Académique Documents
Professionnel Documents
Culture Documents
Nucleation
Transféré par
Mohamed AbdullaCopyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Nucleation
Transféré par
Mohamed AbdullaDroits d'auteur :
Formats disponibles
Nucleation
The rate of nucleation is the rate of new particles formed per unit
time per unit volume of magma or solids-free mother liquor.
Nucleation is split into two mechanisms, primary nucleation and
secondary nucleation.
Primary nucleation
Is independent of crystal presence split into two subsections,
homogenous (spontaneous) and heterogeneous (facilitated by alien
substances).
Homogenous nucleation:
Nucleation without preferential nucleation sites, homogeneous
nucleation occurs spontaneously and randomly, but it requires
superheating or super cooling of the medium. Nuclei are in a state
of unstable equilibrium; if a nucleas looses units it dissolves and if it
gains units it becomes a crystal.
Cluster Embryo Nucleus Crystal
Ostwald ripening is a concept that explains nucleation,
thermodynamically the difference between a smaller crystal and a
large one is that the smaller one contains a much larger surface
area per unit mass this causes an unstable equilibrium with a
supersaturated solution causing the small crystal to dissolve and
leaving the larger one to grow.
The solubility of a substance is related to it particle size by Kelvin
equation:
ln =
4Vm
vRTL
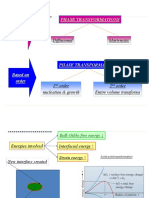
Heterogeneous Nucleation
Heterogeneous nucleation occurs much more often than
homogeneous nucleation. Heterogeneous nucleation applies to the
phase transformation between any two phases of gas, liquid, or
solid, typically for example, condensation of gas/vapor, solidification
from liquid, and bubble formation from liquid. Theoretically the
nucleus wets the surface of the catalyst and the work of nucleus
formation is reduced by a fraction that is proportional to the angle of
wetting.
Vous aimerez peut-être aussi
- CRYSTALLIZATIONDocument53 pagesCRYSTALLIZATIONKshitiz KumarPas encore d'évaluation
- Crystallization SBDocument60 pagesCrystallization SBMirza Salman BaigPas encore d'évaluation
- Crystallization PDFDocument74 pagesCrystallization PDFMohammed Akif ShaikhPas encore d'évaluation
- Mass Transfer Operation 1 (2150501) : Crystallization Concept, Techniques and ProcessesDocument18 pagesMass Transfer Operation 1 (2150501) : Crystallization Concept, Techniques and Processesvashu patelPas encore d'évaluation
- MEE1005 Materials Engineering and Technology: Dr. Ashish Kumar Saxena, Assistant Professor, CIMR Ashishkumar@vit - Ac.inDocument10 pagesMEE1005 Materials Engineering and Technology: Dr. Ashish Kumar Saxena, Assistant Professor, CIMR Ashishkumar@vit - Ac.inKD GamerPas encore d'évaluation
- Cry Equi 2Document13 pagesCry Equi 2Shreya JoshiPas encore d'évaluation
- Crystallization: DR - Sangeetha SubramanianDocument20 pagesCrystallization: DR - Sangeetha SubramanianYoshita SinghPas encore d'évaluation
- L2 - Nucleation and GrowthDocument14 pagesL2 - Nucleation and GrowthJagdeep Rahul100% (1)
- I - 8. Solidification & NucleationDocument19 pagesI - 8. Solidification & NucleationVarshni VsPas encore d'évaluation
- Types of CrystalsDocument29 pagesTypes of CrystalsPratap TanariPas encore d'évaluation
- Crystallisation - Faculty LectureDocument24 pagesCrystallisation - Faculty Lecturesoumitra hazraPas encore d'évaluation
- Module 2Document21 pagesModule 2jithin7025089793Pas encore d'évaluation
- Lecture 2 - Nucleation and Growth of NanomaterialsDocument17 pagesLecture 2 - Nucleation and Growth of NanomaterialsRachit Goyal100% (1)
- Important Parameters and Basics in LyophilisationDocument41 pagesImportant Parameters and Basics in LyophilisationVaruni ChowdaryPas encore d'évaluation
- Crystallization EASILY DESCRIBEDDocument4 pagesCrystallization EASILY DESCRIBEDFrancess Liene YagoPas encore d'évaluation
- Solvate: Extremely Pure FormDocument22 pagesSolvate: Extremely Pure FormNayan HalderPas encore d'évaluation
- Separation Process 2 SMJC 3283: DR Khairunnisa Binti Mohd. Paad 13 April 2020 MondayDocument53 pagesSeparation Process 2 SMJC 3283: DR Khairunnisa Binti Mohd. Paad 13 April 2020 MondaynorsiahPas encore d'évaluation
