Académique Documents
Professionnel Documents
Culture Documents
Acid-Base Study Guide: Memorize These
Transféré par
backch90110 évaluation0% ont trouvé ce document utile (0 vote)
15 vues4 pagesAcids and Bases
Titre original
Acids and Bases
Copyright
© © All Rights Reserved
Formats disponibles
PDF, TXT ou lisez en ligne sur Scribd
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentAcids and Bases
Droits d'auteur :
© All Rights Reserved
Formats disponibles
Téléchargez comme PDF, TXT ou lisez en ligne sur Scribd
0 évaluation0% ont trouvé ce document utile (0 vote)
15 vues4 pagesAcid-Base Study Guide: Memorize These
Transféré par
backch9011Acids and Bases
Droits d'auteur :
© All Rights Reserved
Formats disponibles
Téléchargez comme PDF, TXT ou lisez en ligne sur Scribd
Vous êtes sur la page 1sur 4
Acid-Base Study Guide
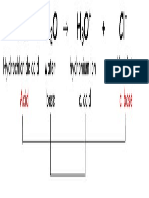
Acid = proton donor
Base = proton acceptor
We write the formula of an acid with a H out front to help you
identify it as an acid. Strong bases will contain hydroxide ion
(OH ) in the formula.
Remember that H+ and H3O+ are equivalent.
Strong Acids (= strong electrolytes)
Note: each reaction has water implied as a reactant, and the
H+ is donated to it to form H3O+. For example, these two
equations are equivalent:
HNO3 H+ + NO3-
and HNO3 + H2O H3O+ + NO3-
MEMORIZE THESE:
HNO3
H2SO4
HClO4
HCl
HBr
HI
Nitric Acid
Sulfuric Acid
Perchloric Acid
Hydrochloric Acid
Hydrobromic Acid
Hydroiodic Acid
HNO3 H+ + NO3H2SO4 2H+ + SO42HClO4 H+ + ClO4HCl H+ + ClHBr H+ + BrHI H+ + I-
Strong Bases (= strong electrolytes)
MEMORIZE THESE:
Group IA hydroxides LiOH, NaOH, KOH, etc
LiOH Li+ + OHNaOH Na+ + OHKOH K+ + OHand from Group IIA Ba(OH)2
Ba(OH)2 Ba2+ + 2OH-
Weak Acids (= weak electrolytes)
If the acid is not one of the strong acids above, you can
safely assume its a weak acid.
Note: like the strong acid reactions given above, each
reaction has water as a reactant, and the H+ is donated to it
to form H3O+.
EXAMPLES FROM NOMENCLATURE THAT YOU NEED
TO MEMORIZEHNO2
H2SO3
Nitrous Acid
Sulfurous Acid
H2CO3
Carbonic Acid
H3PO4
Phosphoric Acid
HNO2 W H+ + NO2H2SO3 W H+ + HSO3HSO3- W H+ + SO32H2CO3 W H+ + HCO3HCO3- W H+ + CO32H3PO4 W H+ + H2PO4H2PO4- W H+ + HPO42HPO42- W H+ + PO43-
HClO3, HClO2, HClO (and Br and I equivalents) Chloric,
Chlorous, and hypochlorous acids
Weak Bases (= weak electrolytes)
Memorize NH3 only
NH3 + H2O W NH4+ + OH-
Amphiteric (Amphiprotic) Substances - can
behave like an acid or a base
Substances that have a H out front and have a negative
charge can behave like an acid or base, for example
Acid:
HCO3- + H2O
W H3O+ + CO32-
Base:
HCO3- + H2O
W H2CO3 + OH-
Vous aimerez peut-être aussi
- 3.ii. BASES AND ACIDS OF CHOICEDocument110 pages3.ii. BASES AND ACIDS OF CHOICEKeith OmwoyoPas encore d'évaluation
- Acid Base ConceptDocument20 pagesAcid Base Conceptyadavamlesh045Pas encore d'évaluation
- Topic 12 NotesDocument31 pagesTopic 12 NotesHassan 2Pas encore d'évaluation
- Topic 12 NotesDocument31 pagesTopic 12 NotesHK Nova ChiuPas encore d'évaluation
- ACID BASE THEORY by FS ShahDocument24 pagesACID BASE THEORY by FS Shahfarooq shah shabbirPas encore d'évaluation
- Chapter 3 Acid - BaseDocument96 pagesChapter 3 Acid - BaseAnh NhamPas encore d'évaluation
- Chapter 3 Acid - BaseDocument98 pagesChapter 3 Acid - BaseNhan PhướcPas encore d'évaluation
- Ionic EquilibriumDocument60 pagesIonic EquilibriumVermavinay3940 vinay394080% (5)
- PPP1A 8.1 Arrhenius and NamingDocument13 pagesPPP1A 8.1 Arrhenius and NamingRichard LindemannPas encore d'évaluation
