Académique Documents
Professionnel Documents
Culture Documents
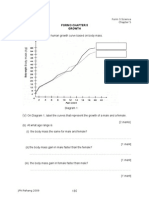
Chapter 4 Carbon Compound Student Copy Science Form 5
Transféré par
Angie Kong Su MeiDescription originale:
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Chapter 4 Carbon Compound Student Copy Science Form 5
Transféré par
Angie Kong Su MeiDroits d'auteur :
Formats disponibles
Chapter 4: Carbon Compounds:
Carbon compounds:
Organic compounds
Inorganic Compounds
Example
Similarities:
1)
2)
3)
4)
5)
Differences:
Originates from living things
Burn in air producing Carbon
dioxide
Size of molecules or
compounds
Solubility
Example
What is hydrocarbon?
Examples:
4.2 Alcohol
- is organic compounds made of ___________________________________
Ethanol
Process: _____________________
Chemical equation:
Physical characteristics of alcohol:
1)
2)
3)
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
However, _____________________
Example: methanol _________________
Butanol ______________________
Alcohol burns easily. Colour of flame __________ with _______________ of air
Equation: _______________________________
Exposed to air and become _______________, Oxidised by air to form : ________________________
Equation: _________________________
Alcohol can react with __________________, presence of catalyst _________________, to produce ______
Ester is _______________ with ____________ found in __________________ and _________________
Equation: _________________________
Example: ______________________________
Uses of alcohol:
1) Alcoholic drinks : _________________
2) Antiseptic to _______________
Such as__________
3) Used in ____________ such as ___________
4) Iodine _____________________
5) As solvent such as__________________________
6) Ingredient in ___________________________
7) Ethanediol ____________________________to ________________________
8) As _______________ for _________________
Example: butanol in _____________________
Effects of alcohol
1) A type of ___________________________
2) Frequently consumed cause _____________
3) Slows down ________________
4) Lead to _____________________________
5) Cause liver disease such as_____________
6) Baby may have ___________________
Symptoms such as: ______________________
7) Social problems such as
1) _________
2) __________
3) ___________
Vous aimerez peut-être aussi
- Secondary Growth of PlantsDocument13 pagesSecondary Growth of PlantsAngie Kong Su Mei50% (2)
- Molecular Models of Covalent Compounds WorksheetDocument2 pagesMolecular Models of Covalent Compounds WorksheetJames Dauray100% (1)
- E&i QC Inspector Resum and DocumentsDocument24 pagesE&i QC Inspector Resum and DocumentsIrfan 786pakPas encore d'évaluation
- FWN Magazine 2018 - Leonor VintervollDocument48 pagesFWN Magazine 2018 - Leonor VintervollFilipina Women's NetworkPas encore d'évaluation
- Form 1 Chapter 2 Cell As A Unit of LifeDocument5 pagesForm 1 Chapter 2 Cell As A Unit of LifeJames WongPas encore d'évaluation
- Chapter 15 I Trigonometry II StudentDocument41 pagesChapter 15 I Trigonometry II StudentAngie Kong Su MeiPas encore d'évaluation
- Sce Form 2Document22 pagesSce Form 2williamkong123Pas encore d'évaluation
- Chemistry Module Form 4 Complete SetDocument197 pagesChemistry Module Form 4 Complete SetDawana Nasuha100% (2)
