Académique Documents
Professionnel Documents
Culture Documents
Water System Validation Example
Transféré par
dvdynamic1Description originale:
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Water System Validation Example
Transféré par
dvdynamic1Droits d'auteur :
Formats disponibles
FACILITY QUALIFICATION PROTOCOL
Water for Injection System Validation
ORIGINAL __X____ AMENDMENT _______
Qualification Number: _MVP-2002-002____
Special ___Normal_X__
FACILITY: Manufacture, Package, Test and Distribution
LOCATION:
Mahwah, New Jersey, Building 1
SYSTEM: Water for Injection (WFI)
CRITICAL: YES
Qualification Protocol Approvals:
Department
Name
Signature
Date
Originator
1. Purpose: To demonstrate through documented evidence that the Water for Injection system is
validated for use.
2. Roles and Responsibilities:
Activity
Responsible Department
Protocol Execution
Report Preparation
Report Approval
3. Diagram of System indicating sampling points:
4. Materials of Construction/Composition: (list here the composition of the various pieces of equipment. I
usually us a table format)
Equipment/Process element
Water tank (5,000 US gallon)
Transportation Piping
Tank piping
Coupling joint - tank
Composition
316 electropolished stainless steel
with smooth welded surfaces
PVFC 1 with support brackets
every foot
316 electropolished stainless steel
with smooth welded surfaces
316 electropolished stainless steel
Page 1 of 6
Drawing/print reference
Drawing a-1 dated 11/14/06
Drawing b-1`9 dated
11/18/06
Drawing a-1 dated 11/14/06
Drawing a-1 dated 11/14/06
FACILITY QUALIFICATION PROTOCOL
Water for Injection System Validation
ORIGINAL __X____ AMENDMENT _______
Equipment/Process element
Coupling joint Transportation
lines
Composition
PVFC 1 with support brackets
every foot
Drawing/print reference
Drawing b-1`9 dated
11/18/06
5. Procedures/Reference Documents (including drawings)
Document
Type
SOP
Working
Instruction
Working
Instruction
Working
Instruction
SOP
SOP
SOP
SOP
SOP
SOP
Procedure /Document Title
Facility Qualification
Installation Qualification
Document
Number
SOP 09-14
WI 09-03
Revision
Level
Original
Original
Operational Qualification
WI 09-04
Original
Process Qualification
WI 09-05
Original
Water for Injection Testing
Cleaning Validation
Incoming Municipal Water Testing
PM of Resin Beds
PM of Water Tank
PM of Water Transportation Lines
SOP 10-01
SOP 09-16
SOP 10 04
Original
Original
Original
6. Training Requirements:
Training Requirements
Individuals Trained
(List Doc # /Rev Level)
See attached list with final
report
See attached list with final report
Training Performed
By/Date
See attached list with final report
7. Calibration and Preventative Maintenance (attach supportive evidence):
Equipment/Item
Name
Water Tank
Ion exchangers
Resin beds
UV light source
Etc
Asset #
(refer to drawing)
70
21, 22
41, 42
Calibration
verified
N/A
Yes
N/A
N/A
N/A
Yes
Yes
Yes
8. Risk Analysis Determination
Page 2 of 6
PM verified
Signature & Date
FACILITY QUALIFICATION PROTOCOL
Water for Injection System Validation
ORIGINAL __X____ AMENDMENT _______
Attach risk documentation and customer requirements You should mock one up for whatever system
you are challenging.
9. Test Plan and Rationale:
Samples to be tested:
Samples will be chosen based on the locations identified on the drawing. Locations are based on slope,
distance from water source, frequency and type of use.
Attached is a comprehensive listing of all water uses and their source.
Test plan:
1. A cleaning validation will be performed in conjunction with the system validation to ensure
that the system validation results reflect normal use.
