
Aromaticity (Organic Chemistry)
Transféré par
noneDescription originale:
Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Aromaticity (Organic Chemistry)
Transféré par
noneDroits d'auteur :
Formats disponibles
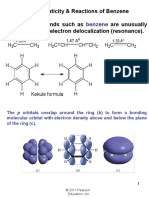
Aromatic compounds are unusually stable
Aromatic structures are more stable than their open-
chain counterparts. For example, benzene is more stable than 1,3,5-hexatriene
CH2 CH2
To be classified as aromatic, a compound must meet both of the following criteria:
1. It must have an uninterrupted cyclic cloud of pi
electrons (often called a cloud) above and below the plane of the molecule.
For the pi cloud to be cyclic, the molecule must be
cyclic. For the pi cloud to be uninterrupted, every atom in the ring must have a p orbital. For the pi cloud to form, each p orbital must overlap with the p orbitals on either side of it. Therefore, the molecule must be planar.
2. The pi cloud must contain an odd number of pairs of pi electrons.
Huckels Rule or the (4n+2) rule
The rule states that for a planar, cyclic compound to be aromatic, its
uninterrupted cloud must contain (4n+2) pi electrons, where n is any whole number. According to Hckels rule, then, an aromatic compound must have 2 (n=0), 6 (n=1), 10(n=2), 14(n=3), 18(n=4) etc., electrons. Systems with 4n pi electrons, with n an integer, are ANTIAROMATIC. For example cyclobutadiene (4 pi electrons)
Antiaromatic compounds are highly unstable
Aromaticity is characterized by stability, whereas antiaromaticity is
characterized by instability. An antiaromatic compound is planar, cyclic and has an uninterrupted ring of p orbital-bearing atoms, and has an even pair of pi electrons.
CH2 CH2
Less stable More
stable
Aromatic Heterocylcic Compounds
Classify each as aromatic, antiaromatic, or nonaromatic
Convince yourselves that these structures are AROMATIC
CH
-
CH
CH
CH CH
-
N H
Vous aimerez peut-être aussi
- Aromaticity With Huckle's RuleDocument7 pagesAromaticity With Huckle's RuleSk ZPas encore d'évaluation
- HidrokarbonDocument2 pagesHidrokarbonSilvi TatianPas encore d'évaluation
- Properties and Reactions of Haloalkanes: Bimolecular Nucleophilic SubstitutionDocument48 pagesProperties and Reactions of Haloalkanes: Bimolecular Nucleophilic SubstitutionKunjal100% (1)
- Icse X - Chemistry: Board Paper - 2011Document8 pagesIcse X - Chemistry: Board Paper - 2011DhrumilPas encore d'évaluation
- Feed Data PDFDocument2 pagesFeed Data PDFahmedPas encore d'évaluation
- JP XII Organic Chemistry (01) - 1Document4 pagesJP XII Organic Chemistry (01) - 1Ashish RanjanPas encore d'évaluation
- Lab Report Sensory Analysis GustationDocument5 pagesLab Report Sensory Analysis Gustationapi-246827236Pas encore d'évaluation
- AAEDR-F-005 Rev 1 (Extended Storage PA)Document6 pagesAAEDR-F-005 Rev 1 (Extended Storage PA)Jose G LopezPas encore d'évaluation
- Essential Organic Chemistry: Aromaticity: Reactions of Benzene and Substituted BenzenesDocument75 pagesEssential Organic Chemistry: Aromaticity: Reactions of Benzene and Substituted BenzeneschurvaloooPas encore d'évaluation
- Tiaft Drug Concentration Reference TableDocument20 pagesTiaft Drug Concentration Reference TablerodrigoPas encore d'évaluation
- Organic Chemistry - Chapter 15 Benzene & Aromatic CompoundsDocument9 pagesOrganic Chemistry - Chapter 15 Benzene & Aromatic CompoundsSairille ManejaPas encore d'évaluation