- Ramrajya 2025Document39 pagesRamrajya 2025maxabs121Pas encore d'évaluation
- Blood Circulation and Transport ModuleDocument15 pagesBlood Circulation and Transport ModuleSENSPas encore d'évaluation
- Science Form 3 Chapter 2 - Blood Circulation PDFDocument4 pagesScience Form 3 Chapter 2 - Blood Circulation PDFFarah Sofea Razali91% (11)
- 1.2.1 Angles and Lines II, PT3 Practice - PT3 MathematicsDocument9 pages1.2.1 Angles and Lines II, PT3 Practice - PT3 MathematicsPrince NeshPas encore d'évaluation
- Form 3 Chapter 5Document4 pagesForm 3 Chapter 5naza9775100% (3)
- Quiz f3Document10 pagesQuiz f3Hafizah PakahPas encore d'évaluation
- Chemistry Form 5 Carbon Mind MapDocument23 pagesChemistry Form 5 Carbon Mind MapAngie Kong Su MeiPas encore d'évaluation
- Chapter 8 Electromagnet Teacher's Guide 2009Document48 pagesChapter 8 Electromagnet Teacher's Guide 2009Mohd Khairul Anuar100% (18)
- Form 3 Chapter 8Document8 pagesForm 3 Chapter 8naza9775100% (6)
- Chapter 7 - Light - Colour and SightDocument9 pagesChapter 7 - Light - Colour and SightHHK88893% (15)
- SPM Chemistry Form 5Document5 pagesSPM Chemistry Form 5Aileen PoLyPas encore d'évaluation
- Chemistry Perfect Score Module Form 4 Set 2Document19 pagesChemistry Perfect Score Module Form 4 Set 2alanisln100% (1)
- Chapter 7 Linear InequalitiesDocument8 pagesChapter 7 Linear InequalitiesROSMAWATI BINTI MOHAMED -Pas encore d'évaluation
- Enhancement GravitationDocument5 pagesEnhancement GravitationCart KartikaPas encore d'évaluation
- Chapter 3 Exercise Physics Form 4Document6 pagesChapter 3 Exercise Physics Form 4Wan Nabil Fikri100% (1)
- PBD Assessment Physics Form 4 Chapter 2Document18 pagesPBD Assessment Physics Form 4 Chapter 2Nor ShuhadaPas encore d'évaluation
- Science Form 4 Chapter 5Document20 pagesScience Form 4 Chapter 5cellea98rose100% (2)
- Reviewer 1 Chemical Engineering Day 1Document13 pagesReviewer 1 Chemical Engineering Day 1Romevie Prado100% (2)
- RELATIVE CLAUSES 1º Bachillerato and KeyDocument3 pagesRELATIVE CLAUSES 1º Bachillerato and Keyrapitanoroel0% (2)
- Granulometry of ClinkerDocument18 pagesGranulometry of ClinkerNael100% (12)
- Thermochemistry SPM Form 5Document18 pagesThermochemistry SPM Form 5Azie Nurul AkhtarPas encore d'évaluation
- Form 5 Science SPM Chapter 4 Carbon CompoundsDocument18 pagesForm 5 Science SPM Chapter 4 Carbon Compoundsangie0812Pas encore d'évaluation
- Form 5 Chapter 2Document38 pagesForm 5 Chapter 2Azie Nurul AkhtarPas encore d'évaluation
- Pt3 Science Seminar ModuleDocument28 pagesPt3 Science Seminar ModuleSuntharan Muniandy100% (5)
- Answers Math MF2 Chapter 2 (Squares, Square Roots, Cubes and Cube Roots)Document2 pagesAnswers Math MF2 Chapter 2 (Squares, Square Roots, Cubes and Cube Roots)compeil100% (2)
- Mathematics T For New STPM SyallabusDocument43 pagesMathematics T For New STPM SyallabusLoo Soo Yong100% (3)
- Blood Circulation and Transport Learning Objective: 2.1 Understanding The Transport System in HumansDocument14 pagesBlood Circulation and Transport Learning Objective: 2.1 Understanding The Transport System in HumansJames WongPas encore d'évaluation
- STF Mid Year Science Form 1 2009 Paper2Document12 pagesSTF Mid Year Science Form 1 2009 Paper2Syahrul67% (3)
- Biology Form 4 RevisionDocument18 pagesBiology Form 4 RevisionAyu YunusPas encore d'évaluation
