Académique Documents
Professionnel Documents
Culture Documents
2015
Transféré par
Ankur DhirDescription originale:
Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
2015
Transféré par
Ankur DhirDroits d'auteur :
Formats disponibles
4.
Systemic requirements
4.1 Organizational requirements
4.1.1 Establish a QMS.
Develop a QMS for your organization.
Meet all applicable ISO 13485 requirements.
Meet all applicable regulatory requirements.
Document your organization's QMS.
Implement your organization's QMS.
Carry out all required QMS activities.
Carry out all required ISO 13485 activities.
Carry out all required regulatory activities.
Follow all required QMS procedures.
Follow all required ISO 13485 procedures.
Follow all required regulatory procedures.
Apply all required QMS arrangements.
Apply all required ISO 13485 arrangements.
Apply all required regulatory arrangements.
Maintain your organization's QMS.
4.1.2 Clarify structure.
Consider the roles that regulators expect you to perform.
Determine the processes that your organization's QMS needs.
Clarify how QMS processes are applied throughout your
organization.
Apply a risk based approach to your organization's QMS
processes.
4.1.3 Support processes.
Support each QMS process.
Support QMS process operations.
Support QMS process monitoring.
Support QMS process measuring.
Support QMS process analysis.
Support QMS process record keeping.
4.1.4 Manage changes.
Manage your QMS processes.
Comply with ISO 13485 process management requirements.
Manage changes to your organization's QMS processes.
Comply with regulatory process management requirements.
Comply with regulatory change control requirements.
4.1.5 Control outsourcing.
Monitor outsourced processes that affect product conformity.
Control outsourced processes that affect product conformity.
4.1.6 Validate software.
Develop a procedure to validate and revalidate your QMS software.
Develop an approach that is proportional to the risk that is being
taken.
Use your procedure to validate and revalidate software
applications.
Validate computer software applications for their intended use.
Validate software whenever its intended use changes (as
appropriate).
Maintain a record of your software validation and revalidation
activities.
4.2 Documentation requirements
4.2.1 Establish quality documents.
4.2.1.1 Clarify documentation requirements.
Consider your documentation requirements.
Include all required documents and records.
Include the documents and records that regulations
require.
Include the documents and records that ISO 13485 2015
requires.
Include the documents and records that your organization
requires.
4.2.1.2 Clarify file management requirements.
Establish a file for each medical device or family of medical
devices.
Maintain a file for each medical device or family of medical
devices.
4.2.2 Develop a quality manual.
Prepare a quality manual for your QMS.
Define the scope of your organization's QMS.
Outline the structure of your QMS documentation.
Include your QMS procedures or refer to them.
Describe how your QMS processes interact.
4.2.3 Control quality documents.
Establish a procedure to control QMS documents.
Document your QMS document control procedure.
Implement your QMS document control procedure.
4.2.4 Maintain quality records.
Establish records for your organization's QMS.
Develop procedures to control QMS records.
Document your record control procedures.
Implement your record control procedures.
Define methods to protect QMS health records.
5. Management requirements
5.1 Commitment requirements.
Demonstrate your ongoing commitment.
Support the development of your QMS.
Support the implementation of your QMS.
Support the maintenance of your QMS.
5.2 External requirements.
Ensure that external requirements are determined.
Ensure that external requirements are being met.
5.3 Policy requirements.
Plan your quality policy.
Draft your quality policy.
Apply your quality policy.
Review your quality policy.
5.4 Planning requirements
5.4.1 Establish quality objectives.
Establish quality objectives for your organization.
Set objectives needed to meet product requirements.
Set objectives needed to meet regulatory requirements.
5.4.2 Carry out quality planning.
Plan how you're going to develop your QMS.
Plan how you're going to document your QMS.
Plan how you're going to structure your QMS.
Plan how you're going to manage your QMS.
Plan how you're going to monitor your QMS.
Plan how you're going to control your QMS.
Plan how you're going to implement your QMS.
Plan how you're going to maintain your QMS.
5.5 Managerial requirements
5.5.1 Clarify responsibility and authority.
Define QMS responsibilities and authorities.
Document QMS responsibilities and authorities.
Define how QMS personnel are interrelated.
5.5.2 Appoint management representative.
Appoint a member of management to oversee your QMS.
Give management representative authority and responsibility.
Assign authority and responsibility for documenting your QMS.
Assign authority and responsibility for reporting to top
management.
Assign authority and responsibility for promoting corporate
awareness.
5.5.3 Establish internal communications.
Establish appropriate internal communication processes.
Encourage communication about the effectiveness of your QMS.
5.6 Review requirements
5.6.1 Perform regular management reviews.
Schedule your reviews at planned intervals.
Document your QMS review requirements.
Review your QMS at planned intervals.
Keep a record of management reviews.