- Organic ChemistryDocument1 pageOrganic ChemistryPybRajeshKumarPas encore d'évaluation
- 13 PneumaticsDocument18 pages13 PneumaticsEbied Yousif AlyPas encore d'évaluation
- Title 22 CombinedDocument254 pagesTitle 22 CombinedWaldi RahmanPas encore d'évaluation
- CHEM 4341 Syllabus Fall 2016 PDFDocument5 pagesCHEM 4341 Syllabus Fall 2016 PDFCarmen FloresPas encore d'évaluation
- The Mundell-Fleming Model (Topic 1)Document30 pagesThe Mundell-Fleming Model (Topic 1)Anonymous Ptxr6wl9DhPas encore d'évaluation
- In The Presence of Allah, His Prophet, and The RulerDocument19 pagesIn The Presence of Allah, His Prophet, and The Rulertips and tricksPas encore d'évaluation
- Organic Chemistry NotesDocument3 pagesOrganic Chemistry NotesAmin MukhlisPas encore d'évaluation
- Assignment Description Rubric For Queen Mab ProjectDocument2 pagesAssignment Description Rubric For Queen Mab Projectapi-339890913Pas encore d'évaluation
- Practice Questions-Conformational AnalysisDocument4 pagesPractice Questions-Conformational AnalysisHarry Zgambo100% (1)
- 210 Fa 15 Exam 2 KEYDocument19 pages210 Fa 15 Exam 2 KEYdsarathy1Pas encore d'évaluation
- JR Tucker Hs 10-12 Course Options 21-22Document2 pagesJR Tucker Hs 10-12 Course Options 21-22api-4657936540% (1)
- Chap 06Document17 pagesChap 06Anand Kumar SumanPas encore d'évaluation
- ICT LSN Plan Lisan HortatoriDocument6 pagesICT LSN Plan Lisan Hortatorinofri_excerPas encore d'évaluation
- Course Title: Organic Chemistry-I Course Code: Chm-553, Chm-507 Semester: MSC 1, Bs 5Document18 pagesCourse Title: Organic Chemistry-I Course Code: Chm-553, Chm-507 Semester: MSC 1, Bs 5Mian Naveed AhmedPas encore d'évaluation
- Tutorial 1 @stereochemistry PDFDocument5 pagesTutorial 1 @stereochemistry PDFMoulindu Kundu50% (2)
- Electron Delocalization and ResonanceDocument9 pagesElectron Delocalization and ResonanceMariana LizethPas encore d'évaluation
- Organic Chemistry Practice ProblemsDocument1 pageOrganic Chemistry Practice ProblemsSushant KumarPas encore d'évaluation
- Jan. 26 CSLEA v. DPA Ruling, 3rd District Court of AppealDocument29 pagesJan. 26 CSLEA v. DPA Ruling, 3rd District Court of Appealjon_ortizPas encore d'évaluation
- M.sc. - II, Organic ChemistryDocument15 pagesM.sc. - II, Organic ChemistryDeepak50% (2)
- Organic ChemistryDocument24 pagesOrganic ChemistryNivas KaruppananPas encore d'évaluation
- PolimerDocument22 pagesPolimerDhea Kana ZhafiraPas encore d'évaluation
- PUR 3000 Fall2010 - FarberDocument4 pagesPUR 3000 Fall2010 - FarberMark FiorentinoPas encore d'évaluation
- Organic Chemistry AlcoholsDocument20 pagesOrganic Chemistry AlcoholsDante Luis SilvaPas encore d'évaluation
- AFJ Supplement The Jamaica GleanerDocument8 pagesAFJ Supplement The Jamaica Gleanercchung01Pas encore d'évaluation
- Benzene and Derivatives Members GroupDocument57 pagesBenzene and Derivatives Members GroupHaris KhanPas encore d'évaluation
- Final Rationale EssayDocument9 pagesFinal Rationale Essaycubalove18Pas encore d'évaluation
- Loudon, Organic Chemistry ErrataDocument13 pagesLoudon, Organic Chemistry Errataig12345Pas encore d'évaluation
- Alcohol, Phenol, and Ethers:: "Their Structures, Physical Properties and Nomenclature"Document33 pagesAlcohol, Phenol, and Ethers:: "Their Structures, Physical Properties and Nomenclature"AmanPas encore d'évaluation
