Académique Documents
Professionnel Documents
Culture Documents
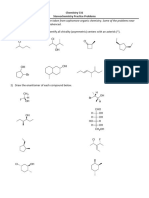
Stereochemistry Problem Set 1) Indicate Whether The Following Structures Are
Transféré par
kevinamyTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Stereochemistry Problem Set 1) Indicate Whether The Following Structures Are
Transféré par
kevinamyDroits d'auteur :
Formats disponibles
Stereochemistry Problem Set
1) Indicate whether the following structures are chiral or achiral (no asymmetric centers or meso); if chiral then assign R or S to each stereogenic center.
Br H R Br
CO2H
a) R
h) OH n) HO CHO R
R
H OH R
H Br H Cl Me HO H S
b) i) S Me
S
R R H
HO H H H
HO Br H OH
Br Cl Me
c) S j) S R R o) not chiral
R H
HO H Br H
OH
S S
d) not chiral
p)
HO H H OH CHO
k) H OH R HO
R H OH R
e)
not stereogenic Me
HO Br H Me HO H
S S
CO2H q)
f) Br S S
l) OH
H OH S carbon w/ Cl has higher
HO H H OH HO Cl R priority than CO2H
H
g) CO2H
H OH S CHO Me OH
m) S
Br H R HO H S r)
not stereogenic not stereogenic
CHO H H HO H
H OH R
Me
2) Draw structures which correspond to the following names: More than one picture may be correct.
OH
Br Br
a) (R) 2-bromobutane OR f) meso -1,2-dihydroxycyclohexane
OH
I
b) (S) 2-iodopentane g) (R) 3-bromononane
Br
Br
c) (2R, 3S) 2-bromo-3-iodoheptane h) (S) 4-ethyldecane
I
d) (S) 3-methylhexane i) meso- 1,3-dimethylcyclohexane
NO2
OH
e) (3S, 5S) 3-hydroxy-5-methyloctane j) (S) 2-nitropentane (nitro = NO2)
(hydroxy = OH)
3) Draw i) an enantiomer; ii) a diastereomer, for each of the following H H HO
OH
CH2Cl Br Br Me Me
a) H Cl b) H Me Me H c) H H
d)
HO
OH
H Me Me H O
Cl H CN CN OH OH O
Me enantiomer enantiomer enantiomer
CH2Cl
CH2Cl Me H OH
Cl H H Me
Cl H Me one one
Cl H H Br diastereomer diastereomer
OH OH
H Cl Me CN
H O
Me one one
enantiomer diastereomer diastereomer
4) You have a sample of the enantiomerically pure (3S)-3-chloro-1-butene and carry out the non-stereospecific addition reaction shown below.
H Cl H Cl Me Me
a) What happens to the absolute configuration of the existing stereocenter? unchanged RXN
b) Draw Fisher projection(s) of the product(s). HO H H OH
c) How many product spots would you expect to see by TLC? 2 OH H Cl H Cl
d) Which of the compounds corresponding to the TLC spots would be optically active? both Me Me
Vous aimerez peut-être aussi
- Homework Problems: Structure, Bonding & Hybridization 1. The Molecule Shown Below Is Griseofulvin, An Antifungal CompoundDocument8 pagesHomework Problems: Structure, Bonding & Hybridization 1. The Molecule Shown Below Is Griseofulvin, An Antifungal CompoundPrachi KaushikPas encore d'évaluation
- 4.5 Answers To ExercisesDocument4 pages4.5 Answers To Exercisesloly62006Pas encore d'évaluation
- Reaksi AlkenaDocument1 pageReaksi Alkenajoel13Pas encore d'évaluation
- Problem Set 1: Review Questions Chemistry 260 Organic ChemistryDocument3 pagesProblem Set 1: Review Questions Chemistry 260 Organic ChemistrydddddPas encore d'évaluation
