Académique Documents
Professionnel Documents
Culture Documents
009 C 187 D 74
Transféré par
Alicia ShortTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
009 C 187 D 74
Transféré par
Alicia ShortDroits d'auteur :
Formats disponibles
Organic Chemistry I Exam 4 20101 Name KEY
Multiple Choice - Circle the letter of the best choice for the answer to the question. (2 1/3 Points each) 1. The correct IUPAC name for the following compound is:
Br
A) 2-Bromo-4-methylenehexane C) E) 2-(2-Bromopropyl)-1-butene 4-Bromo-2-ethyl-1-pentene
B) 2-Bromo-4-ethyl-1-pentene D) 2-Bromo-4-ethyl-4-pentene
2.
Give the IUPAC name for
A) 3-Methyl-4-hexyne C) E) 4-Methyl-2-hexyne 2-Ethyl-3-pentyne
B) 4-Ethyl-2-pentyne D) 3-Methyl-2-hexyne
3.
The correct IUPAC name for the following compound is:
Cl
Br
A) (E)-2-Bromo-3-chloro-5-methyl-2hexene C) (E)-2-Bromo-3-chloro-5-methyl-3hexene E) (Z)-2-Bromo-3-chloro-5-methyl-3hexene
B) (Z)-2-Bromo-3-chloro-5-methyl-2hexene D) (E)-2-Methyl-5-bromo-4-chloro-4hexene
4.
What is the correct IUPAC name for the following compound?
CH3 CH3CHOHCHCHCH(CH3)2 CH3
A) 4-isopropyl-3,4-dimethyl-2-butanol C) E) 2,3,4-trimethyl-4-pentanol 1,1,2,3-tetramethyl-4-pentanol
B) 3,4,5-trimethyl-2-hexanol D) 3,4,5,5-tetramethyl-2-pentanol
5.
Methyl tert-butyl ether has the IUPAC name:
CH3 CH3 O C CH3 CH3
A) methyl dimethylethyl ether C) E) methyoxy-t-butane 2-methoxy-2-methylpropane
B) t-butoxymethane D) 1,1-dimethyl-1methoxyethane
6.
Which of the following carbocations would NOT be likely to undergo rearrangement? A) C)
CH3CHCHCH3 CH3 CH3
CH3CHCCH3 CH3
B) D)
CH3 CH3CHCH2 CH3
CH3CCHCH2CH3 CH3
E)
CH3 CH3CCH2CH3
7.
What would be the major product of the following reaction?
HCl
Cl Cl
Cl Cl
I
Cl
II
Cl
III
IV
A) I C) E) III V
B) II D) IV
8.
The reaction of Br2/CCl4 to cyclohexene would produce the compound(s) represented by structure(s):
H H Br Br Br H H Br Br H Br H
I
A) I alone C) E) III alone II and III (enantiomers)
II
III
B) II alone D) I , II and II
9.
What product would result from the following reaction?
KMnO4, H2O cold, dilute
?
CO2H O K MnO4 OH OH
II
III
OH OH
OH OH
IV
A) I C) E) III V
B) II D) IV
10.
Select the structure of the major product formed in the following reaction.
2 HCl
Cl Cl
Cl Cl
I
Cl Cl
II
Cl Cl
III
IV
A) I C) E) III V
V
B) II D) IV
11.
Which of these is not formed when cyclopentene reacts with an aqueous solution of bromine (Br2 in H2O)?
Br OH Br OH Br Br Br Br OH OH
II
III
IV
A) I C) E) III V
B) II D) IV
12.
How many compounds are possible from the addition of bromine to CH2=CHCH2CH3 (counting stereoisomers separately)? A) One C) E) Three Five B) Two D) Four
13.
In general, when the addition of an unsymmetrical electrophilic reagent to an unsymmetrical alkene forms the product predicted by Markovnikov's rule, that occurs because: A) the product is statistically favored. C) E) steric hindrance favors its formation. All of the above are reasons. B) it is the more/most stable product. D) it is formed via the more/most stable carbocation.
14.
Which of these compounds belongs to the class of substances commonly known as "halohydrins"? A) BrCH2CH2Cl C) E) ClCH2CO2H ICH2CH2OH B) FCH2CH2NH2 D) HOCH2 COCl
15.
