Académique Documents
Professionnel Documents
Culture Documents
Aldol Condensation Discussion
Transféré par
Denisse Watt CuarterosCopyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Aldol Condensation Discussion
Transféré par
Denisse Watt CuarterosDroits d'auteur :
Formats disponibles
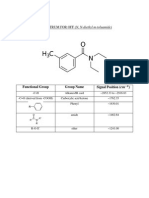
Discussion for Lab #8: Aldol Condensation
The result of performing an aldol condensation leads into the formation of unsaturated
ketone products since ketones are less reactive than aldehydes in aldol condensation because the
carbonyl of the ketones are histerically hindered and is electronically not as susceptible to
nucleophilic attack. Another reason would be because the aldehyde used, dont have -
hydrogens, so they cannot react with themselves; one such example is the experiment performed.
Also, dehydration is more favorable because the product has extended conjugations and
precipitates out of the solution. The aldehyde derivative used was trans-cinnamaldehyde and the
ketone derivative was cyclopentanone. Since the ketone derivative used was cyclopentanone and
is a symmetrical ketone, it will undergo double aldol condensation since it will be able to react
with two molecules of another carbonyl compound under basic conditions. Since the product is
highly conjugated, the hydration will be facile. The reaction of cyclopentanone and trans-
cinnamaldehyde (1:2) leads into the formation of the aldol product, (2E,5E)-2,5-Bis[(2E)-3-
phenyl-2-propen-1-ylidene]cyclopentanone. The aldol product is yellow-colored due to the
narrow HOMO-LUMO gap because of the extensive conjugation which is the reason why the
falls in the visible portion of the spectrum.
The solvent used was 20 mL of 80% toluene (16 mL) and 20% ethanol (4 mL). Hexanes
and acetone doesnt dissolve the product both in room temperature and after heating. One of the
reasons why hexanes and acetone wont dissolve the product is because of the differences in
polarity. Although the product is an unsaturated ketone, acetone doesnt have conjugated double
bonds and therefore, its ability to dissolve the aldol is hindered. Since hexane only exhibits
vander waals, like acetone, it doesnt have the ability to dissolve the aldol because the aldol can
exhibit dipole-dipole interactions. However, both toluene and ethanol dissolved some of the
crude product but not completely. Toluene dissolves most of the product since it also contains a
Discussion for Lab #8: Aldol Condensation
phenyl group like aldol. Ethanol also dissolves some of the product but since aldol is not very
polar unlike ethanol, using either toluene or ethanol alone wont be sufficient. The reason why
the solvent used was 80% toluene 20% ethanol, is because the aldol product doesnt completely
dissolve in pure toluene and pure ethanol at room temperature and when heated separately (with
the crude product). By mixing both solvents together, most of the crude product dissolved at
room temperature and the rest dissolved when heated and after evaporating the ethanol. After the
solution was cooled at room temperature, most of the crystals developed and was then
recrystallized through vacuum filtration.
After obtaining the recrystallized product, an IR spectrum, m.p, and NMR were obtained
to verify the results. The melting point of the product obtained is at 234C while the theoretical
value is 235 C. Because the obtained melting point is lower than the theoretical value, it means
that the recrystallized product still has impurities. The result of the IR spectra verifies that the
product has a carbonyl peak (C=O) at ~1676
which identifies the ketone group (R-C=O-
R). Another important peak that was identified was the peak at ~1586
which corresponds
to the phenyl group (
. However, both the IR peaks identified were not as sharp and
stretched because while the IR was not performed fast enough and ketones are volatile. The
NMR obtained shows all the significant peaks that correspond to the obtained aldol product,
(2E,5E)-2,5-Bis[(2E)-3-phenyl-2-propen-1-ylidene]cyclopentanone. However, traces of
impurities can still be traced like the singlet peak at ~2.92 ppm which is not supposed to be on
the NMR. According to the coupling pattern analysis of the product, it is not supposed to have
any singlet peak because it can only contain doublets and triplets. Due to its chemical shift and
coupling pattern, the peak might correspond to ethanol, since ethanol was also used as one of the
solvents in recrystallization. If the ethanol was completely evaporated from the solution, maybe a
much better result can be obtained from the NMR and m.p.