- Isomerism: One or More Than One Answer Type Questions: 1. Which One of The Following Pairs of Isomers Are EnentiomersDocument11 pagesIsomerism: One or More Than One Answer Type Questions: 1. Which One of The Following Pairs of Isomers Are EnentiomerskamalPas encore d'évaluation
- B1.2 ProteinsDocument8 pagesB1.2 ProteinslittleianlauPas encore d'évaluation
- Uploads381938195043alcohols - Worksheet - PDF 2Document2 pagesUploads381938195043alcohols - Worksheet - PDF 2John Rey FlandezPas encore d'évaluation
- Uploads381938195043alcohols Worksheet PDFDocument2 pagesUploads381938195043alcohols Worksheet PDFRyan RamlawiPas encore d'évaluation
- Homework 1 (Original)Document1 pageHomework 1 (Original)hanna liuPas encore d'évaluation
- TABORADA - Act8 (Ketones and Aldehydes)Document4 pagesTABORADA - Act8 (Ketones and Aldehydes)Justin Habaña TaboradaPas encore d'évaluation
- NMR Problems Dec 2012Document8 pagesNMR Problems Dec 2012Biswajit Gopal RoyPas encore d'évaluation
- Chiral CompoundsDocument20 pagesChiral CompoundsShubham RampalliwarPas encore d'évaluation
- CHM3201 Tutorial 1 Basic ConceptsDocument3 pagesCHM3201 Tutorial 1 Basic ConceptsAkmalZharifAbdullahPas encore d'évaluation
- Organic Chemistry I: CH H C CH DDocument4 pagesOrganic Chemistry I: CH H C CH DSankar AdhikariPas encore d'évaluation
- Chapter 2 Lecture Slides PDFDocument108 pagesChapter 2 Lecture Slides PDFjoseph changPas encore d'évaluation
- DrawingsDocument1 pageDrawingsJAGTAP UTKARSH ASHOKRAOPas encore d'évaluation
- 531 - Stereochem PracticeDocument4 pages531 - Stereochem Practiceyigermalamanuel32Pas encore d'évaluation
- Antibiotici: StreptomicinDocument13 pagesAntibiotici: StreptomicinMediha DedicPas encore d'évaluation
- Ejercicios Axial EcuatorialDocument1 pageEjercicios Axial EcuatorialJulio Cesar Boada MartinezPas encore d'évaluation
- 315 Acid Cat MechsDocument13 pages315 Acid Cat MechsDeepak N SPas encore d'évaluation
- MechanismsDocument5 pagesMechanismsnajifaahmed223Pas encore d'évaluation
- Activity 3 - The Cahn-Ingold-Prelog Rules - 0Document5 pagesActivity 3 - The Cahn-Ingold-Prelog Rules - 0Naved AnjoomPas encore d'évaluation
- OH A, A Is: : CHN 1 EqvDocument4 pagesOH A, A Is: : CHN 1 EqvAtharva GanjuPas encore d'évaluation
- Solutions: OH R OH ODocument32 pagesSolutions: OH R OH ONGR SRGPas encore d'évaluation
- Introduction To Organic Chemistry: C C C CDocument18 pagesIntroduction To Organic Chemistry: C C C CPedro Moreno de SouzaPas encore d'évaluation
- Msds Etil AsetatDocument12 pagesMsds Etil AsetatNurAfifahPas encore d'évaluation
- Organic Tutorial 1Document2 pagesOrganic Tutorial 1karthik chinnaPas encore d'évaluation
- IB Past Paper Questions: Carbohydrates, Lipids, Proteins and Nucleic Acids (Incl DNA Replication)Document12 pagesIB Past Paper Questions: Carbohydrates, Lipids, Proteins and Nucleic Acids (Incl DNA Replication)John Osborne78% (9)
- Mechanisms 1-10: CHEM 725: Davey 1Document7 pagesMechanisms 1-10: CHEM 725: Davey 1Bradley DaveyPas encore d'évaluation
- Addition To CCDocument17 pagesAddition To CCHadiPas encore d'évaluation