The most resistant compound to the action of hot alkaline KMnO4 is: A) Pentane C) E) 1-Pentene 2-Pentene B) 2-Pentyne D) Cyclopentene
16.
Consider the ozonolysis products obtained from all the unbranched and unsymmetrical isomers of heptene. The reaction product in each case would consist of: A) a single aldehyde. C) E) an aldehyde and a ketone. two different ketones. B) two different aldehydes. D) a single ketone.
17.
In the presence of light, ethane (1 mol) reacts with chlorine (1 mol) to form which product(s)? A) CH2ClCHCl2 C) E) CH3CHCl2 CH3CH2Cl (one point for this one) B) ClCH2CH2Cl D) All of these
18.
Select the structure of the major product formed in the following reaction.
CH3
Br2 h
?
Br CH3
CH2Br
Br
CH3
CH3 Br
Br
II
III
IV
A) I C) E) III V
B) II D) IV
19.
Which of the following combinations of reactants can provide a demonstrable example of anti-Markovnikov addition? A) CH2=CHCH3 + HCl + ROOR C) E) CH3CH=CH2 + H2O + Cl2 CH3CH=CHCH3 + HBr + ROOR B) CH3CH2CH=CH2 + HBr + ROOR D) CH3CH2CH=CH2 + Br2 + ROOR
20.
What sequence of reactions could be used to prepare cis-1,2-cyclopentanediol from cyclopentane? (1) Cl2, h; (2) t-BuOK/t-BuOH; (3) OsO4; (4) NaHSO3/H2O (1) t-BuOK/t-BuOH; (2) Cl2, h; (3) NaOH/H2O (1) Cl2, h; (2) t-BuOK/t-BuOH; (3) H2O2 (1) NaOH/H2O; (2) Br2; (3) NaNH2(2eq.)/liq.NH3; (4) KMnO4, NaOH/H2O, 5C E) (1) Cl2, h; (2) t-BuOK/t-BuOH; (3) Hg(OAc)2, H2O (4) NaBH4, H3O+ A) B) C) D)
21.
The p-orbital of a methyl radical carbon, CH3, contains how many electrons? A) 1 C) E) 3 0 B) 2 D) 4
22.
Which of the following free radicals is the most stable? A)
CH2 CH3CHCH2CH3
B)
CH3 CH3CHCHCH3
C)
CH3 CH3CHCH2CH2
D)
CH3 CH3CCH2CH3
E)
CH3 CH2CHCH2CH3
23.
Free radicals can be produced by: A) use of high temperatures. C) E) irradiation with light. all of A), B) and C). B) reaction of a molecule with another free radical. D) both A) and B).
24.
What is the final product, C, obtained via the following reaction sequence?
Br2 h A t-BuOK t-BuOH heat
OH
i) BH3, THF ii) H2O2, OH
OH
OH
OH
OH OH
II
III
IV
A) I C) E) III V
B) II D) IV
25.
Which of the following reactions would serve as a synthesis of butyl bromide? A)
CH3CH2CH2CH2OH + HBr reflux
B) CH CH CH CH OH + PBr 3 2 2 2 3 reflux C) CH3CH2CH2CH2OH + NaBr D) CH CH CH CH OH + Br 3 2 2 2 2 E) Answers A) and B) only (one point for A or B)
26.
The product(s) of the following reaction
CH2 CH2 O CH2
excess HBr
CH2
heat
is/are:
CH3CH2OCH2CH3 I
CH3CH2CH2CH2OH and CH3CH2CH2CH2Br II
CH2 CHBr CH2 O
BrCH2CH2CH2CH2OH and BrCH2CH2CH2CH2Br III
A) I C) E) III (Excess HBr) None of these (1 point for this) B) II D) IV
CH2
IV
27.
Which product(s) would you expect to obtain from the following sequence of reactions?
CH3
1. BH3-THF 2. H2O2, NaOH
?
H3C O
CH3 CH3 OH OH
CH3 OH
CH2OH
+ enantiomer I II
+ enantiomer III
+ enantiomer IV V
A) I C) E) III (Syn addition of H and OH) V
B) II D) IV
28.
Select the structure of the major product formed from the following reaction.
CH3
1. Hg(OOCCH3)2 THF, H2O 2. NaBH4, NaOH
CH3 OH
CH3
CH2OH
HO CH3
CH3
OH OH
II
III
IV
A) I C) E) III V
B) II D) IV
29.