- Alcohols, Phenols and EthersDocument99 pagesAlcohols, Phenols and EthersSanya VermaPas encore d'évaluation
- Introduction To MatlabDocument45 pagesIntroduction To MatlabSivaraman ChidambaramPas encore d'évaluation
- Benzene & Aromatic Compound: Jully Tan School of EngineeringDocument41 pagesBenzene & Aromatic Compound: Jully Tan School of EngineeringSàtz ÑÖÑïtPas encore d'évaluation
- Help IPA EnglishDocument12 pagesHelp IPA EnglishEkoPas encore d'évaluation
- Ether: Navigation Search AetherDocument7 pagesEther: Navigation Search AetherMuhammad Wahyu Nugraha0% (1)
- Organic Chem U-2 AlcoholDocument33 pagesOrganic Chem U-2 Alcoholsinte beyuPas encore d'évaluation
- Organic Chemistry IIDocument83 pagesOrganic Chemistry IINaveen KumarPas encore d'évaluation
- CEI Planet - Summer 2016Document16 pagesCEI Planet - Summer 2016Competitive Enterprise InstitutePas encore d'évaluation
- Basics of Organic Chemistry 1 QPDocument8 pagesBasics of Organic Chemistry 1 QPMuhammad YousafPas encore d'évaluation
- Welcome To Chem 206: Fall Term, 2005, David A. EvansDocument22 pagesWelcome To Chem 206: Fall Term, 2005, David A. EvanseraborPas encore d'évaluation
- ALKANES2Document41 pagesALKANES2Shiki Asagami BrunestedPas encore d'évaluation
- Sn1 MechanismDocument24 pagesSn1 MechanismDian MustikasariPas encore d'évaluation
- Revised Organic ChemistryDocument90 pagesRevised Organic ChemistryMinh TieuPas encore d'évaluation
- Organic Chemistry 2 - Syllabus - USTHDocument3 pagesOrganic Chemistry 2 - Syllabus - USTHMinh MinhPas encore d'évaluation
- Annual LeaveDocument3 pagesAnnual LeaveDzul Azhar SalimPas encore d'évaluation
- Lab ManualDocument19 pagesLab Manualanon_467104036Pas encore d'évaluation
- Chemistry Tutorial: Aromaticity: Based On A Chemistry 14C Honors ProjectDocument15 pagesChemistry Tutorial: Aromaticity: Based On A Chemistry 14C Honors Projectgel18Pas encore d'évaluation
- Organic Chem 2 by HaftomDocument285 pagesOrganic Chem 2 by Haftomdereje dawitPas encore d'évaluation
- Aromatic CompoundsDocument55 pagesAromatic CompoundsNadine Bacalangco100% (1)
- Aromaticity TutorialDocument15 pagesAromaticity TutorialAlex-Mihai Ciubara100% (2)
- Aromatic Compounds: © 2006 Thomson Higher EducationDocument103 pagesAromatic Compounds: © 2006 Thomson Higher Educationbrigittanwp putriPas encore d'évaluation
- 1 Year: - ECG Reading Exercise (NOT DONE)Document1 page1 Year: - ECG Reading Exercise (NOT DONE)nonePas encore d'évaluation
- 3) Metabolism and BioenergeticsDocument41 pages3) Metabolism and BioenergeticsnonePas encore d'évaluation
- SDSD Form 06 Parents Guardian ConformeDocument2 pagesSDSD Form 06 Parents Guardian ConformenonePas encore d'évaluation
- Lecture 1. Introduction To BiotechnologyDocument54 pagesLecture 1. Introduction To BiotechnologynonePas encore d'évaluation
- Nervous System of The FrogDocument38 pagesNervous System of The Frognone57% (7)
- Sample Written ReportDocument3 pagesSample Written ReportnonePas encore d'évaluation
- Book-Advanced English For ChemistryDocument65 pagesBook-Advanced English For ChemistryPhuong NguyenPas encore d'évaluation
- Retron DefinitionDocument12 pagesRetron Definitionveromendo100% (1)
- Woodward Fischer ProblemsDocument26 pagesWoodward Fischer ProblemsAyesha RashidPas encore d'évaluation
- Essential Organic Chemistry: Aromaticity: Reactions of Benzene and Substituted BenzenesDocument75 pagesEssential Organic Chemistry: Aromaticity: Reactions of Benzene and Substituted BenzeneschurvaloooPas encore d'évaluation