5.6.2 Examine management review inputs.
Examine information about your QMS (inputs).
Examine previous management reviews.
Examine the results of previous audits.
Examine product conformity information.
Examine process performance feedback.
Examine corrective and preventive actions.
Examine recommendations for improvement.
Examine feedback about requirements (from 8.2.1).
Examine new or revised regulatory requirements.
Examine changes that could affect your QMS.
5.6.3 Generate management review outputs.
Generate your management review outputs.
Generate decisions and actions to improve your QMS.
Generate decisions and actions to enhance your products.
Generate decisions and actions to deal with regulatory changes.
Generate decisions and actions to address relevant resource
needs.
Establish a record of your management reviews.
6. Resource requirements
6.1 Allocation requirements.
Determine the resources that your QMS needs.
Provide the resources that your QMS needs.
Provide the resources needed to implement your QMS.
Provide the resources needed to meet regulatory requirements.
Provide the resources needed to meet customer requirements.
6.2 Personnel requirements
6.2.1 Ensure the competence of workers.
Identify personnel who can affect product safety or performance.
Identify work that can affect product safety or performance.
Make sure that these workers are competent.
6.2.2 Meet all competence requirements.
Establish a process to manage the competence of medical
device workers.
Use your process to manage the competence of medical device
workers.
Identify suitable methods for evaluating training and awareness
activities.
Maintain records that document the competence of medical device
workers.
6.3 Infrastructure requirements
6.3.1 Document the infrastructure that you need.
Document the infrastructure that your QMS needs.
Provide the infrastructure that your QMS needs.
Provide the buildings and utilities that your organization needs.
Provide the workspaces and equipment that your organization
needs.
Provide the support services that your organization needs.
Maintain the infrastructure that your QMS needs.
6.3.2 Document your maintenance requirements.
Identify maintenance activities that may affect product safety or
performance.
Establish maintenance requirements for these maintenance
activities.
Document maintenance requirements for these maintenance
activities.
Document maintenance requirements for equipment (as
appropriate).
Maintain equipment in accordance with your maintenance
requirements.
6.4 Environment requirements
6.4.1 Define your medical device work environment.
6.4.1.1 Document your medical device environment.
Identify the work environment needed to meet product
requirements.
Document the work environment needed to meet product
requirements.
Establish the work environment needed to meet product
requirements.
6.4.1.2 Document your environmental requirements.
Identify work environments that affect product safety or
performance.
Document your requirements for these work
environments.
Identify the environmental conditions that affect how work
is performed.
Identify working conditions that affect product safety or
performance.
Document procedures to monitor and control working
conditions.
6.4.1.3 Document your cleanliness expectations.
Identify personnel who could affect product safety or
performance.
Identify those who come into contact with related work
environments.
Establish suitable health, cleanliness, and clothing
requirements.
6.4.1.4 Document your contamination controls.
Establish arrangements to control products that could be
contaminated.
Document arrangements to control products that could be
contaminated.
6.4.2 Define your sterile medical device environment.
Establish work environment requirements for sterile medical
devices.
Comply with your work environment requirements for medical
devices.
7. Realization requirements
7.1 Planning requirements.
Plan the processes you need to realize products.
Develop the processes that you need to realize products.
Develop a risk management process for product realization.
Plan how you're going to realize each product.
Formulate quality objectives for your product.
Clarify specific product realization requirements.
Generate product realization planning outputs.
Establish records of product realization activities.
7.2 Customer requirements
7.2.1 Determine product requirements.
Clarify your product requirements.
Identify requirements specified by your customers.
Identify requirements dictated by your product's intended use.
Identify requirements imposed by your regulatory bodies.
Identify requirements defined by your organization.
7.2.2 Review your product requirements.
Evaluate product requirements before you accept orders.
Review product requirements before you supply products.
Verify product requirements before you agree to accept orders.
Confirm that product requirements can be met before you
proceed.
Maintain a record of your product requirement reviews.
7.2.3 Communicate product requirements.
7.2.3.1 Communicate with customers.
Establish arrangements to communicate with customers.
Document your customer communication arrangements.
7.2.3.2 Communicate with regulators.
Establish arrangements to communicate with regulatory
authorities.
7.3 Development requirements
7.3.1 Prepare design and development procedures.
Establish procedures for design and development.
Document procedures for design and development.
7.3.2 Organize design and development activities.
Plan the design and development of your products.
Document your product design and development plans.
Maintain your design and development planning documents.
Control the design and development of your products.
7.3.3 Determine design and development inputs.
Determine product design and development inputs.
Review your product design and development inputs.
Approve your product design and development inputs.
Maintain a record of design and development inputs.
7.3.4 Generate design and development outputs.
Generate suitable design and development outputs.
Verify your product design and development outputs.
Approve your product design and development outputs.
Maintain records of design and development outputs.
