Académique Documents
Professionnel Documents
Culture Documents
Heat Transfer Experiment
Transféré par
Jeanne Kamille Evangelista PiniliCopyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Heat Transfer Experiment
Transféré par
Jeanne Kamille Evangelista PiniliDroits d'auteur :
Formats disponibles
I.
Heat Transfer Conduction the transfer of heat through the transmission of energy between particles during collisions Convection the transfer of heat in liquids and gases through convection currents brought by the rising of heated particles and sinking of cooler and denser particles Radiation the transfer of heat energy by electromagnetic waves
Conduction occurs as the energized particles transfer energy through contact; gas particles near source of heat solid particles in the beaker, tripod and wire gauze liquid particles near the base of the beaker. Convection currents are formed as the water near the base of the liquid expands and becomes less dense due to heat. The heated liquid rises as the denser and cooler liquid fills up the vacuum left by risen liquid. The liquid that sank becomes heated as well and rises. The cycle continues as long as theres any temperature difference. Gases near the mouth of the beaker also heats up and rises, their place filled up by cooler air. Heat transfer also occurs through radiation as energized gas particles near the mouth of the gas lamp speeds up, leaving small spaces of vacuum where heat travels through electromagnetic waves.
II. Effects of Heat (Thermal Expansion)
At room temperature, the metal ball fit the hole easily. When the metal ball was heated, the movement of the particles became more rapid due to the accumulation of
energy. The rapid movement caused the particles to veer farther from one another, leading it to expand and increase in volume. It didnt fit the hole then. Once the ball was cooled, the particles contracted.
Vous aimerez peut-être aussi
- Scott Foresman Heat 4 12Document10 pagesScott Foresman Heat 4 12api-238216496Pas encore d'évaluation
- Conduction Convection Radiation PowerpointDocument27 pagesConduction Convection Radiation PowerpointApet Satusembilansembilan JiePas encore d'évaluation
- Conduction Convection Radiation PowerpointDocument18 pagesConduction Convection Radiation PowerpointRavichandran G100% (1)
- A Piece of the Sun: The Quest for Fusion EnergyD'EverandA Piece of the Sun: The Quest for Fusion EnergyÉvaluation : 3 sur 5 étoiles3/5 (4)
- Water and Wastewater Treatment in The Alcoholic BeverageDocument33 pagesWater and Wastewater Treatment in The Alcoholic BeverageJeanne Kamille Evangelista PiniliPas encore d'évaluation
- Design of Sand FilterDocument5 pagesDesign of Sand FilterJeanne Kamille Evangelista Pinili100% (1)
- Golden Ratio Geometry and The Fine-Structure Constant2Document8 pagesGolden Ratio Geometry and The Fine-Structure Constant2Michael A. SherbonPas encore d'évaluation
- MATLAB: Rocket Launcher SimulationDocument4 pagesMATLAB: Rocket Launcher SimulationAhsan NaeemPas encore d'évaluation
- Stability of ColloidDocument27 pagesStability of ColloidHendrawan SaputraPas encore d'évaluation
- Activity No. 1: Modes of Heat TransferDocument7 pagesActivity No. 1: Modes of Heat TransferSquidward TentaclesPas encore d'évaluation
- Interface Mass TraDocument26 pagesInterface Mass TraWahid AliPas encore d'évaluation
- Modesoftransferofheat 160103051008Document23 pagesModesoftransferofheat 160103051008Evangelene Esquillo SanaPas encore d'évaluation
- Plate Tectonics Section 2 Reading SummaryDocument1 pagePlate Tectonics Section 2 Reading SummaryDarlene AmpocPas encore d'évaluation