- Synthesis and Fragrance of Macrocyclic Musks PDFDocument8 pagesSynthesis and Fragrance of Macrocyclic Musks PDF21PHT122 Suhani GargPas encore d'évaluation
- Corey Et Al 2002 General Methods of Synthetic Analysis Strategic Bond Disconnections For Bridged Polycyclic StructuresDocument9 pagesCorey Et Al 2002 General Methods of Synthetic Analysis Strategic Bond Disconnections For Bridged Polycyclic Structurescaiohenriquelins1998Pas encore d'évaluation
- ch1 PDFDocument4 pagesch1 PDFAdriano RayhanPas encore d'évaluation
- Aromatic Chemistry - J. Hepworth, Et Al., (RSC, 2002) BBS PDFDocument180 pagesAromatic Chemistry - J. Hepworth, Et Al., (RSC, 2002) BBS PDFKevin Wang50% (2)
- Green Chemistry in Drug Discovery - From Academia To Industry-Humana (2021)Document624 pagesGreen Chemistry in Drug Discovery - From Academia To Industry-Humana (2021)Nitin BagraPas encore d'évaluation
- Vicky Chahar Vicky Chahar: HomoaromaticityDocument20 pagesVicky Chahar Vicky Chahar: HomoaromaticityVicky ChaharPas encore d'évaluation
- STK 1233 Organic Chemistry 1: LU 5.1: Aromatic CompoundsDocument37 pagesSTK 1233 Organic Chemistry 1: LU 5.1: Aromatic CompoundsArllen Joy AlbertPas encore d'évaluation
- Aromatic CompoundDocument256 pagesAromatic CompoundLuc LePas encore d'évaluation
- UntitledDocument9 pagesUntitled章晴昱Pas encore d'évaluation
- Research Progress of Polycyclic Polyprenylated AcylphloroglucinolsDocument51 pagesResearch Progress of Polycyclic Polyprenylated AcylphloroglucinolsHamid MatariPas encore d'évaluation
- Organic Chemistry - WikipediaDocument11 pagesOrganic Chemistry - Wikipediatsvmpm1765Pas encore d'évaluation
- Expt 7 Classification Tests For HydrocarbonsDocument7 pagesExpt 7 Classification Tests For HydrocarbonsRizzalaine Caringal87% (30)
- Forensic ChemistryDocument6 pagesForensic ChemistryPrincess Mae EstabilloPas encore d'évaluation
- Astm e 204Document16 pagesAstm e 204luisPas encore d'évaluation
- Pub 496Document102 pagesPub 496Bimo Ary Pujangga PutraPas encore d'évaluation
- Chem Volume 3Document170 pagesChem Volume 3Marc Laurenze CelisPas encore d'évaluation
- Stereochemistry by Qari Abdullah SiddiqueDocument48 pagesStereochemistry by Qari Abdullah SiddiqueABDULLAH0% (1)
- First Year Academic Schedule 2023-2024Document16 pagesFirst Year Academic Schedule 2023-2024Piyush SahooPas encore d'évaluation
- Chapter 15 - Organic - 2Document24 pagesChapter 15 - Organic - 2Omar JbaraPas encore d'évaluation
- X Rbse Science-2 Free Notes: Carbon and Important Compounds of CarbonDocument9 pagesX Rbse Science-2 Free Notes: Carbon and Important Compounds of CarbonBhaveshPas encore d'évaluation
- Conformations of AlkanesDocument25 pagesConformations of AlkanesBalakrishna Arpula100% (1)
- SCH 402 Replacement Nomenclature of HeterocyclesDocument11 pagesSCH 402 Replacement Nomenclature of Heterocyclessomon pierre GAHIMBAREPas encore d'évaluation
- 7 Benzene and AromaticsDocument72 pages7 Benzene and Aromaticshamdy solimanPas encore d'évaluation
- Hydrocarbons UsesDocument3 pagesHydrocarbons UsesAj Benito MalidomPas encore d'évaluation
- Laboratory Test For HydrocarbonsDocument2 pagesLaboratory Test For HydrocarbonsdhonaPas encore d'évaluation
- V Aro HydrocarbonsDocument15 pagesV Aro HydrocarbonsSnehalata MishraPas encore d'évaluation