7.3.5 Carry out design and development reviews.
Plan your organization's design and development reviews.
Perform reviews in accordance with planned arrangements.
Maintain records of your design and development reviews.
7.3.6 Perform design and development verifications.
Plan your design and development verification activities.
Document your design and development verification plans.
Perform verifications in accordance with planned arrangements.
Keep records of your design and development verification
activities.
7.3.7 Conduct design and development validations.
Plan your organization's design and development
validation activities.
Document your organization's design and development validation
plans.
Perform validations in accordance with your planned
arrangements.
Keep records of design and development validation activities.
7.3.8 Plan design and development transfers.
Plan product design and development transfers.
Document design and development transfer plans.
Use your plans and procedures to make transfers.
Keep records of design and development transfers.
7.3.9 Control design and development changes.
7.3.9.1 Establish control of your changes.
Establish processes to control design and development
changes.
Use processes to control changes that affect your medical
devices.
7.3.9.2 Identify and record your changes.
Identify design and development changes.
Approve changes before you implement them.
Record design and development changes.
7.3.9.3 Review and evaluate your changes.
Review and evaluate design and development changes.
Review and evaluate the effect changes could have.
7.3.9.4 Maintain a record of your reviews.
Record results of your review of design and development
changes.
7.3.10 Maintain design and development files.
Maintain a design and development file for each device or family of
devices.
Include or reference records that document compliance and
changes.
7.4 Purchasing requirements
7.4.1 Manage purchasing process.
7.4.1.1 Prepare purchase procedures.
Establish procedures to control product purchases.
Document procedures to control product purchases.
7.4.1.2 Control selection of suppliers.
Plan the selection and evaluation of your suppliers.
Establish supplier selection and evaluation criteria.
Evaluate and select your organization's suppliers.
Approve suppliers that meet your selection and
evaluation criteria.
Approve suppliers that meet or can meet all relevant
requirements.
7.4.1.3 Monitor supplier performance.
Plan how you're going to monitor supplier performance.
Monitor the performance of your organization's suppliers.
Use monitoring results to re-evaluate supplier performance.
Take remedial actions that are proportional to your risk.
7.4.1.4 Establish performance records.
Establish a record of supplier monitoring, evaluation, and
re-evaluation.
7.4.2 Clarify purchasing information.
Plan your organization's product purchases.
Review purchase requirements before sharing them.
Maintain your organization's purchasing documents and records.
7.4.3 Verify purchased products.
Develop methods and activities to verify purchased products.
Make arrangements to verify the products you plan to purchase.
Verify that your purchased products meet purchase requirements.
Consider what to do when changes are made to purchased
products.
Establish and maintain a record of purchased product
verifications.
7.5 Production requirements
7.5.1 Control medical device production and service provision.
7.5.1.1 Plan and perform production and service provision.
Plan your production and service provision activities.
Carry out production and service provision activities.
Monitor production and service provision activities.
Control production and service provision activities.
7.5.1.2 Clarify cleanliness, installation, and servicing expectations.
7.5.1.2.1 Define requirements for cleanliness or contamination
controls.
Define cleanliness or contamination control
requirements for products that you clean
prior to sterilization or use.
Define cleanliness or contamination control
requirements for products that cannot be
cleaned prior to sterilization or use.
Define cleanliness or contamination control
requirements for products supplied non-sterile
to be cleaned before sterilization or use.
Define cleanliness or contamination control
requirements for process agents that must be
removed from products during manufacture.
7.5.1.2.2 Specify product installation and verification
requirements.
Establish product installation requirements.
Document your product installation
requirements.
Establish installation verification requirements.
Document your installation verification
requirements.
7.5.1.2.3 Develop servicing procedures and reference
materials.
Establish servicing procedures and reference
materials.
Document your servicing procedures and reference
materials.
Use procedures and materials to control servicing
activities.
Maintain a record of your organization's servicing
activities.
Identify servicing complaints and improvement
opportunities.
7.5.1.3 Maintain a record of sterilization process parameters.
Establish a record of your sterilization process parameters.
Record sterilization parameters for each batch of medical
devices.
7.5.2 Validate processes used for production and service provision.
7.5.2.1 Validate processes and software if outputs aren't being
verified.
Identify processes that generate outputs that are
not or cannot be verified by subsequent monitoring
and measurement until it's too late.
Establish procedures to validate production and
service delivery processes and software applications
that could affect your products and services.
Develop plans to validate production and service
delivery processes that generate outputs that are
not or cannot be verified until it's too late.
Validate processes and software applications that
could generate output deficiencies and could affect
your products and services.
7.5.2.2 Validate sterilization processes and product packaging
processes.
Prepare procedures for validating sterilization processes.
Document your sterilization process validation
procedures.
Prepare procedures to validate packaging for sterile barrier
systems.
Document your packaging process validation procedures.
