Académique Documents
Professionnel Documents
Culture Documents
Energetics: By: Jose Suter
Transféré par
Jose Gregorio SuterTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Energetics: By: Jose Suter
Transféré par
Jose Gregorio SuterDroits d'auteur :
Formats disponibles
Energetics
BY: JOSE SUTER
Exothermic and Endothermic
When chemical reactions take place, chemical bonds are broken in the reactants and new chemical bonds are formed in the products. It is as a result of these processes that a reaction is overall exothermic (energy is given out to the surroundings) or overall endothermic (energy is absorbed from the surroundings).
Bond breaking as endothermic and bond forming as exothermic
As breaking bonds requires energy the process is endothermic - energy is used up in breaking the bonds, so the surroundings get cooler as heat energy is used up.
As the bonds form to create the energetically products, energy is released, which means that the surroundings warm up, so thats exothermic.
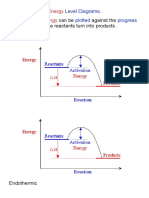
Exothermic Energy Level Diagram
During a chemical reaction energy is either taken in or given out. This means that the energy of the products will be lower than the energy of the reactants. This can be represented using an energy level diagram.
Endothermic Energy Level Diagram
During endothermic reactions energy is taken in. This means the energy of the products will be higher than the energy of the reactants. This energy-level diagram above shows energy has been absorbed in the reaction - is it endothermic.
Activation Energy
The energy curve goes up from the reactants energy level to begin with, then drops to the products energy level. This is because many reactions need an input of energy to start the reaction off. This is energy is called the activation energy. It is represented on an energy level diagram as the difference between the reactants energy level and the top of the curve.
Reminder
Breaking bonds requires energy, you have to put heat in - it is endothermic. This is why melting and boiling are endothermic. Making bonds gives out energy - it is exothermic. This is why freezing and condensing are exothermic In a chemical reaction you need to put energy in to break bonds in the reactants. You get energy out when new bonds are formed to make the products.
Vous aimerez peut-être aussi
- Chemical EnergeticsDocument11 pagesChemical EnergeticsMerab FarooqPas encore d'évaluation
- Chemical EnergeticsDocument11 pagesChemical EnergeticsSafwan MahmudPas encore d'évaluation
- 7-Chemical EnergiticsDocument6 pages7-Chemical EnergiticshusseinrabihhijaziPas encore d'évaluation
- Energetics CHEMDocument12 pagesEnergetics CHEMEgg BreadPas encore d'évaluation
- Energy Changes:: Change in Energy During Chemical ReactionDocument4 pagesEnergy Changes:: Change in Energy During Chemical ReactionadnanPas encore d'évaluation
- Chemical EnergeticsDocument13 pagesChemical EnergeticsVenusCrazy 550Pas encore d'évaluation
- Energy Level Diagrams - WorksheetDocument3 pagesEnergy Level Diagrams - WorksheetLuisPas encore d'évaluation
- Endothermic and ExothermicDocument37 pagesEndothermic and Exothermicactive learning educationPas encore d'évaluation
- Chapter 7Document13 pagesChapter 7Shafiqah AiradzPas encore d'évaluation
- Calorimetry + Bond Energies Study NotesDocument9 pagesCalorimetry + Bond Energies Study Noteszadinova.tereza16Pas encore d'évaluation
- Bond Energy W Endo-Exo Booklet 2 Breakup 1Document9 pagesBond Energy W Endo-Exo Booklet 2 Breakup 1dukethetoxic0Pas encore d'évaluation