Discussion for Lab #8: Aldol Condensation
References
Bruice, P. Organic Chemistry, 7
th
ed.; Pearson: New Jersey, 2013.
Padias, A. Making The Connections, 2nd ed.; Hayden-McNeil Publishing: Plymouth, MI, 2013.
Padias, A. Organic Chemistry Laboratory Manual, 4th ed.; Hayden-McNeil Publishing:
Plymouth, MI, 2013.
Vous aimerez peut-être aussi
- Engineering Chemistry Unit III(A) - Definition and Types of LubricantsDocument25 pagesEngineering Chemistry Unit III(A) - Definition and Types of Lubricantssri aknth100% (1)
- Determination of Fluoride Concentration Using Ion Selective ElectrodeDocument7 pagesDetermination of Fluoride Concentration Using Ion Selective ElectrodeAmanda WangPas encore d'évaluation
- IJSO Chemistry Module-2Document300 pagesIJSO Chemistry Module-2Ikhbaat Atiqur Rehman100% (2)
- An Extraction of SpinachDocument4 pagesAn Extraction of SpinachDenisse Watt Cuarteros67% (3)
- E6 AtqDocument2 pagesE6 AtqSOUPINESSPas encore d'évaluation
- Experiment 4 Palvi FinalDocument8 pagesExperiment 4 Palvi FinalSara AliPas encore d'évaluation
- Lab Report (Final Editied)Document8 pagesLab Report (Final Editied)Alexia Channer100% (4)
- ASAL Chemistry CB Executive Preview DigitalDocument68 pagesASAL Chemistry CB Executive Preview DigitalAlisha AhmedPas encore d'évaluation
- Staphylococcus AureusDocument3 pagesStaphylococcus AureusDenisse Watt CuarterosPas encore d'évaluation
- Experiment 8 The Preparation of AcetanlideDocument12 pagesExperiment 8 The Preparation of AcetanlideRadhwanPas encore d'évaluation
- Chemical Reactions of Copper LabDocument5 pagesChemical Reactions of Copper Labrikubean100% (1)
- Water Purity Standards for Laboratory AnalysisDocument8 pagesWater Purity Standards for Laboratory Analysisade muchlasPas encore d'évaluation
- Synthesis of Tert-Butyl Chloride from AlcoholDocument7 pagesSynthesis of Tert-Butyl Chloride from AlcoholFerdinand Tamayo Cayabyab Jr.Pas encore d'évaluation
- Anthranilic acid: precursor to tryptophanDocument20 pagesAnthranilic acid: precursor to tryptophanGlibPas encore d'évaluation
- KHP LabDocument5 pagesKHP LabSantino MusaPas encore d'évaluation
- ###Best Practices Hydrogen Storage DOEDocument579 pages###Best Practices Hydrogen Storage DOEAnshul GuptaPas encore d'évaluation
- Di Benz Al AcetoneDocument3 pagesDi Benz Al AcetoneKristine Mae De GuzmanPas encore d'évaluation
- Practical Guide EdexcelDocument43 pagesPractical Guide EdexcelUsman BokhariPas encore d'évaluation
- Exp 6Document8 pagesExp 6KaVisha AShaPas encore d'évaluation
- Application of Statistical Concepts in The Determination of Weight Variation in Coin SamplesDocument3 pagesApplication of Statistical Concepts in The Determination of Weight Variation in Coin SamplesMicah PeraltaPas encore d'évaluation
- Solving The Bacterial UnknownDocument14 pagesSolving The Bacterial UnknownDenisse Watt Cuarteros100% (1)
- Inorganic Prac 2Document3 pagesInorganic Prac 2Ray DyerPas encore d'évaluation
- Aldol CondensationDocument10 pagesAldol CondensationVanessa Nguyen0% (1)
- Aldol Condensation LabDocument6 pagesAldol Condensation LabChristian AmpePas encore d'évaluation
- F325 Redox Equations and TitrationsDocument9 pagesF325 Redox Equations and TitrationsDoc_Croc100% (1)
- Practice 2Document30 pagesPractice 2Najmul Puda PappadamPas encore d'évaluation