7.5.3 Identify medical device products and establish traceability.
7.5.3.1 Preserve product identity throughout product realization.
Prepare procedures and systems to control identify of
medical devices.
Establish procedures to maintain identify during product
realization.
Document your product identification systems and
procedures.
7.5.3.2 Establish suitable traceability procedures and records.
7.5.3.2.1 Establish suitable product traceability procedures.
Clarify your product traceability requirements.
Establish your product traceability procedures.
Maintain a record of your traceability activities.
7.5.3.2.2 Establish suitable records for implantable devices.
Establish traceability records for implantable
medical devices.
Expect suppliers and distributors to have
traceability records.
7.5.3.3 Maintain product status throughout product realization.
Identify the status of medical devices throughout product
realization.
Maintain the status of medical devices throughout product
realization.
Use status information to control the disposition of medical
devices.
7.5.4 Protect property supplied for medical devices by customers.
Identify property supplied by customers to be used by medical
devices.
Verify property supplied by customers to be used by your medical
devices.
Safeguard property supplied by customers to be used by medical
devices.
Maintain a record of customer property that is lost, damaged, or
unsuitable.
Report lost, damaged, or unsuitable customer property to your
customers.
7.5.5 Preserve medical device products and components.
7.5.5.1 Develop procedures to preserve product conformity.
Establish procedures to preserve the conformity of
products.
Document and maintain your product preservation
procedures.
Use your procedures to preserve the conformity of
products.
7.5.5.2 Prevent product damage, alteration, and contamination.
Prevent medical device damage, alteration, and
contamination.
Protect products when exposed to hazards and expected
conditions.
7.6 Measurement requirements
Identify monitoring and measurement requirements.
Select suitable monitoring and measurement equipment.
Establish your monitoring and measurement procedures.
Prepare calibration and verification plans and procedures.
Protect your monitoring and measurement equipment.
Validate your monitoring and measurement software.
Apply your monitoring and measurement procedures.
8. Remedial requirements
8.1 Planning requirements
Plan monitoring, measurement, and analytical processes..
Plan how monitoring methods will be used ensure conformity and
effectiveness.
Plan how measurement will be used to ensure conformity and
effectiveness.
Plan how analytics will be used to ensure conformity and
effectiveness.
8.2 Research requirements
8.2.1 Establish suitable feedback methods and procedures.
8.2.1.1 Develop feedback methods and gather information.
Establish feedback methods and procedures.
Establish customer feedback methods and procedures.
Establish production feedback methods and
procedures.
Examine the information you have gathered.
Use your feedback to measure QMS effectiveness.
Use your feedback to facilitate risk management.
Use your feedback to support product realization.
8.2.1.2 Investigate complaints, take action, and report results.
8.2.1.2.1 Develop and document complaint handling
procedures.
Establish your complaint handling procedures.
Document your complaint handling procedures.
Document related responsibilities and
requirements.
8.2.1.2.2 Develop and document complaint reporting
procedures.
Establish procedures for reporting complaints to
regulators.
Report complaints to regulators when adverse
events occur.
Maintain a record of your regulatory reports and
notifications.
8.2.2 Plan and perform internal audits at planned intervals.
Plan your organization's internal audit program.
Carry out your internal audits at planned intervals.
Maintain a record of audit plans and performance.
Eliminate all detected nonconformities and causes.
Follow-up on steps taken to resolve nonconformities.
8.2.3 Find out whether processes achieve planned results.
Establish suitable methods to monitor and measure each QMS
process.
Implement suitable methods to monitor and measure each QMS
process.
Determine whether or not each QMS process is achieving
planned results.
Take remedial action whenever processes fail to achieve planned
results.
8.2.4 Monitor and measure medical device characteristics.
8.2.4.1 Verify that all medical device requirements are being met.
Monitor and measure your organization's product
characteristics.
Establish a record of product monitoring and measurement
activities.
Complete all planned arrangements before you release your
products.
8.2.4.2 Keep a record of implantable device testers and inspectors.
Establish a record of implantable medical device testing
and inspection.
Identify the people who test or inspect implantable
medical devices.
8.3 Product requirements
8.3.1 Prevent unintended delivery or use of nonconforming products.
Clarify how you intend to prevent unintended product delivery or
use.
Prevent the unintended delivery or use of your nonconforming
products.
Establish a record of your organization's nonconforming product
activities.
8.3.2 Deal with pre-delivery nonconformities and keep suitable records.
Deal with nonconforming products prior to delivery.
Take action to eliminate detected nonconformities.
Prevent the product's original intended use or application.
Authorize nonconforming product use, release, or acceptance.
8.3.3 Address post-delivery nonconformities and issue advisory notices.
8.3.3.1 Respond to post-delivery nonconformities and keep records.
Identify nonconforming products after delivery or after use
has started.
Take action that is appropriate to the effects that have been
identified.