- NGSS3D MSPS ThermalEnergyTransfer EXPLAIN STEMscopediaDocument7 pagesNGSS3D MSPS ThermalEnergyTransfer EXPLAIN STEMscopediaSivekiPas encore d'évaluation
- Heat ActivityDocument3 pagesHeat ActivitySittie Jivaiza Malanji MalantocPas encore d'évaluation
- 1 Heat TransferDocument3 pages1 Heat TransferAdam HartmannPas encore d'évaluation
- Sci Oly 2.0Document12 pagesSci Oly 2.0arinaitwejoshua24Pas encore d'évaluation
- 11-Transfer of Thermal EnergyDocument46 pages11-Transfer of Thermal Energyrodel.verzosaPas encore d'évaluation
- Introduction Transfer of HeatDocument17 pagesIntroduction Transfer of HeatNazakat HussainPas encore d'évaluation
- 3rd Quarter SCIENCE 4 WEEK 6 Heat EnergyDocument25 pages3rd Quarter SCIENCE 4 WEEK 6 Heat EnergyCharlesJames MaruquezPas encore d'évaluation
- Conduction, Convection, RadiationDocument4 pagesConduction, Convection, RadiationChloe LiewPas encore d'évaluation
- HE BackDocument2 pagesHE BackJoshua BrightPas encore d'évaluation
- Heat Transfer TransesDocument2 pagesHeat Transfer TransesERICKA MAE CANTOSPas encore d'évaluation
- 7.2 Heat FlowDocument50 pages7.2 Heat FlowNITIASSWAREPas encore d'évaluation
- Heat Enegy TransferDocument6 pagesHeat Enegy TransferManya SharmaPas encore d'évaluation
- Heat Transfer Methods 2017 Class Notes AutosavedDocument39 pagesHeat Transfer Methods 2017 Class Notes AutosavedItsMe SirMJPas encore d'évaluation
- Project in ScienceDocument4 pagesProject in ScienceArsharip Hasim TaupPas encore d'évaluation
- Moving Particles: Heat Energy Is in Volcanoes and IceDocument5 pagesMoving Particles: Heat Energy Is in Volcanoes and IceRovejane S. SalvacionPas encore d'évaluation
- Act 8a. Convection Current ArticleDocument2 pagesAct 8a. Convection Current ArticleGUILLER BELENPas encore d'évaluation
- 07 - Temperature & HeatDocument18 pages07 - Temperature & HeatKrisha PatelPas encore d'évaluation
- Lecture 4 Transmission of HeatDocument18 pagesLecture 4 Transmission of Heatdinesh11rPas encore d'évaluation
- 2.3 Thermal ProcessesDocument6 pages2.3 Thermal ProcesseshaiderPas encore d'évaluation
- Heat Energy (GZ) 2017Document20 pagesHeat Energy (GZ) 2017kaviPas encore d'évaluation
- Temperature and HeatDocument25 pagesTemperature and HeatAlessandro YumangPas encore d'évaluation
- Energy Transfer ConductionconvectionradiationDocument14 pagesEnergy Transfer ConductionconvectionradiationalongsilatPas encore d'évaluation
- Energy Transfer ConductionconvectionradiationDocument14 pagesEnergy Transfer Conductionconvectionradiationkumpul tugasPas encore d'évaluation
- Formative Assessment 8Document1 pageFormative Assessment 8bashadelacruz7Pas encore d'évaluation
- Thermal Energy Transfer PPDocument20 pagesThermal Energy Transfer PPvenkat_09Pas encore d'évaluation
- TheoryDocument2 pagesTheoryapi-368165787Pas encore d'évaluation
- Heatvs Tem2Document18 pagesHeatvs Tem2Ross Adrales GeleraPas encore d'évaluation
- Energy TransferDocument6 pagesEnergy TransferSamin YasarPas encore d'évaluation
- Heat TransferDocument24 pagesHeat TransferABEGAIL SOLIMANPas encore d'évaluation
- Heat For Form 1 ADocument61 pagesHeat For Form 1 AChrise RajPas encore d'évaluation
- Thermal Physics Project AssignmentDocument3 pagesThermal Physics Project Assignmentsparkle-A TenchPas encore d'évaluation
- Thermal Energy ReadingDocument5 pagesThermal Energy Readingapi-189616674Pas encore d'évaluation
- HeatDocument16 pagesHeatapi-233194737Pas encore d'évaluation
- Heat Energy Transfer BookletDocument4 pagesHeat Energy Transfer BookletLaurenPas encore d'évaluation
- Review of Past Terms:: - Define "Energy"Document26 pagesReview of Past Terms:: - Define "Energy"Tapish GoelPas encore d'évaluation