2. All the testing that will be used and then routinely performed is found in SOP 10-01, USP
Testing of Water for Injection
3. Samples will be drawn from each location at the frequency listed below. Volumes of
samples will be as specified within SOP and will be taken aseptically as required within the
SOP 10-01. Samples will be refrigerated upon receipt and testing within 24 hours of
receipt.
4. The sampling will reflect initial installation, and potential seasonal changes to the public
water supply input. This facility qualification with the specific water system validation will
be ongoing for a period of 1 year to reflect seasonal influences.
5. The equipment settings for each series of tests will be recorded on the attached data sheets
when samples are taken.
6. Cleaning will be performed initially prior to any routine water sample for this protocol.
Sample Frequency:
Site
Frequency
Daily for one year (when plant is in operation)
Daily for 2 weeks
Once/week for 2 weeks
Once/month for remainder of year
Daily for 2 weeks
Once/week for 2 weeks
Once/month for remainder of year
Daily for 2 weeks
Once/week for 2 weeks
Once/month for remainder of year
Daily for 2 weeks
Once/week for 2 weeks
Once/month for remainder of year
Daily for 2 weeks (in order of nearest to farthest
Incoming to plant
Prior to resin beds
After resin beds
Before holding tank
After holding tank
Identified points of use
Page 3 of 6
FACILITY QUALIFICATION PROTOCOL
Water for Injection System Validation
ORIGINAL __X____ AMENDMENT _______
Site
Frequency
point from tank and always prior to use)
Daily for points of active use and once every 2
weeks for non-active use
Sample Frequency Rationale:
Sample Identification and Labeling Instructions:
10. Criteria and Results:
Raw data sheets should be attached.
Attribute
Inspection/Test
Method
Acceptance
Criteria
Sample
Size
pH
Appearance
Particulates
Microbial load
11. Comments, Deviations and Revisions
12. Sample Disposition:
13. Control Post Validation (to monitor state of control challenged in the original activity):
14. Final Report summary (attach all data sheets, etc):
Attribute
Inspection/Test
Acceptance
Method
Criteria
Sample
Size
Result
(Pass/Fail/Justify*)
*If Justify, provide
rationale
pH
Appearance
Particulates
Microbial load
Page 4 of 6
FACILITY QUALIFICATION PROTOCOL
Water for Injection System Validation
ORIGINAL __X____ AMENDMENT _______
15. Conclusion: (Indicate if the system is considered validated or not and if not, what other activities/steps will be taken)
16. Report Approval and Concurrence:
Department
Originator
Name
Signature
Operations
Quality Assurance
Page 5 of 6
Date
FACILITY QUALIFICATION PROTOCOL
Water for Injection System Validation
ORIGINAL __X____ AMENDMENT _______
SUPPLEMENTAL DATA SHEETS
Page 6 of 6
Vous aimerez peut-être aussi
- Water System PQDocument46 pagesWater System PQVeChrist PharmacistoLshop100% (3)
- Water Sys ProtocolDocument14 pagesWater Sys Protocolasit_m100% (8)
- EPC Progress Measurement SystemDocument6 pagesEPC Progress Measurement SystemSubhojit AdhikaryPas encore d'évaluation
- Data Science EssayDocument2 pagesData Science EssayAbdelhak100% (1)
- Clean Val Protocol 1Document8 pagesClean Val Protocol 1krishnavkkPas encore d'évaluation
- Cleaning Validation Protocol-1Document22 pagesCleaning Validation Protocol-1Bejoy Karim100% (4)