Establish and maintain a record of the actions that have
been taken.
8.3.3.2 Implement post-delivery advisory notices and keep records.
Clarify how advisory notices should be issued and
implemented.
Keep a record of actions taken when advisory notices are
issued.
8.3.4 Follow procedures and instructions when products are reworked.
Clarify how product rework should be performed.
Clarify how product rework should be retested.
Clarify how product rework should be reviewed.
Clarify how product rework should be approved.
Clarify how product rework should be recorded.
8.4 Analytical requirements
Consider how you plan to evaluate your QMS.
Establish procedures to evaluate your QMS.
Gather information and data about your QMS.
Analyze information and data about your QMS.
8.5 Improvement requirements
8.5.1 Take action to change QMS and products.
Identify any changes that must be made.
Identify changes that maintain QMS suitability and effectiveness.
Identify changes that maintain product safety and performance.
Make any changes that must be made.
8.5.2 Take action to correct actual nonconformities.
Document a corrective action procedure.
Specify how actual problems will be investigated.
Specify how corrective actions will be developed.
Specify how corrective actions will be verified.
Specify how corrective action will be taken.
Specify how corrective action will be reviewed.
Implement your corrective action procedure.
Maintain records of corrective action taken.
8.5.3 Take action to prevent potential nonconformities.
Document a preventive action procedure.
Specify how potential problems will be investigated.
Specify how preventive actions will be developed.
Specify how preventive actions will be verified.
Specify how preventive action will be taken.
Specify how preventive action will be reviewed.
Implement your preventive action procedure.
Maintain records of preventive action taken.
Also see ISO 14971 Risk Management Standard for Medical Devices (in Plain
English).
Vous aimerez peut-être aussi
- Beyond Compliance Design of a Quality System: Tools and Templates for Integrating Auditing PerspectivesD'EverandBeyond Compliance Design of a Quality System: Tools and Templates for Integrating Auditing PerspectivesPas encore d'évaluation
- Data Integrity and Compliance: A Primer for Medical Product ManufacturersD'EverandData Integrity and Compliance: A Primer for Medical Product ManufacturersPas encore d'évaluation
- Eu MDRDocument34 pagesEu MDRgobu269104100% (1)
- ISO 14971 A Complete Guide - 2021 EditionD'EverandISO 14971 A Complete Guide - 2021 EditionÉvaluation : 1 sur 5 étoiles1/5 (1)
- Eu GMP Guide PDFDocument2 pagesEu GMP Guide PDFSteve0% (1)
- Risk Management for Medical Device Manufacturers: [MD and IVD]D'EverandRisk Management for Medical Device Manufacturers: [MD and IVD]Pas encore d'évaluation
- GHTF Supplier Controlsg3final N17Document21 pagesGHTF Supplier Controlsg3final N17freelovefestPas encore d'évaluation
- ISO 13485 Quality Management System A Complete Guide - 2020 EditionD'EverandISO 13485 Quality Management System A Complete Guide - 2020 EditionPas encore d'évaluation
- Achieving 6 Sigma Quality in Medical Device Manufacturing by Use of DoE and SPCDocument9 pagesAchieving 6 Sigma Quality in Medical Device Manufacturing by Use of DoE and SPCispam28Pas encore d'évaluation
- GHTF Sg3 n99 8 Guidance On Quality 990629Document48 pagesGHTF Sg3 n99 8 Guidance On Quality 990629BALAJIPas encore d'évaluation
- SS ISO 10993-1-2018 - PreviewDocument14 pagesSS ISO 10993-1-2018 - PreviewmarkPas encore d'évaluation
- Good Distribution Practices A Complete Guide - 2021 EditionD'EverandGood Distribution Practices A Complete Guide - 2021 EditionPas encore d'évaluation
- Design Documentation L4Document27 pagesDesign Documentation L4KOFI BROWNPas encore d'évaluation
- Capa-UpDocument22 pagesCapa-UpChristinaPas encore d'évaluation
- Overview PF CPGPDocument26 pagesOverview PF CPGPmmmmm0% (1)
- How Medical Devices Regulated in EuropeDocument7 pagesHow Medical Devices Regulated in Europebasakerpolat100% (1)
