Académique Documents
Professionnel Documents
Culture Documents
Determine Rate Law by Method of Initial Rates
Transféré par
Tinni TapawanDescription originale:
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Determine Rate Law by Method of Initial Rates
Transféré par
Tinni TapawanDroits d'auteur :
Formats disponibles
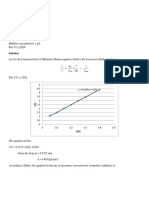
4/11/2016
ChemTeam:Kinetics:determineratelawbymethodofinitialrates
Determineratelawbymethodofinitialrates
ReturntoKineticsMenu
Problem#1:Ratedatawereobtainedforfollowingreaction:
A+2B>C+2D
Exp.
InitialA InitialB Init.RateofFormation
(mol/L) (mol/L)
ofC(Mmin1)
0.10
0.10
3.0x104
0.30
0.30
9.0x104
0.10
0.30
3.0x104
0.20
0.40
6.0x104
Whatistheratelawexpressionforthisreaction?
Solution:
1)compareexp.1andexp.3.AremainsconstantandBistripled.Theratefrom1to3
remainsconstant.Conclusion:Bisnotintheratelawexpression.
2)compareexp1toexp2.TheconcentrationofAtriples(andwedon'tcarewhat
happenstoB).Theratetriples.Conclusion:firstorderinA.
3)wecanalsoshowfirstorderinAbycomparingexp1toexp4.TheconcentrationofA
doublesandtheratedoubles.Remember,Bisnotpartoftheratelaw,sowedon'tpayany
attentiontoitatall.
rate=k[A]
Problem#2:ForthereactionA+B>products,thefollowinginitialrateswerefound.Whatisthe
ratelawforthisreaction?
Trial1:[A]=0.50M[B]=1.50MInitialrate=4.2x103M/min
Trial2:[A]=1.50M[B]=1.50MInitialrate=1.3x102M/min
Trial3:[A]=3.00M[B]=3.00MInitialrate=5.2x102M/min
Solution:
1)OrderwithrespecttoA:
Lookattrial1andtrial2.BisheldconstantwhileAtriples.Theresultisthattherate
triples.Conclusion:Aisfirstorder.
http://www.chemteam.info/Kinetics/WSKineticsmethodofinitialrates.html
1/8
4/11/2016
ChemTeam:Kinetics:determineratelawbymethodofinitialrates
2)OrderwithrespecttoB:
Lookattrials2and3.ThekeytothisisthatwealreadyknowthattheorderforAisfirst
order.
Bothconcentrationsweredoubledfrom2to3andtherategoesupbyafactorof4.Since
Aisfirstorder,weknowthatadoublingoftherateisduetotheconcentrationofAbeing
doubled.
So,welookattheconcentrationchangeforB(adoubling)andtheconsequentrate
change(anotherdoublingremembertheoverallincreasewasafactorof4thinkof4as
beingadoubleddoubling).
Conclusion:theorderforBisfirstorder.
Theratelawisrate=k[A][B]
Comment:theabovemannerofmakingtherateorderdeterminationforBabitmorecomplexisa
commontechnique.Lookforittobeusedonyourtest!
Problem#3:Thefollowingdatawereobtainedforthischemicalreaction:A+B>products
Exp.
InitialA InitialB Init.RateofFormation
(mmol/L) (mmol/L) ofproducts(mMmin1)
4.0
6.0
1.60
2.0
6.0
0.80
4.0
3.0
0.40
(a)Determinetheratelawforthisreaction.
(b)Findtherateconstant.
Solution:
1)Lookatexperiments2and1.From2to1,weseethatAisdoubled(whileBisheld
constant).Adoublingoftheratewithadoublingoftheconcentrationshowsthatthe
reactionisfirstorderwithrespecttoA.
2)Nowcompareexperiments1and3.TheconcentrationofAisheldconstantwhilethe
concentrationofBiscutinhalf.WhenBiscutinhalf,theoverallrateiscutbyafactor
of4(whichisthesquareof2).ThisshowsthereactionissecondorderinB.
3)Theratelawisthis:rate=k[A][B]2
4)Notethatthecomparisonin(2)canbereversed.ConsiderthattheconcentrationofBis
doubledasyougofromexp.3toexp.1.Whentheconcentrationisdoubled,therategoes
upbyafactorof4(whichis22).