- Chemistry Form 4 - Paper 1Document13 pagesChemistry Form 4 - Paper 1adikmuk0% (1)
- Short Note Chemistry Form 5-Chapter 4 ThermochemistryDocument4 pagesShort Note Chemistry Form 5-Chapter 4 Thermochemistrysalamah_sabri100% (2)
- Module Science Pt3Document11 pagesModule Science Pt3lccjane8504Pas encore d'évaluation
- Form 3 Chapter 2Document9 pagesForm 3 Chapter 2Mariana AhmadPas encore d'évaluation
- Modul 2 Science Form 1 Chapter 2: Cell As A Unit of Life What Is Cell?Document22 pagesModul 2 Science Form 1 Chapter 2: Cell As A Unit of Life What Is Cell?Nurhuda MitiePas encore d'évaluation
- SPM Form 4 Exercise Periodic Table of ElementsDocument3 pagesSPM Form 4 Exercise Periodic Table of Elementsasparagus1996Pas encore d'évaluation
- Exercises Chapter 1 Physics Form 5 WavesDocument15 pagesExercises Chapter 1 Physics Form 5 Wavesnurulfalah100% (3)
- Form 3 Chapter 9Document3 pagesForm 3 Chapter 9naza9775100% (5)
- Mathematics Form 3Document24 pagesMathematics Form 3Amal SufiahPas encore d'évaluation
- Science Form 2 Revision QuestionsDocument3 pagesScience Form 2 Revision QuestionsDorcas Chiang100% (1)
- M013-Consumer Mathematics (Taxation)Document5 pagesM013-Consumer Mathematics (Taxation)Tan Jun YouPas encore d'évaluation
- Science Form 4 Chapter 5Document7 pagesScience Form 4 Chapter 5Suryakala Sundram Sivaananda Sundram100% (2)
- Chapter 6Document1 pageChapter 6cikguanuar100% (1)
- Science Form 1 Chapter 6Document9 pagesScience Form 1 Chapter 6Syazwani Radzi100% (1)
- Mathematics Form 3Document24 pagesMathematics Form 3Amal SufiahPas encore d'évaluation
- Chemistry Module Form 4Document17 pagesChemistry Module Form 4mohd faisol67% (3)
- Biology Topical Exercise Form 4 Chapter 2Document13 pagesBiology Topical Exercise Form 4 Chapter 2SanjeefKumrIIPas encore d'évaluation
- Nota Padat Math f1Document2 pagesNota Padat Math f1qq235100% (16)
- SPM Physics Exercise - Chapter 2 Force QuestionsDocument10 pagesSPM Physics Exercise - Chapter 2 Force QuestionsElyse Kymberly Teoh50% (2)
- Chemistry Form 5Document3 pagesChemistry Form 5alliey75% (8)
- Solid Geometry 2 AnswerDocument15 pagesSolid Geometry 2 AnswerHazri Mohd HamzahPas encore d'évaluation
- Form 4 Additional Mathematics Revision PatDocument7 pagesForm 4 Additional Mathematics Revision PatJiajia LauPas encore d'évaluation
- Kimia Module 1 5 Diagnostik f4 PDFDocument70 pagesKimia Module 1 5 Diagnostik f4 PDFJuan DavisPas encore d'évaluation
- 40 Chemistry Question For RevisionDocument5 pages40 Chemistry Question For RevisionSathish Sarma SathianarayananPas encore d'évaluation
- Additional Science 2010 SPMDocument15 pagesAdditional Science 2010 SPMArthur JonnesPas encore d'évaluation
- Science 9 Q2 Episode 8 SLMDocument4 pagesScience 9 Q2 Episode 8 SLMJunefer MalinisPas encore d'évaluation
- Alcohol Homologous SeriesDocument5 pagesAlcohol Homologous SeriesChia Sann Roo CynthiaPas encore d'évaluation
- Organic Chemistry Tutorial Lesson 1 - Worksheet (AutoRecovered)Document4 pagesOrganic Chemistry Tutorial Lesson 1 - Worksheet (AutoRecovered)BakasukaPas encore d'évaluation
- Ta Ia2 Chemistry m20 Chap Carbonyl GroupDocument1 pageTa Ia2 Chemistry m20 Chap Carbonyl GroupRafit BiswasPas encore d'évaluation