- 9 C Validation Protocol TABLETDocument20 pages9 C Validation Protocol TABLETMohammed ZubairPas encore d'évaluation
- Validation of Water System For Pharmaceutical UseDocument25 pagesValidation of Water System For Pharmaceutical Usedesaiurvish100% (2)
- Cleanroom Technology: Fundamentals of Design, Testing and OperationD'EverandCleanroom Technology: Fundamentals of Design, Testing and OperationPas encore d'évaluation
- VALIDATION MASTER PLAN (Repaired)Document56 pagesVALIDATION MASTER PLAN (Repaired)aman pathania100% (3)
- IQ Purified WaterDocument127 pagesIQ Purified Wateranon-204001100% (11)
- F03qa038-00 VMPDocument24 pagesF03qa038-00 VMPMeet Vermaa100% (1)
- VAL 080 Validation Master Plan Sample PDFDocument3 pagesVAL 080 Validation Master Plan Sample PDFsiva sankar100% (1)
- Datastage Interview Questions & AnswersDocument8 pagesDatastage Interview Questions & AnswersVamsi KarthikPas encore d'évaluation
- Operational Qualification For Compressed Air System.Document11 pagesOperational Qualification For Compressed Air System.BREWSKI50% (2)
- Validation VialWasher OQ NIHDocument30 pagesValidation VialWasher OQ NIHcongacon3aPas encore d'évaluation
- Validation Protocol PDFDocument63 pagesValidation Protocol PDFmarwa100% (1)
- Purified Water System ValidationDocument2 pagesPurified Water System Validationankur_haldarPas encore d'évaluation
- OQ FormatDocument13 pagesOQ FormatAliqahwash100% (1)
- Validation Master Plan TemplateDocument17 pagesValidation Master Plan TemplateNadine100% (4)
- Validation Plan For Purified Water Generation and Distribution SystemDocument49 pagesValidation Plan For Purified Water Generation and Distribution SystemPrashansa Shrestha100% (2)
- Process Performance Qualification Protocol For Autoclave - Pharmaceutical Guidelines 2Document12 pagesProcess Performance Qualification Protocol For Autoclave - Pharmaceutical Guidelines 2MykolaPas encore d'évaluation
- Standard Operating Procedure: Validation of Heating Ventilation and Air Conditioning (Hvac) SystemDocument20 pagesStandard Operating Procedure: Validation of Heating Ventilation and Air Conditioning (Hvac) SystemMaryanthony Namyalo100% (3)
- URS For Water For Injection Generation SystemDocument14 pagesURS For Water For Injection Generation Systemyogendra100% (2)
- OQ Protocol For Purified WaterDocument5 pagesOQ Protocol For Purified WaterNorhasma Ismail100% (4)
- ArevaDocument10 pagesArevamkbpgcilPas encore d'évaluation
- Sample Cleaning Validation ProtocolDocument9 pagesSample Cleaning Validation ProtocolOryza SativaPas encore d'évaluation
- Validation ProtocolDocument63 pagesValidation ProtocolIndústria Petys64% (22)
- DI IQ OQ ReportDocument11 pagesDI IQ OQ ReportVemulapalli SaibabuPas encore d'évaluation
- PQ For Purified Water Generation SystemDocument29 pagesPQ For Purified Water Generation SystemDilawar Bakht100% (1)
- Man Diesel Primeserv Academy Introduction To The Me EngineDocument17 pagesMan Diesel Primeserv Academy Introduction To The Me EngineRoman SydorovPas encore d'évaluation
- Validation of Water SystemDocument25 pagesValidation of Water Systemsukanya100% (2)
- Performance Qualification Protocol For Water For Injection (WFI) System - Pharmaceutical GuidelinesDocument3 pagesPerformance Qualification Protocol For Water For Injection (WFI) System - Pharmaceutical GuidelinesJayesh PatidarPas encore d'évaluation