- CIE Chemistry Chapter 5 - Chemical EnergeticsDocument12 pagesCIE Chemistry Chapter 5 - Chemical EnergeticsIt's futfutiPas encore d'évaluation
- General Chemistry Ii: Thermochemistry: Energy Changes in A Chemical ReactionDocument35 pagesGeneral Chemistry Ii: Thermochemistry: Energy Changes in A Chemical ReactionRonalda GuevarraPas encore d'évaluation
- Cambridge IGCSE Chemistry Topic 6: Chemical EnergeticsDocument3 pagesCambridge IGCSE Chemistry Topic 6: Chemical EnergeticsTesar DzikrullohPas encore d'évaluation
- 6.1. Energetics of A Reaction SummaryDocument3 pages6.1. Energetics of A Reaction SummaryWilliam TsuiPas encore d'évaluation
- Cambridge IGCSE Chemistry Topic 6: Chemical EnergeticsDocument3 pagesCambridge IGCSE Chemistry Topic 6: Chemical EnergeticsDakwan InPas encore d'évaluation
- 7.01 Endothermic and ExothermicDocument4 pages7.01 Endothermic and ExothermicYangelis Martinez50% (2)
- General Chemistry2 - Lesson3Document3 pagesGeneral Chemistry2 - Lesson3Ronalda GuevarraPas encore d'évaluation
- Examples of Exothermic ReactionsDocument14 pagesExamples of Exothermic ReactionsshaliniPas encore d'évaluation
- Energetics Note 1Document15 pagesEnergetics Note 1shaliniPas encore d'évaluation
- IGCSE Chemistry Energy ChangesDocument11 pagesIGCSE Chemistry Energy ChangesahmedPas encore d'évaluation
- Energy and Chemical Change Grade 11Document14 pagesEnergy and Chemical Change Grade 11Reitumetse MolefePas encore d'évaluation
- Chemistry Week 7Document5 pagesChemistry Week 7EDUARDO lll NADATEPas encore d'évaluation
- Cambridge IGCSE Chemistry Topic 6: Chemical EnergeticsDocument3 pagesCambridge IGCSE Chemistry Topic 6: Chemical EnergeticsretaPas encore d'évaluation
- Energy ChangesDocument18 pagesEnergy ChangesPatricia CadacioPas encore d'évaluation
- Energy Calculations: Flash Notes: Comparing The Energy Produced by FuelsDocument3 pagesEnergy Calculations: Flash Notes: Comparing The Energy Produced by Fuelsapi-25909541Pas encore d'évaluation
- Exit Task Energy ChangesDocument2 pagesExit Task Energy ChangesPriya Elizabeth Aruldass HenryPas encore d'évaluation
- Chemical Energetics AssignmentDocument3 pagesChemical Energetics AssignmentGovindi BahadurPas encore d'évaluation
- Unit 1.2 Chemistry As Book EditedDocument20 pagesUnit 1.2 Chemistry As Book EditedJamsheed KakarPas encore d'évaluation
- Endothermic and Exothermic ReactionDocument5 pagesEndothermic and Exothermic ReactionMuhammad Umar SalmanPas encore d'évaluation
- Chemical Energy PDFDocument12 pagesChemical Energy PDFfarsxdchgPas encore d'évaluation
- Activation Energy and Reaction ProfilesDocument5 pagesActivation Energy and Reaction Profileswama ojhaPas encore d'évaluation
- EnergeticsDocument12 pagesEnergeticsAliyah HamiltonPas encore d'évaluation
- 7049df2e 10 Chemical EnergeticsDocument17 pages7049df2e 10 Chemical EnergeticsMuhammad UzairPas encore d'évaluation
- 4 5 Energy ChangesDocument3 pages4 5 Energy ChangesAmmaarPas encore d'évaluation
- Exothermic ReactionsDocument25 pagesExothermic ReactionsazilaPas encore d'évaluation
- Chemical EnergyDocument12 pagesChemical EnergyMathews ZimbaPas encore d'évaluation
- Chemical EnergyDocument12 pagesChemical EnergyFaiza RizwanPas encore d'évaluation
- Activation Energy and Reaction ProfileDocument18 pagesActivation Energy and Reaction Profilesayma_akhter5074Pas encore d'évaluation
- Cooling TowerDocument31 pagesCooling TowerAhmed GadPas encore d'évaluation