- NaBH4 Reduction of Cyclohexanone to Cyclohexanol (87Document8 pagesNaBH4 Reduction of Cyclohexanone to Cyclohexanol (87hahadindongPas encore d'évaluation
- Oxalate TitrationDocument10 pagesOxalate Titrationlushu851648Pas encore d'évaluation
- Chem003 - Spectrophotometry - Determination of Wavelength of Maximum AbsorbanceDocument4 pagesChem003 - Spectrophotometry - Determination of Wavelength of Maximum Absorbancejuvy022088100% (1)
- Simultaneous determination of chromium and manganeseDocument35 pagesSimultaneous determination of chromium and manganeseVatra ReksaPas encore d'évaluation
- Phase Diagram of Three-Component Liquid SystemDocument11 pagesPhase Diagram of Three-Component Liquid SystemVanessa Denise Aguilar100% (2)
- Transition Metal ReactionsDocument11 pagesTransition Metal ReactionsFarahSyazwani100% (1)
- Phase Diagram of a Three-Component Liquid SystemDocument7 pagesPhase Diagram of a Three-Component Liquid SystemEllaine TejadaPas encore d'évaluation
- Preparation of Acetaline Notes PDFDocument6 pagesPreparation of Acetaline Notes PDFAnonymous Wwxatt3oIK100% (1)
- Review KTT212Document92 pagesReview KTT212Mohd HisyamPas encore d'évaluation
- Partition Coefficient DeterminationDocument4 pagesPartition Coefficient DeterminationMostafa HamawandyPas encore d'évaluation
- Recrystallization PDFDocument5 pagesRecrystallization PDFMikee GutierrezPas encore d'évaluation
- Lab Report Exp 2Document8 pagesLab Report Exp 2api-384913960Pas encore d'évaluation
- Hexane and Toluene Simple and Fractional DistillationDocument12 pagesHexane and Toluene Simple and Fractional Distillationrodneyperu0% (1)
- Fluoride Ion Selective ElectrodeDocument14 pagesFluoride Ion Selective ElectrodeMihEugen100% (1)
- Exp 11: Analysis of (Co (NH3) 5Cl) Cl2Document6 pagesExp 11: Analysis of (Co (NH3) 5Cl) Cl2Marla Basa50% (2)
- EXPERIMENT 2 Reduction of CamphorDocument2 pagesEXPERIMENT 2 Reduction of CamphorDania FaridPas encore d'évaluation
- LabDocument7 pagesLabLiz HackettPas encore d'évaluation
- Acid Base Titration Lab 6Document11 pagesAcid Base Titration Lab 6Jose Cencič0% (1)
- Objectives: FIGURE A: Example of Coordination CompoundsDocument7 pagesObjectives: FIGURE A: Example of Coordination CompoundsNurul izzatiPas encore d'évaluation
- Identification of Alcohols and Phenols Using Chemical TestsDocument6 pagesIdentification of Alcohols and Phenols Using Chemical Testsh1iraqPas encore d'évaluation
- Lab Report 11Document3 pagesLab Report 11PaulPas encore d'évaluation
- Ester Synthesis LabDocument6 pagesEster Synthesis LabMuhammad Abdur RokhimPas encore d'évaluation
- NITRATION OF METHYL BENZOATE (ELECTROPHILIC AROMATIC SUBSTITUITION - Idayu Razali - Academia - Edu PDFDocument7 pagesNITRATION OF METHYL BENZOATE (ELECTROPHILIC AROMATIC SUBSTITUITION - Idayu Razali - Academia - Edu PDFyawsPas encore d'évaluation
- Diels Alder LabDocument8 pagesDiels Alder Labfatevilcow0% (1)
- pH Electrode Titration Curve AnalysisDocument14 pagespH Electrode Titration Curve AnalysisMina VoPas encore d'évaluation
- Chem 31.1 FR1 SantosDocument5 pagesChem 31.1 FR1 SantosClaire SantosPas encore d'évaluation
- Exer2 PrelabDocument3 pagesExer2 Prelabkarinadegoma100% (1)
- Lab Activity 5Document5 pagesLab Activity 5Jasmin CeciliaPas encore d'évaluation