- HW 1Document11 pagesHW 1sajajPas encore d'évaluation
- CHE 1000 Tutorial Sheet 12 - Organic ChemistryDocument3 pagesCHE 1000 Tutorial Sheet 12 - Organic ChemistryStanley SitaliPas encore d'évaluation
- R Vs S AnsDocument2 pagesR Vs S AnsAtul SinghPas encore d'évaluation
- OzonolysisDocument2 pagesOzonolysisKamaraj NaiduPas encore d'évaluation
- Org Chem QDocument9 pagesOrg Chem QchemdopePas encore d'évaluation
- X910 - ALBiology - Assignment - 01 V2Document14 pagesX910 - ALBiology - Assignment - 01 V2Mikila GittensPas encore d'évaluation
- What Is Stereochemistry?Document30 pagesWhat Is Stereochemistry?Tanmoy SamantaPas encore d'évaluation
- Alkenes 2 QPDocument12 pagesAlkenes 2 QPemanPas encore d'évaluation
- Biochem2 Carbs and LipidsDocument69 pagesBiochem2 Carbs and Lipidstml_19672682Pas encore d'évaluation
- Dopamine Powerpoint Presentation: C H NoDocument8 pagesDopamine Powerpoint Presentation: C H NoAluPas encore d'évaluation
- Tutorial 1Document3 pagesTutorial 1Nicholas OwPas encore d'évaluation
- Test 2 Extra Stereochem Practice-AnswersDocument3 pagesTest 2 Extra Stereochem Practice-AnswersA/P SUPAYA SHALINIPas encore d'évaluation
- Organic Chemistry I Homework 2009 Fall Dr. WorkmanDocument15 pagesOrganic Chemistry I Homework 2009 Fall Dr. WorkmanFrancine DinizPas encore d'évaluation
- Problem Set 1 PDFDocument4 pagesProblem Set 1 PDFRay BinasPas encore d'évaluation
- Exm N X11 Chem Biomol ADocument28 pagesExm N X11 Chem Biomol Asumair hejibPas encore d'évaluation
- Adrenaline Powerpoint Presentation: C H NoDocument8 pagesAdrenaline Powerpoint Presentation: C H NoAjarihant OrthoPas encore d'évaluation
- Org Chemistry Alcohol Carboxylic Acid Macromolecules WS AnsDocument8 pagesOrg Chemistry Alcohol Carboxylic Acid Macromolecules WS Ans2tsPas encore d'évaluation
- Organic ChemistryDocument11 pagesOrganic ChemistryHebah TanveerPas encore d'évaluation
- Introduction To Organic Chemistry Stereochemistry - Problem SetDocument3 pagesIntroduction To Organic Chemistry Stereochemistry - Problem SetAbhiPas encore d'évaluation
- Assignment 1 2019 AnswersDocument7 pagesAssignment 1 2019 AnswersDaniel GhiasvandPas encore d'évaluation
- CH 25Document34 pagesCH 25John Nicholson LanderoPas encore d'évaluation
- 01 02 2023 Chemistry - Paper+With+Answer - MorningDocument6 pages01 02 2023 Chemistry - Paper+With+Answer - MorningLanaPas encore d'évaluation
- Mekanisme PDFDocument1 pageMekanisme PDFSoy Viranda KusumaPas encore d'évaluation
- Nomenclature Isomerism Worksheet AnswersDocument3 pagesNomenclature Isomerism Worksheet AnswersYee MeiPas encore d'évaluation
- The Logic of Chemical Synthesis Corey E J Amp Cheng X M 331 463Document133 pagesThe Logic of Chemical Synthesis Corey E J Amp Cheng X M 331 463bann tvPas encore d'évaluation
- Isomers - Worksheet #6Document3 pagesIsomers - Worksheet #6Ann Dayrit0% (1)
- Carbohydrate & Lipid MC QuestionsDocument4 pagesCarbohydrate & Lipid MC QuestionsJohn Osborne100% (2)
- Isomers of Coordination ComplexesDocument12 pagesIsomers of Coordination ComplexesNov IndaPas encore d'évaluation