- TBI Heat TransferDocument7 pagesTBI Heat TransferRobPas encore d'évaluation
- Thermal Physics NotesDocument26 pagesThermal Physics Notesantonettemosweu211Pas encore d'évaluation
- How Heat Is Produced: 4 Grade ScienceDocument20 pagesHow Heat Is Produced: 4 Grade Sciencenoman turkPas encore d'évaluation
- ScienceDocument9 pagesScienceMary Ann B. ChorhangonPas encore d'évaluation
- Conduction, Convection and Radiation: Heat TransferDocument14 pagesConduction, Convection and Radiation: Heat Transferlucky asliPas encore d'évaluation
- Energy Transfer ConductionconvectionradiationDocument14 pagesEnergy Transfer ConductionconvectionradiationMuqeet76Pas encore d'évaluation
- CH 12 SciDocument8 pagesCH 12 SciHend HamedPas encore d'évaluation
- Heat TransfersDocument16 pagesHeat Transfersrahayuutami30Pas encore d'évaluation
- Flow of Heat: Chapter 6 (Physics)Document56 pagesFlow of Heat: Chapter 6 (Physics)Pratham KanwraniPas encore d'évaluation
- Heat TransferDocument2 pagesHeat TransferRitishBoodhunPas encore d'évaluation
- Heat and 3 Heat TransfersDocument31 pagesHeat and 3 Heat TransfersAlfred Cedrix BornelPas encore d'évaluation
- Menoufia UniversityDocument4 pagesMenoufia Universityelking.ahmed1562002Pas encore d'évaluation
- Heat Transfer: Prepared By: T. Analyn M. Pepito Date: February 16, 2009Document20 pagesHeat Transfer: Prepared By: T. Analyn M. Pepito Date: February 16, 2009annuobPas encore d'évaluation
- Hot Globe and No Greenery Courtesy of the Carbon Hater's CultD'EverandHot Globe and No Greenery Courtesy of the Carbon Hater's CultPas encore d'évaluation
- Case-Bart Case StudyDocument4 pagesCase-Bart Case StudyJeanne Kamille Evangelista PiniliPas encore d'évaluation
- Capitalized Cost P50,000 1.5Document7 pagesCapitalized Cost P50,000 1.5Jeanne Kamille Evangelista PiniliPas encore d'évaluation
- Mechanics ReviewerDocument4 pagesMechanics Reviewerrmgc1003Pas encore d'évaluation
- Capitalized Cost P50,000 1.5Document7 pagesCapitalized Cost P50,000 1.5Jeanne Kamille Evangelista PiniliPas encore d'évaluation
- Functional Food Case StudyDocument7 pagesFunctional Food Case StudyJeanne Kamille Evangelista PiniliPas encore d'évaluation
- 5.3 Environmental Management System (EMS)Document1 page5.3 Environmental Management System (EMS)Jeanne Kamille Evangelista PiniliPas encore d'évaluation
- Type of Fermentation Description (+) Advantages/ (-) DisadvantagesDocument2 pagesType of Fermentation Description (+) Advantages/ (-) DisadvantagesJeanne Kamille Evangelista PiniliPas encore d'évaluation
- Jess Anthony B. Dicdiquin Bs Che IvDocument2 pagesJess Anthony B. Dicdiquin Bs Che IvJeanne Kamille Evangelista PiniliPas encore d'évaluation
- Waste Management InitiativesDocument1 pageWaste Management InitiativesJeanne Kamille Evangelista PiniliPas encore d'évaluation
- Banana LiteratureDocument3 pagesBanana LiteratureJeanne Kamille Evangelista PiniliPas encore d'évaluation
- Temperature, °C: SolutionDocument18 pagesTemperature, °C: Solutionمحمد حلمي هاريريPas encore d'évaluation
- ChapterDocument36 pagesChapterJeanne Kamille Evangelista PiniliPas encore d'évaluation
- Double Cone Blender - ChallengerDocument14 pagesDouble Cone Blender - ChallengerJeanne Kamille Evangelista PiniliPas encore d'évaluation
- September 2014 : Friday Saturday Monday Sunday Tuesday Wednesday ThursdayDocument1 pageSeptember 2014 : Friday Saturday Monday Sunday Tuesday Wednesday ThursdayJeanne Kamille Evangelista PiniliPas encore d'évaluation
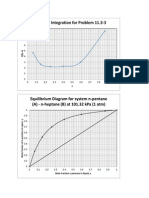
- Numerical IntegrationDocument1 pageNumerical IntegrationJeanne Kamille Evangelista PiniliPas encore d'évaluation