- Half Titration Lab ReportDocument6 pagesHalf Titration Lab Reportapi-20078641867% (3)
- Preparationofpotassiumtrisoxalateferrateiitrihydrate 140328134711 Phpapp02Document15 pagesPreparationofpotassiumtrisoxalateferrateiitrihydrate 140328134711 Phpapp02Guru P MPas encore d'évaluation
- Titus John - Enthalpy Prac ReportDocument12 pagesTitus John - Enthalpy Prac Reportapi-295071132Pas encore d'évaluation
- Qualitative Organic Analysis - Sem 3Document37 pagesQualitative Organic Analysis - Sem 3Reshma SomanPas encore d'évaluation
- Spectrophotometric Determination of Iron Using 1,10-PhenanthrolineDocument9 pagesSpectrophotometric Determination of Iron Using 1,10-Phenanthrolinedawin_mornaPas encore d'évaluation
- Aldehydes and KetonesDocument1 pageAldehydes and KetonesThea Mae Dino0% (1)
- Lab 10 - Diels AlderDocument4 pagesLab 10 - Diels AlderAlison NguyenPas encore d'évaluation
- Preparation and Purification of An Alkyl Halide FRDocument6 pagesPreparation and Purification of An Alkyl Halide FRCamille GrefaldiaPas encore d'évaluation
- Exp 6Document6 pagesExp 6MsShu93100% (1)
- Experiment 4 - Quantitative Analysis of Soda Ash by Double-Indicator TitrationDocument2 pagesExperiment 4 - Quantitative Analysis of Soda Ash by Double-Indicator TitrationMelchiPas encore d'évaluation
- Preparation of Colloidal Dispersion ExperimentsDocument8 pagesPreparation of Colloidal Dispersion ExperimentsFairuz Naim Z100% (1)
- Lab 4 Determination of An Equilibrium Constant 1Document7 pagesLab 4 Determination of An Equilibrium Constant 1Mohammad IzadiPas encore d'évaluation
- S D E C R / E 5: Pectrophotometric Etermination OF THE Quilibrium Onstant OF A Eaction XperimentDocument14 pagesS D E C R / E 5: Pectrophotometric Etermination OF THE Quilibrium Onstant OF A Eaction XperimentKenneth Dionysus SantosPas encore d'évaluation
- Coordination Chemistry: Invited Lectures Presented at the 20th International Conference on Coordination Chemistry, Calcutta, India, 10-14 December 1979D'EverandCoordination Chemistry: Invited Lectures Presented at the 20th International Conference on Coordination Chemistry, Calcutta, India, 10-14 December 1979D. BanerjeaPas encore d'évaluation
- Nitration of Methyl Benzoate (Worksheet)Document1 pageNitration of Methyl Benzoate (Worksheet)Denisse Watt CuarterosPas encore d'évaluation
- N, N-Diethyl M-Toluamide (OFF)Document3 pagesN, N-Diethyl M-Toluamide (OFF)Denisse Watt CuarterosPas encore d'évaluation
- IR Spectrum For OFF (Mosquito Repellant)Document1 pageIR Spectrum For OFF (Mosquito Repellant)Denisse Watt CuarterosPas encore d'évaluation
- Macrocyclic SynthesisDocument3 pagesMacrocyclic SynthesisDenisse Watt CuarterosPas encore d'évaluation
- Oxone Oxidation Analysis (Borneol and Camphor)Document2 pagesOxone Oxidation Analysis (Borneol and Camphor)Denisse Watt CuarterosPas encore d'évaluation
- NMR For Aldol CondensationDocument1 pageNMR For Aldol CondensationDenisse Watt CuarterosPas encore d'évaluation
- 2,3,4,5 TetraphenylcyclopentadienoneDocument6 pages2,3,4,5 TetraphenylcyclopentadienoneDenisse Watt Cuarteros100% (2)
- Fischer EsterificationDocument3 pagesFischer EsterificationDenisse Watt Cuarteros100% (1)
- Chem 243A - Hydrogenation (Catalytic)Document2 pagesChem 243A - Hydrogenation (Catalytic)Denisse Watt CuarterosPas encore d'évaluation