Which is the best way to prepare 3-methoxypentane via the Williamson method? (Hint - Draw the structure of the product first.) A) B) C) D) E) CH3OH + CH3CH2CHOHCH2CH3 + H2SO4, 140C CH3OH + (CH3)2CHCH2CH2OH + H2SO4, 140C CH3ONa + (CH3CH2)2CHBr (Mostly E2) CH3I + (CH3CH2)2CHONa CH3I + (CH3)2CHCH2CH2ONa
30.
Epoxidation followed by reaction with aqueous base converts cyclopentene into which of these?
H OH H H OH OH H OH OH H H H OH OH
II
III
IV
A) I C) III
B) II D) IV
E)
Equal amounts of III and IV
(III and IV are enantiomers)
31.
What would be the final product?
O CH3 RCOOH H3CC CH2
product
CH3OH, HA
final product
A) (CH3)2CHCH2OCH3 B) (CH3)2CCH3
OCH3
C) (CH3)2CCH2OH (the nucleophile attacks the most sub. C in acidic conditions (HA)) D) (CH3)2CCH2OCH3 E)
OH (CH3)2CCH2OCH3
OCH3
OCH3
32.
What would be the major product of the following reaction?
H OH CH3 H H I CH3 H H I H CH3 I H I CH3 H H OSO2I CH3
CH3SO2Cl base
mesylate
NaI ethanol
II
III
IV
A) I C) E) III V
B) II (SN2 backside by I- after mes formed from -OH) D) IV
33.
Which of the compounds listed below would you expect to have the highest boiling point? (They all have approximately the same molecular weight.) A) CH3CH2CH2CH2CH3 C) E) CH3CH2CH2CH2OH CH3CH2CH2OCH3 B) CH3CH2CH2Cl D) CH3CH2OCH2CH3
34.
The following reaction,
HBr CH3CH2CH2CH2OH heat CH3CH2CH2CH2Br + H2O
is probably: A) B) C) D) E) An SN1-type reaction involving the protonated alcohol as the substrate. An SN2-type reaction involving the protonated alcohol as the substrate. An E1-type reaction involving the protonated alcohol as the substrate. An E2-type reaction involving the protonated alcohol as the substrate. An epoxidation reaction.
35.
Which of the following statements is NOT true of ethers? A) Ethers are generally unreactive molecules toward reagents other than certain strong acids. B) Ethers generally have lower boiling points than alcohols of a corresponding molecular weight. C) Ethers cannot H-bond with water. (It can, but not with another ether molecule) D) Ethers can generally be cleaved by heating them with HBr or HI. E) Ethers form peroxides when allowed to stand in the presence of oxygen. (Hint This is true)
36.
Which compound is a tosylate?
O CH3 S O CH3 O Br O S O CH2 CH3 O CH3 O S O CH3
I
O CH3 S CH3
II
O CH3 O S O CH3
III
IV
A) I C) E) III V
B) II D) IV
Mechanisms Write the mechanism for the following reaction. Use curved arrow notation and indicate the removal of any protons if necessary. The reaction of 1-methylcyclohexene with HBr followed by the reaction of the product with t-butoxide in t-butyl alcohol at 60Cto make an alkene different from the starting material. (8 Points) Electron "dots" not shown in solutions to make drawing easier. You should have shown them.