- Bronsted LowryDocument3 pagesBronsted LowryKurniawan RamadaniPas encore d'évaluation
- Chapter 2 Acid - Base - EngDocument98 pagesChapter 2 Acid - Base - Englong.vuongbz188Pas encore d'évaluation
- Acid Base Notes (Use For Chem 123)Document16 pagesAcid Base Notes (Use For Chem 123)Nate JamesPas encore d'évaluation
- Chapter 3 Acid - BaseDocument98 pagesChapter 3 Acid - BasePHƯƠNG ĐẶNG YẾNPas encore d'évaluation
- Module in Acid-BasesDocument8 pagesModule in Acid-BasesPenPen MalayPas encore d'évaluation
- 4.8 Introduction To Acid-Base Reactions StudentDocument3 pages4.8 Introduction To Acid-Base Reactions StudentSyed RazaPas encore d'évaluation
- Chapter 3 Acid - BaseDocument98 pagesChapter 3 Acid - BaseKhoa Nguyen Viet DangPas encore d'évaluation
- Acids, Bases and Non-Aqueous Solvents PDFDocument27 pagesAcids, Bases and Non-Aqueous Solvents PDFak fuad0% (1)
- Acid-Base Equilibrium PPT UPDATED 2022Document96 pagesAcid-Base Equilibrium PPT UPDATED 2022Sara Molinaro100% (1)
- 7.0 Ionic EquilibriaDocument124 pages7.0 Ionic EquilibriaTasya KassimPas encore d'évaluation
- NH 3 H 2 o Oh NH 4 Acid Base Concepts Chapter 15 H Conjugate Acid Base Pairs H Base AcidDocument12 pagesNH 3 H 2 o Oh NH 4 Acid Base Concepts Chapter 15 H Conjugate Acid Base Pairs H Base AcidKhang TrầnPas encore d'évaluation
- HSC Chemistry Lesson Plan 16Document8 pagesHSC Chemistry Lesson Plan 16Ali HaidarPas encore d'évaluation
- Acid. Base. SaltDocument55 pagesAcid. Base. Saltonly. starPas encore d'évaluation
- Chemistry Chapter 10Document31 pagesChemistry Chapter 10Misbah JilaniPas encore d'évaluation
- Bronsted LowryDocument71 pagesBronsted LowryShaina NovicioPas encore d'évaluation
- Ionic Equilibrium Lecture 9 (1st January 2023) Handout and HomeworkDocument402 pagesIonic Equilibrium Lecture 9 (1st January 2023) Handout and Homeworktanishq yadavPas encore d'évaluation
- Modern Theories of Acids & Bases: The Arrhenius and Bronsted-Lowry TheoriesDocument48 pagesModern Theories of Acids & Bases: The Arrhenius and Bronsted-Lowry TheoriesAgung PratamaPas encore d'évaluation
- Ionic EquilibriumDocument23 pagesIonic EquilibriumjamzeethsPas encore d'évaluation
- Ionic Equilibria Acids and Bases NotesDocument21 pagesIonic Equilibria Acids and Bases Notesseanapollomarco.cantosPas encore d'évaluation
- ACID BAse AssignmentDocument11 pagesACID BAse AssignmentMosfiqur Rahman100% (2)
- Bronsted LowryDocument41 pagesBronsted LowryKara BarbosaPas encore d'évaluation
- Chemistry 2: Unit 3 Acids-Bases and SaltDocument96 pagesChemistry 2: Unit 3 Acids-Bases and SaltNoraziah ZulPas encore d'évaluation
- 15.1 Bronsted-Lowry Acids and BasesDocument6 pages15.1 Bronsted-Lowry Acids and BasesPatricia de LeonPas encore d'évaluation
- Unit 3 Ionic EquibliriumDocument63 pagesUnit 3 Ionic EquibliriumFiixaa B OlqabaaPas encore d'évaluation
- Acids and Bases HonorsDocument47 pagesAcids and Bases HonorsAnsh ChaudharyPas encore d'évaluation
- Acid Ionic EqulbrmDocument21 pagesAcid Ionic EqulbrmsheenajerryPas encore d'évaluation
- Chapter 7 Acid-Base ReactionDocument111 pagesChapter 7 Acid-Base ReactionUMMU MARDHIAH ABDUL HALIMPas encore d'évaluation
- Acid-Base Chemistry: Manasi MantriDocument16 pagesAcid-Base Chemistry: Manasi MantriSonam ChhedaPas encore d'évaluation
- 2015 BiotechDocument74 pages2015 BiotechRosa DemliePas encore d'évaluation
- 6-Acids and BasesDocument46 pages6-Acids and Basesnirvanjain212007Pas encore d'évaluation
- 09 Acids and BasesDocument87 pages09 Acids and BasesvincentPas encore d'évaluation
- Cursul 7Document32 pagesCursul 7Bogdan Cel MicPas encore d'évaluation