- Crystal IzationDocument8 pagesCrystal Izationahmed.khallaf1962Pas encore d'évaluation
- Nucleation and GrowthDocument23 pagesNucleation and Growthyinglv67% (3)
- NeutronDocument2 pagesNeutronapi-9101692100% (2)
- Melting Point of Impure Solids3Document1 pageMelting Point of Impure Solids3palmer okiemutePas encore d'évaluation
- Crystallization - MCE 203Document31 pagesCrystallization - MCE 203samuelodeyemi3000Pas encore d'évaluation
- MLB 1-4 2022 BorderDocument50 pagesMLB 1-4 2022 BorderWilly BillyPas encore d'évaluation
- General Chemistry 2Document23 pagesGeneral Chemistry 2St. DymphaMaralit, Joyce Anne L.Pas encore d'évaluation
- 8 CrystallizationDocument36 pages8 CrystallizationSydney KombePas encore d'évaluation
- CrystallizationDocument6 pagesCrystallizationPulok HasanPas encore d'évaluation
- Lecture 4 CRYSTALLIZATION 2017 PDFDocument31 pagesLecture 4 CRYSTALLIZATION 2017 PDFPritam PatilPas encore d'évaluation
- CrystallizationDocument14 pagesCrystallizationAbhimanyu AwasthiPas encore d'évaluation
- Week 1: Kinetic Molecular Model of Liquids and SolidsDocument31 pagesWeek 1: Kinetic Molecular Model of Liquids and SolidsCrizza Mae CuregPas encore d'évaluation
- MSE 210: Microstructural Engineering: Jan 2024 4 CreditsDocument10 pagesMSE 210: Microstructural Engineering: Jan 2024 4 Creditsabhishek.kumarPas encore d'évaluation
- Nucleation of Crystals From Solution in Microgravity Usml-1 Glovebox (GBX) Investigation)Document10 pagesNucleation of Crystals From Solution in Microgravity Usml-1 Glovebox (GBX) Investigation)hesrinivasPas encore d'évaluation
- Hetrogeneous NucleationDocument9 pagesHetrogeneous NucleationRohanPas encore d'évaluation
- Nucleation: Theory and Applications To Protein Solutions and Colloidal SuspensionsDocument27 pagesNucleation: Theory and Applications To Protein Solutions and Colloidal SuspensionsRosni hasanPas encore d'évaluation
- CrystallizationDocument7 pagesCrystallizationKhaqan AminPas encore d'évaluation
- PHYSCIEreviewerDocument6 pagesPHYSCIEreviewerMaria Lorraine AninoPas encore d'évaluation
- 4.0 Solid-State Nucleation and Growth PDFDocument17 pages4.0 Solid-State Nucleation and Growth PDFLEONARD NYIRONGOPas encore d'évaluation
- Basic Chemistry II ColloidDocument11 pagesBasic Chemistry II ColloidvenaPas encore d'évaluation
- Lecture-08 Nuclear ModelsDocument19 pagesLecture-08 Nuclear ModelsAbdul Jabbar Abdul JabbarPas encore d'évaluation
- 03 Movement of Substances NotesDocument7 pages03 Movement of Substances Notestarankaur401Pas encore d'évaluation
- Unit 2 Thermal Physics CIEDocument27 pagesUnit 2 Thermal Physics CIEShrirang ChandankhedePas encore d'évaluation
- Ice Nucleation in NatureDocument11 pagesIce Nucleation in Nature00gp1200rPas encore d'évaluation
- Difference Between Nuclear Fusion and Fission Reading-1Document2 pagesDifference Between Nuclear Fusion and Fission Reading-1Tula RmahPas encore d'évaluation
- CrystallizationDocument12 pagesCrystallizationAmol RastogiPas encore d'évaluation
- State of Matter InvestigationDocument3 pagesState of Matter Investigationadiraii ordoezPas encore d'évaluation
- Manohar Shivaji Lad .: Nucleation and GrowthDocument16 pagesManohar Shivaji Lad .: Nucleation and GrowthManohar LadPas encore d'évaluation
- Mass CrystallizationDocument15 pagesMass Crystallizationمعاذ المجيولPas encore d'évaluation
- TP1 - Kinetic Theory of Matter 1Document2 pagesTP1 - Kinetic Theory of Matter 1wengiemotshegwePas encore d'évaluation
- Nuclear Fusion & Nuclear FissionDocument7 pagesNuclear Fusion & Nuclear FissionHari KrishnanPas encore d'évaluation
- Crystal Growth: From Wikipedia, The Free EncyclopediaDocument10 pagesCrystal Growth: From Wikipedia, The Free EncyclopediabekkuPas encore d'évaluation
- Crystallization: Vijay Kumar TirukkachiDocument42 pagesCrystallization: Vijay Kumar TirukkachiVIJAY KUMAR TIRUKKACHI80% (5)
- PhysicalscienceDocument3 pagesPhysicalsciencenicole vinluanPas encore d'évaluation
- Liquid Crystals Seminar SMDocument7 pagesLiquid Crystals Seminar SMSmriti100% (1)
- Unit 4 and 5 CombinedDocument205 pagesUnit 4 and 5 CombinednagasudhanPas encore d'évaluation