CH3 H H Br
CH3 Br H H
CH3 Br
H C Br
H H
O-t-but
CH2
Mechanisms Write the mechanism for the following reaction. Use curved arrow notation and indicate the removal of any protons if necessary. The reaction of ethyl alcohol molecules in the presence of concentrated sulfuric acid at 140 to produce diethyl ether. (8 points)
CH3 CH2 OH H H2SO 4 CH3 CH2 OH CH3 CH2 OH2 CH3 CH2 O CH2 CH3
CH3 CH2 OH
CH3 CH2 O CH2 CH3
Vous aimerez peut-être aussi
- Section-I (Single Correct Choice) : HC CH 1.1eq Nanh Nanh Nanh X XDocument14 pagesSection-I (Single Correct Choice) : HC CH 1.1eq Nanh Nanh Nanh X XPriyansh YadavPas encore d'évaluation
- ch8 1Document8 pagesch8 1yonggyePas encore d'évaluation
- Alkanes Alkenes AlkynesDocument10 pagesAlkanes Alkenes AlkynesPanda Boy100% (2)
- Practice Exam I - Organic ChemsitryDocument14 pagesPractice Exam I - Organic ChemsitryDave KowalczykPas encore d'évaluation
- ACS Review 24 PhenolsDocument10 pagesACS Review 24 PhenolsJana BazziPas encore d'évaluation
- ACS Review 18 Enols and EnolatesDocument11 pagesACS Review 18 Enols and EnolatesJana BazziPas encore d'évaluation
- ACS Review 12 Reactions of Arenes - Electrophilic Aromatic SDocument12 pagesACS Review 12 Reactions of Arenes - Electrophilic Aromatic SMohamad HabbabaPas encore d'évaluation
- ACS Review 15 Alcohols Diols and ThiolsDocument10 pagesACS Review 15 Alcohols Diols and ThiolsJana BazziPas encore d'évaluation
- Chapter 5: Structure and Preparation of Alkenes - Elimination ReactionsDocument13 pagesChapter 5: Structure and Preparation of Alkenes - Elimination ReactionsRahma AshrafPas encore d'évaluation
- Organic Chemistry 2 Practice Exam 1Document15 pagesOrganic Chemistry 2 Practice Exam 1KaybidoPas encore d'évaluation
- Alcohols, Phenols and Ethers - MCQs Test - 1Document3 pagesAlcohols, Phenols and Ethers - MCQs Test - 1Prasant Kumar100% (1)
- Organic Chemistry 1 Multiple Choice: Cis TransDocument4 pagesOrganic Chemistry 1 Multiple Choice: Cis Transacb4039Pas encore d'évaluation
- All Year Chemistry Up To 2018 PDFDocument37 pagesAll Year Chemistry Up To 2018 PDFAGAH LUCKYPas encore d'évaluation
- Chem TechDocument181 pagesChem TechDream CakePas encore d'évaluation
- Analytical Chemistry CH 342 20132Document1 pageAnalytical Chemistry CH 342 20132KaizerPas encore d'évaluation
- ch02 Test BankDocument66 pagesch02 Test BankEyaPas encore d'évaluation
- 238 Finial ExamDocument10 pages238 Finial Exammominamin100% (2)
- Analytical ChemistryDocument2 pagesAnalytical ChemistryRochelle Louise SampagaPas encore d'évaluation
- Org Part 1 With AnsDocument7 pagesOrg Part 1 With AnsDeepak PradhanPas encore d'évaluation
- ACS Review 17 Aldehydes and Ketones - Nucleophilic AdditionDocument14 pagesACS Review 17 Aldehydes and Ketones - Nucleophilic AdditionJana Bazzi100% (1)
- Chapter 14Document51 pagesChapter 14Jenny Derne100% (1)
- Organic II Final Exam Practice QuestionsDocument14 pagesOrganic II Final Exam Practice Questionstru99_nl100% (1)
- Organic Chemistry ReviewerDocument10 pagesOrganic Chemistry ReviewerJan Chester ChanPas encore d'évaluation
- ACS Review 20 Carboxylic Acid Derivatives Nucleophilic AcylDocument12 pagesACS Review 20 Carboxylic Acid Derivatives Nucleophilic AcylJana BazziPas encore d'évaluation
- Reaction SummaryDocument5 pagesReaction SummaryShafaqatRahmanPas encore d'évaluation
- Organic Chemistry Practice MidtermDocument7 pagesOrganic Chemistry Practice MidtermAmy HanPas encore d'évaluation
- Stereochemistry QustionsDocument43 pagesStereochemistry QustionsSwaraj Paul100% (1)
- IGCSE Chemistry Organic Chemistry MCDocument11 pagesIGCSE Chemistry Organic Chemistry MCJenkins CK TsangPas encore d'évaluation