- 5.1. Exothermic and Endothermic ReactionsDocument3 pages5.1. Exothermic and Endothermic ReactionsVictoria AdenowoPas encore d'évaluation
- Energy Changes PDFDocument4 pagesEnergy Changes PDFMahmudul Hassan ShuvoPas encore d'évaluation
- Igcse1022 Chemistry 1 2Document9 pagesIgcse1022 Chemistry 1 2Nguyen (Harry) Xuan HoangPas encore d'évaluation
- Week 007 Module ThermochemistryDocument12 pagesWeek 007 Module ThermochemistryFigh terPas encore d'évaluation
- Olevel - Energy ChangesDocument2 pagesOlevel - Energy ChangesFayzanAbdulWasayPas encore d'évaluation
- 5.1 Exothermic & Endothermic ReactionsDocument14 pages5.1 Exothermic & Endothermic ReactionstmmbonelaPas encore d'évaluation
- Chm271 - Chapter 2 Thermochemistry - UpdatedDocument68 pagesChm271 - Chapter 2 Thermochemistry - UpdatedNurfarhanah AsyknPas encore d'évaluation
- 5 1 Exothermic and Endothermic ReactionsDocument6 pages5 1 Exothermic and Endothermic ReactionsNguyenHoangMinhDucPas encore d'évaluation
- Exothermic Vs EndothermicDocument18 pagesExothermic Vs Endothermicapi-449002661Pas encore d'évaluation
- Energy ChangesDocument32 pagesEnergy ChangesABCPas encore d'évaluation
- Video: Exothermic and Endothermic Reactions, Energy Profile Diagram, Activation EnergyDocument2 pagesVideo: Exothermic and Endothermic Reactions, Energy Profile Diagram, Activation EnergyArmaan NooraniPas encore d'évaluation
- 377chemistry Unit 4 Notes CompleteDocument65 pages377chemistry Unit 4 Notes Completemuddasser91100% (3)
- 5.1 EnergeticsDocument8 pages5.1 EnergeticsEldin EnggPas encore d'évaluation
- Endothermic and Exothermic ReactionDocument70 pagesEndothermic and Exothermic Reactionactive learning educationPas encore d'évaluation
- Unit 3 Physical ChemistryDocument16 pagesUnit 3 Physical ChemistryAmna AmerPas encore d'évaluation
- 5 3 Enthalpy Change and Exothermic and Endothermic ReactionsDocument24 pages5 3 Enthalpy Change and Exothermic and Endothermic Reactionsapi-210028385Pas encore d'évaluation
- Practice Makes Perfect in Chemistry: The Physical Behavior of Matter with AnswersD'EverandPractice Makes Perfect in Chemistry: The Physical Behavior of Matter with AnswersPas encore d'évaluation
- “Foundations to Flight: Mastering Physics from Curiosity to Confidence: Cipher 4”: “Foundations to Flight: Mastering Physics from Curiosity to Confidence, #4D'Everand“Foundations to Flight: Mastering Physics from Curiosity to Confidence: Cipher 4”: “Foundations to Flight: Mastering Physics from Curiosity to Confidence, #4Pas encore d'évaluation
- How Do Cities Reduce EcoDocument2 pagesHow Do Cities Reduce EcoJose Gregorio SuterPas encore d'évaluation
- Ecology - Food Webs and Chains: Year 11 Pre-Diploma BiologyDocument3 pagesEcology - Food Webs and Chains: Year 11 Pre-Diploma BiologyJose Gregorio SuterPas encore d'évaluation
- MutualismDocument2 pagesMutualismJose Gregorio SuterPas encore d'évaluation
- Hybridisation QuestionsdDocument2 pagesHybridisation QuestionsdJose Gregorio SuterPas encore d'évaluation
- Lion NicheDocument1 pageLion NicheJose Gregorio SuterPas encore d'évaluation
- Magnetic Field ExperimentDocument5 pagesMagnetic Field ExperimentJose Gregorio SuterPas encore d'évaluation
- Project 2 Information Sources Table: NamesDocument4 pagesProject 2 Information Sources Table: NamesJose Gregorio SuterPas encore d'évaluation
- Spreadsheet ReviewDocument1 pageSpreadsheet ReviewJose Gregorio SuterPas encore d'évaluation
- BrazingDocument13 pagesBrazingjoncattPas encore d'évaluation