- Mechanisms of Nucleation and Growth of Nanoparticles in SolutionDocument21 pagesMechanisms of Nucleation and Growth of Nanoparticles in SolutionMathar BashirPas encore d'évaluation
- Integrated Science NotesDocument26 pagesIntegrated Science NotesGods-star AngelPas encore d'évaluation
- Presentation On Cleavage: by Racheal AkpeneDocument28 pagesPresentation On Cleavage: by Racheal AkpeneAkpene RachealPas encore d'évaluation
- Study Guide KMTDocument6 pagesStudy Guide KMT9Wezen Jowelyn Mae G. TabuzoPas encore d'évaluation
- 2-Thermal PhysicsDocument26 pages2-Thermal PhysicsSamah Hafez100% (1)
- Ostwald RipeningDocument5 pagesOstwald RipeningNurdan AşarPas encore d'évaluation
- Topic-2 3Document13 pagesTopic-2 3Mohamed AbdullaPas encore d'évaluation
- S0032591002002061 - 1 s2.0 S0032591002002061 MainDocument7 pagesS0032591002002061 - 1 s2.0 S0032591002002061 MainMohamed AbdullaPas encore d'évaluation
- Industrial Crystallization and Precipitation From Solutions: State of The TechniqueDocument29 pagesIndustrial Crystallization and Precipitation From Solutions: State of The TechniqueMohamed AbdullaPas encore d'évaluation
- Spherical Agglomeration During Crystallization of An Active Pharmaceutical IngredientDocument7 pagesSpherical Agglomeration During Crystallization of An Active Pharmaceutical IngredientMohamed AbdullaPas encore d'évaluation
- Legalisation Application Form PRINTABLEDocument3 pagesLegalisation Application Form PRINTABLEMohamed AbdullaPas encore d'évaluation
- Li-Fi: Mckinsey Global Institute Report, 2011Document2 pagesLi-Fi: Mckinsey Global Institute Report, 2011Mohamed AbdullaPas encore d'évaluation
- G G G G Effective Rate G Mechanicalattrition Rate DL DT G Crystal GrowthrateDocument4 pagesG G G G Effective Rate G Mechanicalattrition Rate DL DT G Crystal GrowthrateMohamed AbdullaPas encore d'évaluation
- Nozzel DistribeeDocument38 pagesNozzel DistribeeMohamed AbdullaPas encore d'évaluation
- Finllay AppendixDocument6 pagesFinllay AppendixMohamed AbdullaPas encore d'évaluation
- 298 - DemoDocument5 pages298 - DemoMohamed AbdullaPas encore d'évaluation
- School of Management & Languages: Operations Strategy and Process DesignDocument3 pagesSchool of Management & Languages: Operations Strategy and Process DesignMohamed AbdullaPas encore d'évaluation
- 298 - DemoDocument5 pages298 - DemoMohamed AbdullaPas encore d'évaluation
- Time Series Cash Flows Present ValuesDocument2 pagesTime Series Cash Flows Present ValuesMohamed AbdullaPas encore d'évaluation
- Minutes From 12th FebruaryDocument1 pageMinutes From 12th FebruaryMohamed AbdullaPas encore d'évaluation
- Meeting 14Document1 pageMeeting 14Mohamed AbdullaPas encore d'évaluation
- Using A New Life Cycle Assessment A Range Different Nitrogen Concentration Fertilizers Was Analyzed Using A Basis of One Ton of Winter Wheat in Rothamsted EnglandDocument2 pagesUsing A New Life Cycle Assessment A Range Different Nitrogen Concentration Fertilizers Was Analyzed Using A Basis of One Ton of Winter Wheat in Rothamsted EnglandMohamed AbdullaPas encore d'évaluation
- Personal Details: Name: AddressDocument1 pagePersonal Details: Name: AddressMohamed AbdullaPas encore d'évaluation
- CompressorDocument2 pagesCompressorshinejbhPas encore d'évaluation
- PR 20 Int 0.2Document6 pagesPR 20 Int 0.2Mohamed AbdullaPas encore d'évaluation
- ButaneDocument6 pagesButaneRatu Fajrina HanifaPas encore d'évaluation
- Control Engineering Exam 2011-2012 7 No AnswersDocument13 pagesControl Engineering Exam 2011-2012 7 No AnswersMohamed AbdullaPas encore d'évaluation
- Meeting 14Document1 pageMeeting 14Mohamed AbdullaPas encore d'évaluation
- Peer Review FormDocument1 pagePeer Review FormMohamed AbdullaPas encore d'évaluation
- WhatDocument2 pagesWhatMohamed AbdullaPas encore d'évaluation
- Recycling of Waste Tyre Rubber Into Oil AbsorbentDocument15 pagesRecycling of Waste Tyre Rubber Into Oil AbsorbentMohamed AbdullaPas encore d'évaluation
- PR 20 Int 0.2Document6 pagesPR 20 Int 0.2Mohamed AbdullaPas encore d'évaluation
- Pressure Measurement by ManometerDocument7 pagesPressure Measurement by ManometerMohamed AbdullaPas encore d'évaluation
- PR 20 Int 0.2Document6 pagesPR 20 Int 0.2Mohamed AbdullaPas encore d'évaluation
- PR 20 Int 0.2Document6 pagesPR 20 Int 0.2Mohamed AbdullaPas encore d'évaluation