- A) Iron's Molar Mass Must Be Known To Calculate The Moles of Iron in SolutionDocument5 pagesA) Iron's Molar Mass Must Be Known To Calculate The Moles of Iron in SolutionBla NkPas encore d'évaluation
- Chem MCQ FinalDocument258 pagesChem MCQ FinalDare DevilPas encore d'évaluation
- Quiz 3-Practice questions (1) نسخةDocument8 pagesQuiz 3-Practice questions (1) نسخةSabaa AbuzaidPas encore d'évaluation
- Chapter 15 Test BankDocument41 pagesChapter 15 Test BankMeowCat12345678950% (2)
- Analytical Chemistry MC QuestionsDocument27 pagesAnalytical Chemistry MC QuestionsFrank Massiah100% (1)
- MULTIPLE CHOICE QUESTIONS Part 5: Stereochemistry: Topic: Identifications and ComparisonsDocument13 pagesMULTIPLE CHOICE QUESTIONS Part 5: Stereochemistry: Topic: Identifications and ComparisonsErica Chang100% (2)
- Organic Chem Practice Exam by Solomon CH 1-4Document12 pagesOrganic Chem Practice Exam by Solomon CH 1-4Natasha Moo100% (1)
- ACID Base Equil P Test MCDocument5 pagesACID Base Equil P Test MCctyre34Pas encore d'évaluation
- Unit 11 MCQDocument7 pagesUnit 11 MCQJay VermaPas encore d'évaluation
- CH 4 AlkanesDocument16 pagesCH 4 AlkanesBrittnay Marie100% (4)
- Chapter 08 MergedDocument38 pagesChapter 08 MergedreemPas encore d'évaluation
- Test4 Ch19 Electrochemistry Practice ProblemsDocument13 pagesTest4 Ch19 Electrochemistry Practice ProblemsPhysiochemo Chemical100% (1)
- Practice Test Acids BasesDocument4 pagesPractice Test Acids Basesdemetri lanezPas encore d'évaluation
- Quice Review Center: C) The Total Mass of The Atom A) RBDocument5 pagesQuice Review Center: C) The Total Mass of The Atom A) RBMary Francia RicoPas encore d'évaluation
- Exam Three Practice TestDocument13 pagesExam Three Practice TestBUCH203100% (1)
- Acid Base 15Document36 pagesAcid Base 15Imranzo HsnPas encore d'évaluation
- Practical Organic Chemistry III ExamDocument3 pagesPractical Organic Chemistry III ExamTesfahun100% (1)
- PDFDocument3 pagesPDFJude GomezPas encore d'évaluation
- ICSE Chemistry Board Paper19 PDFDocument9 pagesICSE Chemistry Board Paper19 PDFPrajakta DighePas encore d'évaluation
- GRE Chemistry Practice Questions: High Yield GRE Chemistry Questions with Detailed ExplanationsD'EverandGRE Chemistry Practice Questions: High Yield GRE Chemistry Questions with Detailed ExplanationsPas encore d'évaluation
- PMR Spectroscopy: Solved Problems Volume : IID'EverandPMR Spectroscopy: Solved Problems Volume : IIÉvaluation : 5 sur 5 étoiles5/5 (3)
- GRE Chemistry: Inorganic Chemistry Review for GRE Chemistry Subject TestD'EverandGRE Chemistry: Inorganic Chemistry Review for GRE Chemistry Subject TestPas encore d'évaluation
- Practice Ex 3Document10 pagesPractice Ex 3Irene WPas encore d'évaluation
- 09-Final With SolutionsDocument27 pages09-Final With SolutionsDanielle Wood100% (2)
- Practice Exam ChemDocument10 pagesPractice Exam Chemabhijit.salvekarPas encore d'évaluation
- Alkyl Halides, Alcohols, Ethers and Epoxides: 1. What Is The IUPAC Name For CHDocument17 pagesAlkyl Halides, Alcohols, Ethers and Epoxides: 1. What Is The IUPAC Name For CHEllaŠtrbacPas encore d'évaluation
- BIOKMOR N01 3rd ExamDocument8 pagesBIOKMOR N01 3rd ExamMacy MarianPas encore d'évaluation
- TimidDocument13 pagesTimid公孫堂傲100% (2)
- Exam 2bruics PDFDocument29 pagesExam 2bruics PDFsanidaaaPas encore d'évaluation
- Structure and Nomenclature of HydrocarbonsDocument17 pagesStructure and Nomenclature of HydrocarbonsSaurabh ShuklaPas encore d'évaluation
- Palladium Precatalyst Design and Its Applications in Cross-CouplingDocument27 pagesPalladium Precatalyst Design and Its Applications in Cross-CouplingiammouliPas encore d'évaluation