- Ord 384-2020 - ENGLISCHDocument54 pagesOrd 384-2020 - ENGLISCHScribdTranslationsPas encore d'évaluation
- Quality Management System Software A Complete Guide - 2019 EditionD'EverandQuality Management System Software A Complete Guide - 2019 EditionPas encore d'évaluation
- HPS Endoscope Decontamination 2004 PDFDocument190 pagesHPS Endoscope Decontamination 2004 PDFHong-Nam Kim100% (1)
- Thermal Validation Information Manual & GuideDocument10 pagesThermal Validation Information Manual & Guideviethuong96Pas encore d'évaluation
- RDC 16 2013 GMP Requirements For MD and IvdDocument12 pagesRDC 16 2013 GMP Requirements For MD and Ivdnsk79inPas encore d'évaluation
- ISO 14971 A Complete Guide - 2020 EditionD'EverandISO 14971 A Complete Guide - 2020 EditionÉvaluation : 2 sur 5 étoiles2/5 (1)
- Australian Regulatory Guidelines For Medical DevicesDocument337 pagesAustralian Regulatory Guidelines For Medical DevicesLina GamarnikPas encore d'évaluation
- FDA Inspections PDFDocument52 pagesFDA Inspections PDFMajdi Hasan Ayoub100% (1)
- Analyzing The Changes To Risk Management Standard ISO 149712019 PDFDocument5 pagesAnalyzing The Changes To Risk Management Standard ISO 149712019 PDFrakesh marwahPas encore d'évaluation
- Sterilization - Validation, Qualification RequirementsDocument2 pagesSterilization - Validation, Qualification Requirementsgloryclaudia_100% (1)
- Validation Vs QualificationDocument1 pageValidation Vs QualificationKiran ChokshiPas encore d'évaluation
- Validation Change Control Maintaining The Validate State-09-2015Document56 pagesValidation Change Control Maintaining The Validate State-09-2015VaLeritha R. Santa MPas encore d'évaluation
- Unit IV - Complaints and DocumentsDocument103 pagesUnit IV - Complaints and DocumentsIndu VattiPas encore d'évaluation
- Quality by Design Approaches To Analytical Methods - : FDA PerspectiveDocument21 pagesQuality by Design Approaches To Analytical Methods - : FDA PerspectiveJr Zdenko VukojaPas encore d'évaluation
- Validation of Microbiology PDFDocument6 pagesValidation of Microbiology PDFMayur Jadhav0% (1)
- As Far As Possible - en ISO 14971Document19 pagesAs Far As Possible - en ISO 14971Kanwal Jit Singh100% (1)
- Basic Principles of GMP Basic Principles of GMPDocument33 pagesBasic Principles of GMP Basic Principles of GMPTahir KhanPas encore d'évaluation
- Basic Principles of GMP: Qualification and ValidationDocument28 pagesBasic Principles of GMP: Qualification and Validationhyde2520015754100% (1)
- Quality Systems For Pharmacovigilance SME Workshop "Focus On Pharmacovigilance" 19 April 2012, EMA LondonDocument20 pagesQuality Systems For Pharmacovigilance SME Workshop "Focus On Pharmacovigilance" 19 April 2012, EMA LondonHala MohamedPas encore d'évaluation
- Manufacturing Facilities A Complete Guide - 2019 EditionD'EverandManufacturing Facilities A Complete Guide - 2019 EditionPas encore d'évaluation
- Study - ISO 13485 PDFDocument15 pagesStudy - ISO 13485 PDFAnonymous 78Ezy46qvPas encore d'évaluation
- PIT Virtual IAI 2020 Presentation - Data Integrity - Silvia WidyanyDocument28 pagesPIT Virtual IAI 2020 Presentation - Data Integrity - Silvia WidyanyAchmad Fadhil AlmasyhurPas encore d'évaluation
- SS IEC 62366-1-2018 PreviewDocument12 pagesSS IEC 62366-1-2018 PreviewMADDINENI AVANEESHWARPas encore d'évaluation
- Standards Version 1.0Document28 pagesStandards Version 1.0maxPas encore d'évaluation
- Biocompatability, FDA, IsO 10993, IVDDocument31 pagesBiocompatability, FDA, IsO 10993, IVDsam3557Pas encore d'évaluation
- FDA Medical Device Quality Systems Manual - Quality Systems AuditsDocument5 pagesFDA Medical Device Quality Systems Manual - Quality Systems AuditsucbdmoPas encore d'évaluation
- Application Note: Successful Wetting For Filter Integrity Testing in Volume-Restricted SystemsDocument13 pagesApplication Note: Successful Wetting For Filter Integrity Testing in Volume-Restricted SystemsSlavaPas encore d'évaluation
- CPGP CourseDocument7 pagesCPGP CourseantonygamalpharmaPas encore d'évaluation