- Chapter 13 Assertion-Reason QuestionsDocument3 pagesChapter 13 Assertion-Reason Questionsteresa tsoiPas encore d'évaluation
- Welded MasterlinkDocument1 pageWelded MasterlinkWerner SchulzPas encore d'évaluation
- Understanding Cloud Point and Hydrotreating Relationships RevisedDocument4 pagesUnderstanding Cloud Point and Hydrotreating Relationships RevisedAshwani KumarPas encore d'évaluation
- Ryan Mudge ResumeDocument2 pagesRyan Mudge Resumeapi-256246508Pas encore d'évaluation
- Frequently Asked Questions (FAQ) About Hot-Dip Galvanized Reinforcing BarsDocument6 pagesFrequently Asked Questions (FAQ) About Hot-Dip Galvanized Reinforcing Barsali tahaPas encore d'évaluation
- ECE Catalog 2012Document52 pagesECE Catalog 2012xerlinoPas encore d'évaluation
- Effects of Cryogenic Treatment On Cutting Tool DurabilityDocument19 pagesEffects of Cryogenic Treatment On Cutting Tool DurabilityShaheen KunhikrishnanPas encore d'évaluation
- Nom 101Document11 pagesNom 101Anonymous hAAmGohAcPas encore d'évaluation
- Robo TiPTiG Data Sheet FinalDocument2 pagesRobo TiPTiG Data Sheet FinalcwiksjPas encore d'évaluation
- 3003 Aluminum Sheet SuppliersDocument12 pages3003 Aluminum Sheet Supplierssanghvi overseas incPas encore d'évaluation
- Kr274atl - e Ga8731 PartsDocument276 pagesKr274atl - e Ga8731 Partsomar100% (1)
- Asme Section Ii A Sa-351 Sa-351m PDFDocument8 pagesAsme Section Ii A Sa-351 Sa-351m PDFdavid perezPas encore d'évaluation
- Norton Water Stone Users GuideDocument2 pagesNorton Water Stone Users Guidejb71xx100% (1)
- Two Stroke Diesel Engine AyeshaDocument8 pagesTwo Stroke Diesel Engine Ayeshaayesha amjadPas encore d'évaluation
- Standard Cleaning Procedures of Nail Care ToolsDocument3 pagesStandard Cleaning Procedures of Nail Care ToolsIrish Nicole PacionPas encore d'évaluation
- Experiment 2 Electroplating and Galvanic Protection Objectives: Experiment To Demonstrate Electroplating and Galvanic Protection TheoryDocument9 pagesExperiment 2 Electroplating and Galvanic Protection Objectives: Experiment To Demonstrate Electroplating and Galvanic Protection Theoryboatcom100% (1)
- Astm A 681Document14 pagesAstm A 681talhadikenPas encore d'évaluation
- MS For HVAC Ducting Installation and PipingDocument18 pagesMS For HVAC Ducting Installation and PipingProject enghvacPas encore d'évaluation
- JDM A22 - Rev 06-1987Document8 pagesJDM A22 - Rev 06-1987Reginaldo SantosPas encore d'évaluation
- Paint Specification No.: SSPC: The Society For Protective CoatingsDocument6 pagesPaint Specification No.: SSPC: The Society For Protective CoatingsanoopkumarPas encore d'évaluation
- Iso 683 1 2012Document13 pagesIso 683 1 2012Haluk TOKGÖZPas encore d'évaluation
- Petrochemical ProcessDocument20 pagesPetrochemical Processsanjeevs01Pas encore d'évaluation
- VAUTID ASW 145 Engl 041016Document1 pageVAUTID ASW 145 Engl 041016maiquelernPas encore d'évaluation
- Key Facts Typical Wire Analysis: - Bossweld 71T-1Document1 pageKey Facts Typical Wire Analysis: - Bossweld 71T-1cj elec techPas encore d'évaluation
- 3M-Catálogo - Aerospace Surface Protection SolutionsDocument15 pages3M-Catálogo - Aerospace Surface Protection SolutionsAnonymous mq0U43UsPPas encore d'évaluation
- Interlac 665 PDFDocument4 pagesInterlac 665 PDFEngTamerPas encore d'évaluation
- Scribbing Tool BG 17Document11 pagesScribbing Tool BG 17DeniMestiWidiantoPas encore d'évaluation
- Api 571Document41 pagesApi 571majid100% (1)
- Size PressDocument2 pagesSize Presstsvmpm1765Pas encore d'évaluation