- United We Stand, Divided We FallDocument2 pagesUnited We Stand, Divided We FallJeanne Kamille Evangelista PiniliPas encore d'évaluation
- SEM Micrograph of The Diamond Film Surface Deposited Under High Perpendicularity ConditionsDocument2 pagesSEM Micrograph of The Diamond Film Surface Deposited Under High Perpendicularity ConditionsJeanne Kamille Evangelista PiniliPas encore d'évaluation
- Adv Math ProjectDocument19 pagesAdv Math ProjectJeanne Kamille Evangelista PiniliPas encore d'évaluation
- Centre of MassDocument32 pagesCentre of MassR.Yashwanth Raj XBPas encore d'évaluation
- Motion and Types of MotionDocument15 pagesMotion and Types of MotionLudy LynPas encore d'évaluation
- Investigation of Optical and Structural PropertiesDocument14 pagesInvestigation of Optical and Structural PropertiesmarcosdavidPas encore d'évaluation
- Snell's LawDocument9 pagesSnell's Lawnai1690Pas encore d'évaluation
- Flow Loss in Screens: A Fresh Look at Old CorrelationDocument6 pagesFlow Loss in Screens: A Fresh Look at Old CorrelationBenito.camelasPas encore d'évaluation
- Physics: Chapterwise Practise Problems (CPP) For JEE (Main & Advanced) Chapter - Units and MeasurementDocument4 pagesPhysics: Chapterwise Practise Problems (CPP) For JEE (Main & Advanced) Chapter - Units and MeasurementKripa mariam JosephPas encore d'évaluation
- C. de Koninck - Random Reflections On Science & CalculationDocument37 pagesC. de Koninck - Random Reflections On Science & CalculationJoseph TrabbicPas encore d'évaluation
- Acmos 47Document5 pagesAcmos 47Akash VermaPas encore d'évaluation
- Weston 2018Document7 pagesWeston 2018Kartick TarafderPas encore d'évaluation
- Group1 ThermodynamicsDocument4 pagesGroup1 ThermodynamicsMICHAEL ANGELO BRUFALPas encore d'évaluation
- Composite MaterialsDocument2 pagesComposite MaterialsmaheshPas encore d'évaluation
- Thesis Lid DrivenDocument145 pagesThesis Lid DrivenEddieq100% (1)
- A Representative Volume Element Simulation of A Thermo Plastic ElastomerDocument34 pagesA Representative Volume Element Simulation of A Thermo Plastic Elastomervivashwanth paiPas encore d'évaluation
- 1 02 PDFDocument16 pages1 02 PDFircivilcivilPas encore d'évaluation
- Using Labview To Measure Temperature With A ThermistorDocument18 pagesUsing Labview To Measure Temperature With A ThermistorjdaudpotoPas encore d'évaluation
- Mechanics of SolidsDocument4 pagesMechanics of SolidsHiren DesaiPas encore d'évaluation
- PhysicsDocument4 pagesPhysicsramanagopalPas encore d'évaluation
- Skema Fizik Percubaan K1 F5 Kedah 2016Document4 pagesSkema Fizik Percubaan K1 F5 Kedah 2016Cheah Soon TikePas encore d'évaluation
- Electromagnetism STUDocument19 pagesElectromagnetism STUFirdaus BhathenaPas encore d'évaluation
- Inorganic and Organic Chem PrelimsDocument28 pagesInorganic and Organic Chem PrelimsAlly WelchPas encore d'évaluation
- Rutherford's Alpha-Particle Scattering ExperimentDocument10 pagesRutherford's Alpha-Particle Scattering ExperimentAhmad HussainPas encore d'évaluation
- Chapter 3 - Hydrostatic Forces On Submerged SurfacesDocument17 pagesChapter 3 - Hydrostatic Forces On Submerged SurfacesGSaurav DahalPas encore d'évaluation
- 4-1 / 6-1 Energy - Physics and Trilogy: 1.0 A Weightlifter Picks Up A BarbellDocument11 pages4-1 / 6-1 Energy - Physics and Trilogy: 1.0 A Weightlifter Picks Up A BarbellHaleemahPas encore d'évaluation
- Compressible Flow ReviewDocument8 pagesCompressible Flow Reviewthehighlife1080Pas encore d'évaluation
- Kristin Ackerson, Virginia Tech EE Spring 2002Document27 pagesKristin Ackerson, Virginia Tech EE Spring 2002hiteshsoftPas encore d'évaluation
- Kseb Supply Code 2012 PDFDocument134 pagesKseb Supply Code 2012 PDFfaizalpsPas encore d'évaluation