- IR Peaks For Cyclic AnhydrideDocument1 pageIR Peaks For Cyclic AnhydrideDenisse Watt CuarterosPas encore d'évaluation
- Carbon-Coated Co - Rich Cobalt Selenide Derived From ZIF-67 For E Cient Electrochemical Water OxidationDocument6 pagesCarbon-Coated Co - Rich Cobalt Selenide Derived From ZIF-67 For E Cient Electrochemical Water OxidationSeptian Perwira YudhaPas encore d'évaluation
- Sweet Caramel Flavoring Spec SheetDocument1 pageSweet Caramel Flavoring Spec SheetMarhun AlcinaPas encore d'évaluation
- 2017 - Formulation & Adjuvant Technology 17Document1 page2017 - Formulation & Adjuvant Technology 17Catherine TangPas encore d'évaluation
- DLL ElectronegativityDocument2 pagesDLL ElectronegativityCidie Boy BaldoPas encore d'évaluation
- QUES6Document10 pagesQUES6MH MoviezPas encore d'évaluation
- Practical - Gram StainingDocument15 pagesPractical - Gram Stainingsavera saveraPas encore d'évaluation
- Fault Tree Analysis-Air BubblesDocument7 pagesFault Tree Analysis-Air BubblesHeart Touching VideosPas encore d'évaluation
- HPLCDocument8 pagesHPLCMuhammad Koksh Sdiq HussinPas encore d'évaluation
- Chem PaDocument21 pagesChem PaAarushi ShuklaPas encore d'évaluation
- IX WorkSheet-2 (MOLE) With SolutionDocument4 pagesIX WorkSheet-2 (MOLE) With Solutionhridhaan psuedopodiaPas encore d'évaluation
- GPC-IR DatasheetDocument2 pagesGPC-IR DatasheetEdwel GutierrezPas encore d'évaluation
- Report On ICM Project: Rutherford ScatteringDocument10 pagesReport On ICM Project: Rutherford ScatteringSaurabh KumarPas encore d'évaluation
- Cast Cobalt AlloysDocument1 pageCast Cobalt Alloyssanju dhakerPas encore d'évaluation
- Removal of Cobalt and Nickel From Wastewater by UsDocument9 pagesRemoval of Cobalt and Nickel From Wastewater by UsOktavia AnggrainiPas encore d'évaluation
- Penelitian Pengembangan Minyak Atsiri Sebagai Aromaterapi Dan Potensinya Sebagai Produk Sediaan Farmasi MuchtaridiDocument9 pagesPenelitian Pengembangan Minyak Atsiri Sebagai Aromaterapi Dan Potensinya Sebagai Produk Sediaan Farmasi MuchtaridiFitria Dewi 'uthie'Pas encore d'évaluation
- Hypervalent Compounds: G. Sean Mcgrady & Jonathan W. SteedDocument21 pagesHypervalent Compounds: G. Sean Mcgrady & Jonathan W. SteedAndreea DamianPas encore d'évaluation
- 2.2. 5. Relative Density 20205eDocument2 pages2.2. 5. Relative Density 20205evafaashkPas encore d'évaluation
- Class 10 Science MCQ on Periodic Classification of ElementsDocument30 pagesClass 10 Science MCQ on Periodic Classification of ElementsAymen WaelPas encore d'évaluation
- Chemistry Lesson Note For Grade 11Document2 pagesChemistry Lesson Note For Grade 11Milkias BerhanuPas encore d'évaluation
- Araldite: The Original Epoxy AdhesiveDocument3 pagesAraldite: The Original Epoxy Adhesivespahicdanilo100% (1)
- Punjab Hazardous Substances Rules 2018Document56 pagesPunjab Hazardous Substances Rules 2018ahmad tararPas encore d'évaluation
- Soil Science Lab Ion ExchangeDocument2 pagesSoil Science Lab Ion ExchangeJr Bagaporo100% (1)
- Stoichiometry Quiz Answers (ChemistryDocument3 pagesStoichiometry Quiz Answers (ChemistrychampionPas encore d'évaluation
- Corrosion Inspection of Piping CircuitDocument14 pagesCorrosion Inspection of Piping CircuitPrasad ChakkrapaniPas encore d'évaluation