- CIQA Validation Master Plan Sample TemplateDocument4 pagesCIQA Validation Master Plan Sample TemplateSatyam Gupta100% (1)
- From Start To Finish: Pharmaceutical Grade WaterDocument6 pagesFrom Start To Finish: Pharmaceutical Grade Watervenki_bee100% (1)
- Validation of Water SystemDocument51 pagesValidation of Water Systemahmedullah_8100% (3)
- Validation Master Plan A Complete Guide - 2020 EditionD'EverandValidation Master Plan A Complete Guide - 2020 EditionPas encore d'évaluation
- Prot OQ HVACDocument12 pagesProt OQ HVACamrinPas encore d'évaluation
- Cleaning Validation of Sampling ToolsDocument13 pagesCleaning Validation of Sampling Toolsnagendra100% (1)
- Temperature Mapping and Monitoring - A SummaryDocument3 pagesTemperature Mapping and Monitoring - A SummaryEduardPas encore d'évaluation
- A Guide To Validating Purified WaterDocument4 pagesA Guide To Validating Purified WaterDonig Fermanian100% (1)
- Purified Water System Validation - 1Document9 pagesPurified Water System Validation - 1sarada jena100% (1)
- Hold Time Study of 70% IpaDocument5 pagesHold Time Study of 70% IpaAshok Lenka100% (4)
- 02 Cleaning Validation of Double Cone Blender CLV 02Document2 pages02 Cleaning Validation of Double Cone Blender CLV 02Ravi Yadav100% (2)
- HVAC System ValidationDocument4 pagesHVAC System ValidationemonwrePas encore d'évaluation
- Cleaning Validation Rinsing TesDocument5 pagesCleaning Validation Rinsing TesUrsula HillePas encore d'évaluation
- Standard Operating Procedure Somatec: Title: Sop For Pao TestDocument3 pagesStandard Operating Procedure Somatec: Title: Sop For Pao TestMajed HossainPas encore d'évaluation
- PQ Sterile TunnelDocument10 pagesPQ Sterile TunnelReza JafariPas encore d'évaluation
- Water System ValidationDocument49 pagesWater System ValidationJelly Anne Barrera100% (1)
- SOP of Sanitation of PW SystemDocument6 pagesSOP of Sanitation of PW Systemanon_350461302100% (1)
- How To Validate An AutoclaveDocument3 pagesHow To Validate An AutoclaveqhpuongPas encore d'évaluation
- Cleaning Validation Report TEMPLATEDocument9 pagesCleaning Validation Report TEMPLATEnatavcePas encore d'évaluation
- Facility ValidationDocument12 pagesFacility ValidationGhanta Ranjith Kumar100% (1)
- Form URS FPP Vial Washing Machine 050307.odtDocument10 pagesForm URS FPP Vial Washing Machine 050307.odtNur ChamidahPas encore d'évaluation
- Qualification of Purified Water Systems PDFDocument12 pagesQualification of Purified Water Systems PDFMario Vazquez B100% (1)
- Cleaning Validation ProtocolDocument18 pagesCleaning Validation Protocolalfred2000Pas encore d'évaluation
- Validation Protocall For AutoclaveDocument7 pagesValidation Protocall For AutoclaveBalakrishnan S NadarPas encore d'évaluation
- Performance Qualification Protocol (PQP) For (Autoclave), Located inDocument17 pagesPerformance Qualification Protocol (PQP) For (Autoclave), Located inlouayPas encore d'évaluation
- Principles of Cleanroom ValidationDocument8 pagesPrinciples of Cleanroom ValidationNiranjan Lak100% (1)
- URS For HVAC, GPF-2Document3 pagesURS For HVAC, GPF-2Mamun50% (2)
- Cleaning Validation ProtocolDocument3 pagesCleaning Validation Protocolpuneetogupta100% (1)