- DasjkfjdsfjdslDocument9 pagesDasjkfjdsfjdslRhyme CakesPas encore d'évaluation
- Chem 212 Alkyl Halide Problems 5Document1 pageChem 212 Alkyl Halide Problems 5kevinamyPas encore d'évaluation
- Chem 212 Alkyl Halide Problems 2Document1 pageChem 212 Alkyl Halide Problems 2kevinamy100% (1)
- Chem 212 Alkyl Halide Problems 4Document1 pageChem 212 Alkyl Halide Problems 4kevinamyPas encore d'évaluation
- Chem 212 Alkyl Halide Problems 3Document1 pageChem 212 Alkyl Halide Problems 3kevinamyPas encore d'évaluation
- Condensation 1 AnsDocument1 pageCondensation 1 AnskevinamyPas encore d'évaluation
- CH211 ALKYNES Problem Set 1. Provide The Products For TheDocument1 pageCH211 ALKYNES Problem Set 1. Provide The Products For ThekevinamyPas encore d'évaluation
- Chem 212 Provide The Products For The Following Reactions. BeDocument1 pageChem 212 Provide The Products For The Following Reactions. BekevinamyPas encore d'évaluation
- Condensation 2 AnsDocument1 pageCondensation 2 AnskevinamyPas encore d'évaluation
- CarboxylicansDocument1 pageCarboxylicanskevinamyPas encore d'évaluation
- Alkane Stereochemistry 1) For The Molecules Below: A) Provide ADocument3 pagesAlkane Stereochemistry 1) For The Molecules Below: A) Provide AkevinamyPas encore d'évaluation
- Chem 212 Condensation Reactions 3Document1 pageChem 212 Condensation Reactions 3kevinamyPas encore d'évaluation
- Giao Ly For KidsDocument2 pagesGiao Ly For KidskevinamyPas encore d'évaluation
- Energy AnsDocument3 pagesEnergy AnskevinamyPas encore d'évaluation
- Chem 212 Electrophilic Aromatic Substitution 2Document1 pageChem 212 Electrophilic Aromatic Substitution 2kevinamyPas encore d'évaluation
- NMRansDocument1 pageNMRanskevinamyPas encore d'évaluation
- EnolateansDocument1 pageEnolateanskevinamyPas encore d'évaluation
- CHEM211 Problem Set Functional Groups and Reaction Types These AnswersDocument4 pagesCHEM211 Problem Set Functional Groups and Reaction Types These AnswerskevinamyPas encore d'évaluation
- Naming Alkanes AnsDocument1 pageNaming Alkanes AnskevinamyPas encore d'évaluation
- MSansDocument2 pagesMSanskevinamyPas encore d'évaluation
- SpecansDocument2 pagesSpecanskevinamyPas encore d'évaluation
- Synthesis 3 AnsDocument1 pageSynthesis 3 AnskevinamyPas encore d'évaluation
- Organic Chemistry (Alkyl Had., Stereo., Aromat.) (160 Items)Document17 pagesOrganic Chemistry (Alkyl Had., Stereo., Aromat.) (160 Items)S AdiaPas encore d'évaluation
- 2a History of Coordination ChemistryDocument7 pages2a History of Coordination ChemistryFelipe Marçal MorgantiniPas encore d'évaluation
- Chemistry Chapter 20 LECDocument122 pagesChemistry Chapter 20 LECsaxman011Pas encore d'évaluation
- Isomer Bansal InstituteDocument36 pagesIsomer Bansal InstituteVanshaj GuptaPas encore d'évaluation
- Isomers and IsomerismDocument37 pagesIsomers and IsomerismAnwherSehdatPas encore d'évaluation
- Stereochemistry Week-4) : Optical IsomerismDocument27 pagesStereochemistry Week-4) : Optical IsomerismTanya DilshadPas encore d'évaluation