- Goc Iit Jam MaterialDocument15 pagesGoc Iit Jam MaterialjagannivasPas encore d'évaluation
- Hair Straightening CompositionDocument13 pagesHair Straightening Compositionzorro21072107Pas encore d'évaluation
- Lab ReportDocument5 pagesLab Reportsneh1509Pas encore d'évaluation
- Bio MoleculeDocument15 pagesBio MoleculeManish GuptaPas encore d'évaluation
- What Is The Relation Between Normality and MolarityDocument7 pagesWhat Is The Relation Between Normality and MolarityDrAmit VermaPas encore d'évaluation
- Basic Concept of BondingDocument70 pagesBasic Concept of BondingGAURAV PANDEYPas encore d'évaluation
- 4.1: Writing and Balancing Chemical Equations: Learning ObjectivesDocument9 pages4.1: Writing and Balancing Chemical Equations: Learning ObjectivesRicki HanPas encore d'évaluation
- Alcohols & Carboxylic Acids 2 QPDocument11 pagesAlcohols & Carboxylic Acids 2 QPDia GadaPas encore d'évaluation
- Molecular Docking Tostudy Protein-Ligand InteractionDocument5 pagesMolecular Docking Tostudy Protein-Ligand InteractionDr. Kaushal Kishor SharmaPas encore d'évaluation
- Amines Ncert OptDocument20 pagesAmines Ncert OptLokender BhatiPas encore d'évaluation
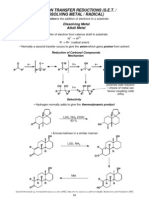
- Metal ReductionDocument11 pagesMetal Reductiondeepthimahanthi sabithaPas encore d'évaluation
- Coursebook Answers: Science in ContextDocument7 pagesCoursebook Answers: Science in ContextAditiPas encore d'évaluation
- Reasoning Ques in Organic ChemistryDocument14 pagesReasoning Ques in Organic ChemistryRIHINBHATNAGAR50% (2)
- Httpsmoodle - Uniba.skpluginfile - Php150161mod Resourcecontent1Drug20analysis Lecture 10 23112022 PDFDocument81 pagesHttpsmoodle - Uniba.skpluginfile - Php150161mod Resourcecontent1Drug20analysis Lecture 10 23112022 PDFSanaPas encore d'évaluation
- Student Organic - AlkanesDocument25 pagesStudent Organic - AlkanesNyein Nu WinnPas encore d'évaluation
- Class12 - Organichemistry - Named ReactionsDocument6 pagesClass12 - Organichemistry - Named ReactionsMᴀïᴢᴍɛɛŋ AŋꜱᴀʀïPas encore d'évaluation
- 9.1 Acid and BasesDocument32 pages9.1 Acid and BasesUmida ZaylobiddinovaPas encore d'évaluation
- TitrationDocument23 pagesTitrationRhyth EtherPas encore d'évaluation
- Assignment No. 6 Carboxylic Acid and DerivativesDocument5 pagesAssignment No. 6 Carboxylic Acid and DerivativesREGINE CUEVASPas encore d'évaluation
- Structure and Functions of Biological Molecules: at The End of The Lesson, You Are Expected ToDocument4 pagesStructure and Functions of Biological Molecules: at The End of The Lesson, You Are Expected ToFrancinne MartinPas encore d'évaluation
- Acidity and Basicity of DrugsDocument52 pagesAcidity and Basicity of DrugsMichelle PatriciaPas encore d'évaluation
- 0-2 7 Teachin - Peroxide LiquidsDocument13 pages0-2 7 Teachin - Peroxide LiquidsWaqas KhanPas encore d'évaluation
- Acid-Base Equilibria and CalculationsDocument48 pagesAcid-Base Equilibria and CalculationsKiss LeviPas encore d'évaluation
- A2-Organic Reactions Spider Diagram HANDOUT (Colour)Document1 pageA2-Organic Reactions Spider Diagram HANDOUT (Colour)udaymohur100% (1)
- WCH02 01 Que 20160610Document24 pagesWCH02 01 Que 20160610anon_569018284Pas encore d'évaluation
- Lecture 3Document32 pagesLecture 3Quang TrườngPas encore d'évaluation
- Chemical Compatibility Reference Chart: PolypropyleneDocument16 pagesChemical Compatibility Reference Chart: PolypropyleneSreesanth SaruvilPas encore d'évaluation
- Chapter 5 - (Philoid-IN) PDFDocument39 pagesChapter 5 - (Philoid-IN) PDFAruna WarkalPas encore d'évaluation