- Quality Data Metrics A Complete Guide - 2020 EditionD'EverandQuality Data Metrics A Complete Guide - 2020 EditionPas encore d'évaluation
- 413-Other Tools-Control PlanDocument8 pages413-Other Tools-Control PlanAnkur DhirPas encore d'évaluation
- Lesson 4: Other Quality Tools Tool #8: BrainstormingDocument4 pagesLesson 4: Other Quality Tools Tool #8: BrainstormingAnkur DhirPas encore d'évaluation
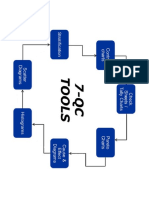
- 7 QC ToolDocument1 page7 QC ToolAnkur DhirPas encore d'évaluation
- 5 S of Lean PosterDocument1 page5 S of Lean PosterAnkur DhirPas encore d'évaluation
- 412-Other Tools-Criteria RatingDocument6 pages412-Other Tools-Criteria RatingAnkur DhirPas encore d'évaluation
- Performance Measurement: Lesson StructureDocument9 pagesPerformance Measurement: Lesson StructureAnkur DhirPas encore d'évaluation
- 410-Other Quality tools-ProcessFlowDocument6 pages410-Other Quality tools-ProcessFlowAnkur DhirPas encore d'évaluation
- Seven QC Tools Tool #7: Stratification: Lesson StructureDocument2 pagesSeven QC Tools Tool #7: Stratification: Lesson StructureAnkur DhirPas encore d'évaluation
- 49-Other Quality Tools-5 WhysDocument4 pages49-Other Quality Tools-5 WhysAnkur DhirPas encore d'évaluation
- Seven QC Tools Tool #5: Part 1-Run ChartDocument6 pagesSeven QC Tools Tool #5: Part 1-Run ChartAnkur DhirPas encore d'évaluation
- Lesson 3: Seven QC Tools Tool #3: Cause & Effect DiagramDocument6 pagesLesson 3: Seven QC Tools Tool #3: Cause & Effect DiagramAnkur DhirPas encore d'évaluation
- Lean Coffee Table Book 8 - 18-5-2k18Document48 pagesLean Coffee Table Book 8 - 18-5-2k18Ankur DhirPas encore d'évaluation
- Seven QC Tools Tool #4: Pareto ChartDocument6 pagesSeven QC Tools Tool #4: Pareto ChartAnkur DhirPas encore d'évaluation
- 1-Problem SolvingDocument8 pages1-Problem SolvingAnkur DhirPas encore d'évaluation
- IRIS Beyond ISO 9001 - HandoutDocument83 pagesIRIS Beyond ISO 9001 - HandoutAnkur DhirPas encore d'évaluation
- 36-7QC Tools-Scatter DiagramDocument6 pages36-7QC Tools-Scatter DiagramAnkur DhirPas encore d'évaluation
- 2-Overview of Quality ToolsDocument3 pages2-Overview of Quality ToolsAnkur DhirPas encore d'évaluation
- Social Responsibility and Performance ExcellenceDocument4 pagesSocial Responsibility and Performance ExcellenceAnkur DhirPas encore d'évaluation
- Readiness Review: ISO RevisionsDocument6 pagesReadiness Review: ISO RevisionsAnkur DhirPas encore d'évaluation
- Session 3 Project Management 2Document15 pagesSession 3 Project Management 2Ankur DhirPas encore d'évaluation
- Best Practices Organizational AgilityDocument23 pagesBest Practices Organizational AgilityAnkur Dhir100% (1)
- Reinventing ExcellenceDocument7 pagesReinventing ExcellenceAnkur DhirPas encore d'évaluation
- Session 1 Office ManagementDocument20 pagesSession 1 Office ManagementAnkur DhirPas encore d'évaluation
- Session 2 Session 2: Introduction To Project Management Introduction To Project ManagementDocument24 pagesSession 2 Session 2: Introduction To Project Management Introduction To Project ManagementAnkur DhirPas encore d'évaluation
- Effective Team SkillsDocument12 pagesEffective Team SkillsAnkur Dhir100% (1)
- Risk Management QMS MethodolgyDocument35 pagesRisk Management QMS MethodolgyAnkur DhirPas encore d'évaluation
- Raci Matrix For Itsms 20000Document3 pagesRaci Matrix For Itsms 20000Ankur DhirPas encore d'évaluation
- Ocp For Fire Fighting Equipment and First Aid BoxesDocument2 pagesOcp For Fire Fighting Equipment and First Aid BoxesAnkur DhirPas encore d'évaluation
- Product Packing Plan: SL NO Item Under Packing Packing Size Weight (In KGS) of Packing Type of PackingDocument1 pageProduct Packing Plan: SL NO Item Under Packing Packing Size Weight (In KGS) of Packing Type of PackingAnkur DhirPas encore d'évaluation
- Synthesis and Characterization of Alumina Zirconia Composite Material Doped With SilicaDocument7 pagesSynthesis and Characterization of Alumina Zirconia Composite Material Doped With SilicaAdvanced Research PublicationsPas encore d'évaluation