- Chemistry Isomerism IIDocument3 pagesChemistry Isomerism IISurya PrakashPas encore d'évaluation
- 10 1002@cptc 201900068Document9 pages10 1002@cptc 201900068Alvaro Putra PrasetyaPas encore d'évaluation
- Isomerism Complete Chapter Notes For Iit-JeeDocument54 pagesIsomerism Complete Chapter Notes For Iit-Jeeasylosaurus71Pas encore d'évaluation
- Carbonyls, Carboxylic Acid and ChiralityDocument23 pagesCarbonyls, Carboxylic Acid and ChiralityAyshath MaaishaPas encore d'évaluation
- A2 Optical IsomerismDocument35 pagesA2 Optical IsomerismteddaboyPas encore d'évaluation
- Organic Chemistry 1 NotesDocument27 pagesOrganic Chemistry 1 Noteszeeshan_haider000Pas encore d'évaluation
- Organic Chemistry ProblemsDocument4 pagesOrganic Chemistry ProblemsIqbal A Mir100% (1)
- Chapter18 CarbohydratesDocument68 pagesChapter18 CarbohydratesMaria Francesca Tiongson0% (1)
- M.SC - BioinformaticsDocument43 pagesM.SC - BioinformaticskrishnanandPas encore d'évaluation
- Carbohydrates - ReviewerDocument6 pagesCarbohydrates - ReviewerMichaella CabanaPas encore d'évaluation
- Chapter 6 - Stereochemisty: ThalidomideDocument42 pagesChapter 6 - Stereochemisty: ThalidomideDaniel McDermottPas encore d'évaluation
- By L. G. Wade, JR.: Organic Chemistry, 7eDocument41 pagesBy L. G. Wade, JR.: Organic Chemistry, 7eKing of KingsPas encore d'évaluation
- Chiral CompoundsDocument20 pagesChiral CompoundsShubham RampalliwarPas encore d'évaluation
- MCAT Organic Summary SheetDocument6 pagesMCAT Organic Summary SheetSpencer Thomas100% (2)
- Stereochemistry 1Document97 pagesStereochemistry 1Babi Kumar KaflePas encore d'évaluation
- Hub. Stereokimia & Aktivitas - 3Document85 pagesHub. Stereokimia & Aktivitas - 3Nia Nurdinia RahmahPas encore d'évaluation
- Separation of Drugs by Packed-Column Supercritical Fluid ChromatographyDocument18 pagesSeparation of Drugs by Packed-Column Supercritical Fluid ChromatographySameh QanadiloPas encore d'évaluation
- CHEM 160 Module 3 Resource 1Document9 pagesCHEM 160 Module 3 Resource 1meyaPas encore d'évaluation
- Stereochemistry: Ranjit Dhillon Inder Pal SinghDocument18 pagesStereochemistry: Ranjit Dhillon Inder Pal SinghIjazPas encore d'évaluation
- Lecture Notes First Semester Yr 2 BPham BMLS BDSDocument57 pagesLecture Notes First Semester Yr 2 BPham BMLS BDSsriPas encore d'évaluation
- Drug InspectorDocument33 pagesDrug InspectorsharaniyasyamPas encore d'évaluation
- Homework 3Document13 pagesHomework 3polypeptidePas encore d'évaluation
- Geometric Isomers Isomers That Differ in The Way The Ligand Is Bound To The Optical Isomers Isomers That Do Not Have Symmetry and Are NotDocument20 pagesGeometric Isomers Isomers That Differ in The Way The Ligand Is Bound To The Optical Isomers Isomers That Do Not Have Symmetry and Are NotSahadath JameelPas encore d'évaluation
- ReagentGuide 8th SynthesticOrganicChemistry MaterialsChemistryDocument396 pagesReagentGuide 8th SynthesticOrganicChemistry MaterialsChemistryyulliarperezPas encore d'évaluation