- Conjugate Acid and Base PairsDocument1 pageConjugate Acid and Base Pairsfelisita wisangPas encore d'évaluation
- Topic 7 Acids and BasesDocument25 pagesTopic 7 Acids and BasesadamskbdPas encore d'évaluation
- CHPT 16Document12 pagesCHPT 16Duaa RajaPas encore d'évaluation
- Acid - Base TheoryDocument19 pagesAcid - Base TheorynrasPas encore d'évaluation
- Acids and BasesDocument34 pagesAcids and BasesAlannah ChadwickPas encore d'évaluation
- CHM271 - Chapter 3 Ionic Equilibrium PDFDocument72 pagesCHM271 - Chapter 3 Ionic Equilibrium PDFMuhd AmirPas encore d'évaluation
- Chapter 9Document4 pagesChapter 9Rochelle Anne BandaPas encore d'évaluation
- 14 Acids BasesDocument165 pages14 Acids BasesManni Piyush SharmaPas encore d'évaluation
- Ionic Equilibrium (Elementary) : Table-1Document19 pagesIonic Equilibrium (Elementary) : Table-1Yogesh GoyalPas encore d'évaluation
- Chapter 18: Don't Drop The AcidDocument17 pagesChapter 18: Don't Drop The AcidRianna MariaPas encore d'évaluation
- Acids and BasesDocument33 pagesAcids and BasesFrancene Badana YepesPas encore d'évaluation
- Acids and BasesDocument17 pagesAcids and Basespadidela swarochishraoPas encore d'évaluation
- PH and BuffersDocument24 pagesPH and BuffersJoshua LewisPas encore d'évaluation
- GC2 Q4 L4stemDocument45 pagesGC2 Q4 L4stemeli candazaPas encore d'évaluation
- Ionic EquilibriumDocument46 pagesIonic EquilibriumPadmalaya paloPas encore d'évaluation
- 13 Ionic Equilibria Notes PDFDocument37 pages13 Ionic Equilibria Notes PDFUchiha YogesPas encore d'évaluation
- Chapter 3 - Concept of Acid-Base NeutralisationDocument58 pagesChapter 3 - Concept of Acid-Base NeutralisationIkmal FikriPas encore d'évaluation
- Chapter4-Konsep Asam BasaDocument50 pagesChapter4-Konsep Asam BasaAnnisah MardiyyahPas encore d'évaluation
- Practice Makes Perfect in Chemistry: Acids, Bases, and SaltsD'EverandPractice Makes Perfect in Chemistry: Acids, Bases, and SaltsPas encore d'évaluation
- Amyporterfield TWN ExitrunwaychecklistDocument3 pagesAmyporterfield TWN Exitrunwaychecklistbackch9011Pas encore d'évaluation
- Minor Fall Major LiftDocument11 pagesMinor Fall Major Liftbackch9011Pas encore d'évaluation
- Toontrack Superior Drummer 3Document11 pagesToontrack Superior Drummer 3backch9011100% (1)
- Getting Started Getting StartedDocument1 pageGetting Started Getting Startedbackch9011Pas encore d'évaluation
- The Sound of The Next Generation - Final Spread PDFDocument17 pagesThe Sound of The Next Generation - Final Spread PDFbackch9011Pas encore d'évaluation
- Chefs Menu December 2018: Aperitif Suggestions BlushDocument1 pageChefs Menu December 2018: Aperitif Suggestions Blushbackch9011Pas encore d'évaluation
- Yamaha Acoustic Guitar Archive v6.4.3 Excel 97 2003Document238 pagesYamaha Acoustic Guitar Archive v6.4.3 Excel 97 2003backch90110% (1)
- Yamaha Acoustic Guitar Archive v6.4.4 FG ALLDocument1 pageYamaha Acoustic Guitar Archive v6.4.4 FG ALLbackch9011Pas encore d'évaluation
- BAUHN AMCD-415+-+Instruction+Manual Tablet Smartphone Mirroring DongleDocument28 pagesBAUHN AMCD-415+-+Instruction+Manual Tablet Smartphone Mirroring Donglebackch9011Pas encore d'évaluation
- Ecco Alcarte 011118Document1 pageEcco Alcarte 011118backch9011Pas encore d'évaluation
- Export ShareDocument1 pageExport Sharebackch9011Pas encore d'évaluation
- Installing Acrylic Mirror: Product DataDocument1 pageInstalling Acrylic Mirror: Product Databackch9011Pas encore d'évaluation
- CarvinDocument21 pagesCarvinbackch9011Pas encore d'évaluation
- He Os Home Cinema EnglishDocument85 pagesHe Os Home Cinema Englishbackch9011Pas encore d'évaluation