- Dr. Ambedkar Institute of Technology: Mandatory Non-Credit NSS Course (22NSN310) Bachelor of Engineering inDocument4 pagesDr. Ambedkar Institute of Technology: Mandatory Non-Credit NSS Course (22NSN310) Bachelor of Engineering inshamalac2004Pas encore d'évaluation
- 4455770110e1 Parts ManualDocument31 pages4455770110e1 Parts ManualSimon AlbertPas encore d'évaluation
- PESTLE Analysis Patanjali Ayurved LTDDocument7 pagesPESTLE Analysis Patanjali Ayurved LTDvaidehi50% (2)
- Modelling and Simulation of Absorption Solar Air Conditioning SystemDocument20 pagesModelling and Simulation of Absorption Solar Air Conditioning SystemShadi KarkabaPas encore d'évaluation
- Building 101 - 25 Tips For A Tropical HomeDocument11 pagesBuilding 101 - 25 Tips For A Tropical HomeMelanie CabforoPas encore d'évaluation
- Whose Online Video Lectures Are Best For The IIT JEE?Document6 pagesWhose Online Video Lectures Are Best For The IIT JEE?Buzz PriyanshuPas encore d'évaluation
- S9300&S9300E V200R001C00 Hardware Description 05 PDFDocument282 pagesS9300&S9300E V200R001C00 Hardware Description 05 PDFmike_mnleePas encore d'évaluation
- 7sd610 CatalogueDocument35 pages7sd610 CatalogueTntngn Petualang100% (1)
- Average - Aptitude MCQ Questions and Solutions Wit - 1597107113795Document6 pagesAverage - Aptitude MCQ Questions and Solutions Wit - 1597107113795Manish ChavannavarPas encore d'évaluation
- Bloomberg Certification FAQ BMC (Bloomberg Market Concepts) : Goizueta Business LibraryDocument3 pagesBloomberg Certification FAQ BMC (Bloomberg Market Concepts) : Goizueta Business LibrarySarah Raquel Bozo Herrera100% (1)
- Trial On CompresorDocument3 pagesTrial On CompresorA JPas encore d'évaluation
- Quick Start Guide: Digital Camera D7000Document2 pagesQuick Start Guide: Digital Camera D7000foosome12Pas encore d'évaluation
- 9500MPR - MEF8 Circuit Emulation ServicesDocument5 pages9500MPR - MEF8 Circuit Emulation ServicesedderjpPas encore d'évaluation
- Poiseuille Lab ExperimentDocument7 pagesPoiseuille Lab ExperimentArjun SinghPas encore d'évaluation
- AIX PowerHA (HACMP) CommandsDocument3 pagesAIX PowerHA (HACMP) CommandsdanilaixPas encore d'évaluation
- 88 Numerical Series BASED TECHNOLOGY AWARENESS Grigory GRABOVOIJADocument5 pages88 Numerical Series BASED TECHNOLOGY AWARENESS Grigory GRABOVOIJAStellaEstel93% (15)
- Group 2 (Oacc39)Document3 pagesGroup 2 (Oacc39)viernazanne.viadoPas encore d'évaluation
- Kristian Tlangau - November, 2016 PDFDocument52 pagesKristian Tlangau - November, 2016 PDFMizoram Presbyterian Church SynodPas encore d'évaluation
- InternshipDocument14 pagesInternshipMohammed Shaheeruddin0% (1)
- David Beard Composer CV ShortDocument2 pagesDavid Beard Composer CV ShortEhsan KarimyPas encore d'évaluation
- Econ 103 - 01Document3 pagesEcon 103 - 01perrerPas encore d'évaluation
- Super CatalogueDocument8 pagesSuper CatalogueITL200_UPas encore d'évaluation
- Tesco, PLC "From Mouse To House"Document13 pagesTesco, PLC "From Mouse To House"Md IrfanPas encore d'évaluation
- Different Types of Steering Systems + ExamplesDocument0 pageDifferent Types of Steering Systems + ExamplesAbhilash NagavarapuPas encore d'évaluation
- A Modified Vince Gingery PlasticDocument13 pagesA Modified Vince Gingery PlasticgeppaPas encore d'évaluation
- NNH4-65C-R6-V2: Electrical SpecificationsDocument4 pagesNNH4-65C-R6-V2: Electrical SpecificationsAntony López GálvezPas encore d'évaluation
- Documents - The New Ostpolitik and German-German Relations: Permanent LegationsDocument5 pagesDocuments - The New Ostpolitik and German-German Relations: Permanent LegationsKhairun Nisa JPas encore d'évaluation
- Cooling Tower 3DTrasar ManualDocument90 pagesCooling Tower 3DTrasar ManualArevaLemaPas encore d'évaluation
- Gudenaaparken (Randers) - All You Need To Know BEFORE You GoDocument8 pagesGudenaaparken (Randers) - All You Need To Know BEFORE You GoElaine Zarb GiorgioPas encore d'évaluation








![Risk Management for Medical Device Manufacturers: [MD and IVD]](https://imgv2-1-f.scribdassets.com/img/word_document/602872428/149x198/825d3b5cd7/1666718194?v=1)