- IQOQ ProtocolDocument4 pagesIQOQ ProtocolVijay RajaindranPas encore d'évaluation
- Qualification Procedure For Vial Washing Machine - Pharmaceutical GuidelinesDocument1 pageQualification Procedure For Vial Washing Machine - Pharmaceutical GuidelinesAli Goutas50% (2)
- RTV Servis HorvatDocument24 pagesRTV Servis Horvatvedran54Pas encore d'évaluation
- Blue and White Corporate Illustrated Blue Connections PresentationDocument26 pagesBlue and White Corporate Illustrated Blue Connections PresentationveroPas encore d'évaluation
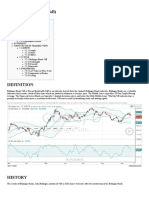
- Bollinger Bands %B (%B) - TradingView DocumentationDocument5 pagesBollinger Bands %B (%B) - TradingView DocumentationFinTPas encore d'évaluation
- Css Interview QuestionsDocument7 pagesCss Interview QuestionsWeb CodPas encore d'évaluation
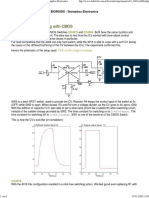
- Hard Vs Soft Switching With CMOS - Bioroids - Stompbox ElectronicsDocument2 pagesHard Vs Soft Switching With CMOS - Bioroids - Stompbox Electronicsch gPas encore d'évaluation
- SyllabusDocument107 pagesSyllabusKonda SumanayanaPas encore d'évaluation
- Money Lender and Pawn BrokerDocument53 pagesMoney Lender and Pawn BrokerAkshay polPas encore d'évaluation
- In-Building Wireless Solutions: Passive Distributed Antenna SystemsDocument44 pagesIn-Building Wireless Solutions: Passive Distributed Antenna SystemsJonas AndradePas encore d'évaluation
- WG Next Generation FirewallsDocument16 pagesWG Next Generation FirewallsVinod GuptaPas encore d'évaluation
- ConfigAdmin AG v2016EEDocument156 pagesConfigAdmin AG v2016EEpatologicoPas encore d'évaluation
- Catalog Easergy Flair 21d 22d 23d Datasheet enDocument4 pagesCatalog Easergy Flair 21d 22d 23d Datasheet ennguyenanhchiPas encore d'évaluation
- Vol 3 Section 6 - Electricity ServicesDocument43 pagesVol 3 Section 6 - Electricity ServicesAndrew SamudaPas encore d'évaluation
- Knoweldge Repository & Academic Searching TechniquesDocument44 pagesKnoweldge Repository & Academic Searching TechniquesSumesh John100% (2)
- Romtip SW PcsDocument5 pagesRomtip SW PcsLaithAl-abbadiPas encore d'évaluation
- Igdtuw Cut Off 2016, 2015Document20 pagesIgdtuw Cut Off 2016, 2015lakshmiPas encore d'évaluation
- L6004L8 - TriacDocument8 pagesL6004L8 - TriacValmir BarbosaPas encore d'évaluation
- Iot Conference Report FinalDocument16 pagesIot Conference Report FinalIman MagzoubPas encore d'évaluation
- 04 - Patrutiu BaltesDocument12 pages04 - Patrutiu BaltesprasadkulkarnigitPas encore d'évaluation
- Ipoque Product Brochure Net-Reporter WebDocument4 pagesIpoque Product Brochure Net-Reporter WebmickysouravPas encore d'évaluation
- Best AI Essay WriterDocument2 pagesBest AI Essay WriterPeter JhonsonPas encore d'évaluation
- RINXs Info SessionDocument21 pagesRINXs Info SessionCherry ChoiPas encore d'évaluation
- Operation ManagementDocument11 pagesOperation ManagementRajni KumariPas encore d'évaluation
- Plant Layout LocationDocument80 pagesPlant Layout LocationShalu TewthiaPas encore d'évaluation
- Better Linux Disk Caching & Performance With VM - Dirty - RatioDocument5 pagesBetter Linux Disk Caching & Performance With VM - Dirty - RatioSelçuk GÜLTEKİNPas encore d'évaluation
- ZKTeco+Fingerprint+Scanner+SDK+Selection+Guide-Ver3 0Document1 pageZKTeco+Fingerprint+Scanner+SDK+Selection+Guide-Ver3 0Evelyn Rosas OreaPas encore d'évaluation