Académique Documents
Professionnel Documents
Culture Documents
Tutorial 2
Transféré par
Nbl KlfCopyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Tutorial 2
Transféré par
Nbl KlfDroits d'auteur :
Formats disponibles
ENG 2223 Properties and Applications of Materials.
Tutorial. Chapter 2 Bonding between atoms structure of engineering materials
1. Give the electron configurations for the following ions: Fe2+ , Al3+ , Cu+ , Ba2+ , Br , O2 , Fe3+ and
S2 .
2. Sodium chloride (NaCl) exhibits predominantly ionic bonding. The Na+ and Cl ions have electron
structures that are identical to which two inert gases?
3. With regard to electron configuration, what do all the elements in Group VIIA of the periodic table
have in common?
4. Without consulting Figure 2.6 or Table 2.2, determine whether each of the electron configurations
given below is an inert gas, a halogen, an alkali metal, an alkaline earth metal, or a transition metal.
Justify your choices.
(a) 1s2 2s2 2p6 3s2 3p6 3d7 4s2
(b) 1s2 2s2 2p6 3s2 3p6
(c) 1s2 2s2 2p5
(d) 1s2 2s2 2p6 3s2
(e) 1s2 2s2 2p6 3s2 3p6 3d2 4s2
(f) 1s2 2s2 2p6 3s2 3p6 4s1
5. (a) What electron subshell is being filled for the rare earth series of elements on the periodic table?
(b) What electron subshell is being filled for the actinide series?
6. For a K+ -Cl ion pair, attractive and repulsive energies EA and ER , respectively, depend on the
distance between the ions r, according to
1.436
r
5.8x106
ER =
r9
EA =
For these expressions, energies are expressed in electron volts per K+ -Cl pair, and r is the distance
in nanometers. The net energy EN is just the sum of the two expressions above.
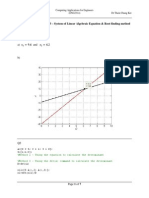
(a) Superimpose on a single plot EN , ER , and EA versus r up to 1.2 nm.
(b) On the basis of this plot, determine (i) the equilibrium spacing r0 between the K+ and Cl ions,
and (ii) the magnitude of the bonding energy E0 between the two ions.
(c) Mathematically determine the r0 and E0 values using the solutions to Problem 2.14 and compare
these with the graphical results from part (b).
7. Compute the percents ionic character of the interatomic bonds for the following compounds: TiO2 ,
ZnTe, CsCl, InSb, and MgCl2 .
Vous aimerez peut-être aussi
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (587)
- Standart Anzi Eyewash PDFDocument12 pagesStandart Anzi Eyewash PDFDwiki Yanuar Ramadhan100% (1)
- Torsion Experiment ReportDocument14 pagesTorsion Experiment ReportNbl KlfPas encore d'évaluation
- PREFCHEM Salary and Leave EntitlementDocument4 pagesPREFCHEM Salary and Leave EntitlementNbl KlfPas encore d'évaluation
- Standart Anzi Eyewash PDFDocument12 pagesStandart Anzi Eyewash PDFDwiki Yanuar Ramadhan100% (1)
- Ansys Assignment 2 (Redo)Document5 pagesAnsys Assignment 2 (Redo)Nbl KlfPas encore d'évaluation
- Test 1 Book Notes Examples-1 PDFDocument65 pagesTest 1 Book Notes Examples-1 PDFRalph Castino87% (15)
- 2 - Friction Loss UNGUIDED Ver2Document4 pages2 - Friction Loss UNGUIDED Ver2Nbl KlfPas encore d'évaluation
- Renewable Energy Tutorial Covers Small Wind SystemsDocument2 pagesRenewable Energy Tutorial Covers Small Wind SystemsNbl KlfPas encore d'évaluation
- 4th Meeting Notes FYP1Document2 pages4th Meeting Notes FYP1Nbl KlfPas encore d'évaluation
- Data TableDocument1 pageData TableNbl KlfPas encore d'évaluation
- Questions Sample 2Document6 pagesQuestions Sample 2Nbl KlfPas encore d'évaluation
- Taylor's University Engineering Students Practice Lagrange InterpolationDocument4 pagesTaylor's University Engineering Students Practice Lagrange InterpolationNbl KlfPas encore d'évaluation
- MT 1Document34 pagesMT 1Vishal VnPas encore d'évaluation
- Internal Combustion Engines & Emissions Revision Questions-Part 1Document6 pagesInternal Combustion Engines & Emissions Revision Questions-Part 1Nbl KlfPas encore d'évaluation
- Project ScopeDocument1 pageProject ScopeNbl KlfPas encore d'évaluation
- Tutorial 8Document2 pagesTutorial 8Nbl KlfPas encore d'évaluation
- ENG2134 - Solution Practice 3-2Document7 pagesENG2134 - Solution Practice 3-2Nbl KlfPas encore d'évaluation
- Failure Analysis FormDocument2 pagesFailure Analysis FormNbl KlfPas encore d'évaluation
- ENG2134 - Solution Practice 3-2Document7 pagesENG2134 - Solution Practice 3-2Nbl KlfPas encore d'évaluation
- Solution of Class Examples - Lecture 4Document4 pagesSolution of Class Examples - Lecture 4Nbl KlfPas encore d'évaluation
- Lab ReportDocument5 pagesLab ReportNbl KlfPas encore d'évaluation
- Solution - Practice 2 A) Relational/Logical Exercises: Refer To MatlabDocument4 pagesSolution - Practice 2 A) Relational/Logical Exercises: Refer To MatlabNbl KlfPas encore d'évaluation
- ENG2314 - Practice 3Document3 pagesENG2314 - Practice 3Nbl KlfPas encore d'évaluation
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (894)
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (399)
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (73)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5794)
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2219)
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (344)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (265)
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (119)
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)