- Organic Chemistry Notes-IntroductionDocument5 pagesOrganic Chemistry Notes-IntroductionPersonnumberunooPas encore d'évaluation
- q2 Law Science 9 Weeks 5 6Document8 pagesq2 Law Science 9 Weeks 5 6Haydee Penalosa AunzoPas encore d'évaluation
- Chemistry Assesment Ch.16 - An Introduction To Organic ChemistryDocument1 pageChemistry Assesment Ch.16 - An Introduction To Organic ChemistryRina RustiyawatiPas encore d'évaluation
- Simple Present Vs ContinuousDocument9 pagesSimple Present Vs ContinuousAngie Kong Su MeiPas encore d'évaluation
- ReproductionDocument3 pagesReproductionRos Mawar MelatiPas encore d'évaluation
- Functions Pure MathsDocument20 pagesFunctions Pure MathsAngie Kong Su MeiPas encore d'évaluation
- Verbs and Past Tense Form 4 EnglishDocument6 pagesVerbs and Past Tense Form 4 EnglishAngie Kong Su MeiPas encore d'évaluation
- Verbs and Past Tense Form 4 EnglishDocument6 pagesVerbs and Past Tense Form 4 EnglishAngie Kong Su MeiPas encore d'évaluation
- CZ Grandmother 1. Loving 3. Attentive 2. Caring 4. Understanding 5. Wonderful / Best Grandmother in The WorldDocument5 pagesCZ Grandmother 1. Loving 3. Attentive 2. Caring 4. Understanding 5. Wonderful / Best Grandmother in The WorldAngie Kong Su MeiPas encore d'évaluation
- Countable NounsDocument5 pagesCountable NounsAngie Kong Su MeiPas encore d'évaluation
- Countable NounsDocument5 pagesCountable NounsAngie Kong Su MeiPas encore d'évaluation
- Stem and Leaf DiagramsDocument1 pageStem and Leaf DiagramsAngie Kong Su MeiPas encore d'évaluation
- Chemistry 5 Carbon Carboxilic Acid and Ester StudentDocument10 pagesChemistry 5 Carbon Carboxilic Acid and Ester StudentAngie Kong Su MeiPas encore d'évaluation
- Essay 2) Identify: To Prepare Soap From Palm Oil and Sodium Hydroxide. Labelled Diagram: 1)Document2 pagesEssay 2) Identify: To Prepare Soap From Palm Oil and Sodium Hydroxide. Labelled Diagram: 1)Angie Kong Su MeiPas encore d'évaluation
- Stem and Leaf DiagramsDocument1 pageStem and Leaf DiagramsAngie Kong Su MeiPas encore d'évaluation
- Verbs and Past Tense Form 4 EnglishDocument6 pagesVerbs and Past Tense Form 4 EnglishAngie Kong Su MeiPas encore d'évaluation
- Form 4 Science Chapter 1: Scientific Investigation Steps in Methods of Scientific InvestigationDocument4 pagesForm 4 Science Chapter 1: Scientific Investigation Steps in Methods of Scientific InvestigationAngie Kong Su MeiPas encore d'évaluation
- Adverbs StudentsDocument4 pagesAdverbs StudentsAngie Kong Su MeiPas encore d'évaluation
- ReproductionDocument2 pagesReproductionAngie Kong Su MeiPas encore d'évaluation
- Science Form 5 Chapter 3 Preservation Conservation StudentDocument8 pagesScience Form 5 Chapter 3 Preservation Conservation StudentAngie Kong Su MeiPas encore d'évaluation
- f3 Science c6 Land and Resources StudentDocument36 pagesf3 Science c6 Land and Resources StudentAngie Kong Su MeiPas encore d'évaluation
- Science Form 5 Chapter 5 MotionDocument46 pagesScience Form 5 Chapter 5 MotionAngie Kong Su Mei0% (1)
- Science Form 5 NutritionDocument5 pagesScience Form 5 NutritionAngie Kong Su MeiPas encore d'évaluation
- Verbs and Past Tense Form 4 EnglishDocument6 pagesVerbs and Past Tense Form 4 EnglishAngie Kong Su MeiPas encore d'évaluation
- Physics Heat F4Ch4studentDocument9 pagesPhysics Heat F4Ch4studentAngie Kong Su MeiPas encore d'évaluation
- Physics 4 Understanding LensesDocument4 pagesPhysics 4 Understanding LensesAngie Kong Su MeiPas encore d'évaluation
- Physics Form 4 Chapter 2.9Document15 pagesPhysics Form 4 Chapter 2.9Farain RashdiPas encore d'évaluation
- Contoh Modul LKS IPADocument12 pagesContoh Modul LKS IPAasep_mulyana_4Pas encore d'évaluation
- Alpha Tech India Limited - FinalDocument4 pagesAlpha Tech India Limited - FinalRahul rPas encore d'évaluation
- Software Requirements SpecificationDocument9 pagesSoftware Requirements SpecificationSu-kEm Tech LabPas encore d'évaluation
- Ansi Numerical CodeDocument6 pagesAnsi Numerical Codekachra13Pas encore d'évaluation
- MC0085 MQPDocument20 pagesMC0085 MQPUtpal KantPas encore d'évaluation
- Finite Element Method For Eigenvalue Problems in ElectromagneticsDocument38 pagesFinite Element Method For Eigenvalue Problems in ElectromagneticsBhargav BikkaniPas encore d'évaluation
- RAMSCRAM-A Flexible RAMJET/SCRAMJET Engine Simulation ProgramDocument4 pagesRAMSCRAM-A Flexible RAMJET/SCRAMJET Engine Simulation ProgramSamrat JanjanamPas encore d'évaluation
- Standards Guide 1021 1407Document8 pagesStandards Guide 1021 1407Anjur SiPas encore d'évaluation
- Personal ComputerDocument3 pagesPersonal ComputerDan Mark IsidroPas encore d'évaluation
- EverServ 7700 M77XX Quick Reference GuideDocument2 pagesEverServ 7700 M77XX Quick Reference GuidetangocharliepdxPas encore d'évaluation
- Role of Quick Response To Supply ChainDocument15 pagesRole of Quick Response To Supply ChainSanuwar RashidPas encore d'évaluation
- 面向2035的新材料强国战略研究 谢曼Document9 pages面向2035的新材料强国战略研究 谢曼hexuan wangPas encore d'évaluation
- The Accreditation Committee Cityland Development CorporationDocument5 pagesThe Accreditation Committee Cityland Development Corporationthe apprenticePas encore d'évaluation
- Industrial Training ReportDocument19 pagesIndustrial Training ReportKapil Prajapati33% (3)
- Travel OrderDocument2 pagesTravel OrderStephen EstalPas encore d'évaluation
- PDF RR Grade Sep ProjectsDocument46 pagesPDF RR Grade Sep ProjectsjunqiangdongPas encore d'évaluation
- Steinecker Boreas: Wort Stripping of The New GenerationDocument16 pagesSteinecker Boreas: Wort Stripping of The New GenerationAlejandro Javier Delgado AraujoPas encore d'évaluation
- Case Study StarbucksDocument2 pagesCase Study StarbucksSonal Agarwal100% (2)
- Almutairy / Musa MR: Boarding PassDocument1 pageAlmutairy / Musa MR: Boarding PassMusaPas encore d'évaluation
- Control Flow, Arrays - DocDocument34 pagesControl Flow, Arrays - DocHARIBABU N SEC 2020Pas encore d'évaluation
- Bangalore Escorts Services - Riya ShettyDocument11 pagesBangalore Escorts Services - Riya ShettyRiya ShettyPas encore d'évaluation
- Cs09 404 Programming Paradigm (Module 1 Notes)Document24 pagesCs09 404 Programming Paradigm (Module 1 Notes)Rohith BhaskaranPas encore d'évaluation
- AXIOM75 50 25 1B - Rev.6 10.000MHzDocument4 pagesAXIOM75 50 25 1B - Rev.6 10.000MHzTürkay PektürkPas encore d'évaluation
- Bai Tap Avtc2 PrepositionsDocument5 pagesBai Tap Avtc2 PrepositionsShy NotPas encore d'évaluation
- Ga-z68p-Ds3 v2.x eDocument104 pagesGa-z68p-Ds3 v2.x ejohnsonlimPas encore d'évaluation
- Fish Siomai RecipeDocument12 pagesFish Siomai RecipeRhyz Mareschal DongonPas encore d'évaluation