5)Wecanuseanysetofvaluestodeterminetherateconstant:
rate=k[A][B]2
http://www.chemteam.info/Kinetics/WSKineticsmethodofinitialrates.html
2/8
4/11/2016
ChemTeam:Kinetics:determineratelawbymethodofinitialrates
1.60mMmin1=k(4.0mM)(6.0mM)2
k=0.011mM2min2
theunitsonkcanberenderedinthismanner:
k=0.067L2mmol2min1
Problem#4:Thefollowingdatawereobtainedforthechemicalreaction:A+B>products
Exp.
InitialA InitialB Init.RateofFormation
(mol/L) (mol/L)
ofproducts(Ms1)
0.040
0.040
9.6x106
0.080
0.040
1.92x105
0.080
0.020
9.6x106
(a)Determinetheratelawforthisreaction.
(b)Findtherateconstant.
(c)Whatistheinitialrateofreactionwhen[A]o=0.12Mand[B]o=0.015
Solution:
1)Examineexps.2and3.AremainsconstantwhileBisdoubledinconcentrationfrom3
to2.Theresultofthischangeisthattherateofthereactiondoubles.Weconcludethat
thereactionisfirstorderinB.
2)Nowwelookatexps1and2.BremainsconstantwhiletheconcentrationofA
doubles.Asaresultofthedoubledconcentration,theratealsodoubles.Conclusion:the
reactionisfirstorderinA.
3)Theratelawforthisreactionis:
rate=k[A][B]
4)Wecanuseanysetofdatatocalculatetherateconstant:
rate=k[A][B]
9.6x106Ms1=k(0.040M)(0.040M)
k=0.0060M1s1
Comment:rememberthatM1s1isoftenwrittenasLmol1s1.
5)Weusetherateconstantalongwiththedatainpart(c)ofthequestion:
rate=(0.0060M1s1)(0.12M)(0.015M)
rate=1.08x105Ms1
http://www.chemteam.info/Kinetics/WSKineticsmethodofinitialrates.html
3/8
4/11/2016
ChemTeam:Kinetics:determineratelawbymethodofinitialrates
Problem#5:ConsiderthereactionthatoccurswhenaClO2solutionandasolutioncontaining
hydroxideions(OH)aremixed,asshowninthefollowingequation:
2ClO2(aq)+2OH(aq)>ClO3(aq)+ClO2(aq)+H2O(l)
WhensolutionscontainingClO2andOHinvariousconcentrationsweremixed,thefollowingrate
datawereobtained:
Determination#1:[ClO2]o=1.25x102M[OH]o=1.30x103M
InitialrateforformationofClO3=2.33x104Ms1
Determination#2:[ClO2]o=2.50x102M[OH]o=1.30x103M
InitialrateforformationofClO3=9.34x104Ms1
Determination#3:[ClO2]o=2.50x102M[OH]o=2.60x103M
InitialrateforformationofClO3=1.87x103Ms1
(a)Writetherateequationforthechemicalreaction.
(b)Calculatetherateconstant,k.
(c)Calculatethereactionrateforthereactionwhen[ClO2]o=8.25x103Mand[OH]o=5.35x
102M.
Solution:
1)Compare#1and#2.TheconcentrationofClO2doubles(hydroxideremainsconstant)
andtherategoesupbyafactoroffour(thinkofitastwosquared).Thismeansthe
reactionissecondorderwithrespecttoClO2.
2)Compare#2and#3.Theconcentrationofhydroxideisdoubledwhilethe[ClO2]
remainsconstant.Theratedoubles,showingthatthereactionisfirstorderinhydroxide.
3)Theratelawis:rate=k[ClO2]2[OH]
4)Calculationfortherateconstant:
1.87x103Ms1=k(2.50x102M)2(2.60x103M)
k=1.15x103M2s1
Oftentherateconstantunitisrenderedthusly:L2mol2s1.
Notethattheoverallorderoftheratelawisthirdorderandthatthisis
reflectedintheunitassociatedwiththerateconstant.
5)Calculationforpart(c):
rate=(1.15x103M2s1)(8.25x103M)2(5.35x102M)
rate=4.19x103Ms1
http://www.chemteam.info/Kinetics/WSKineticsmethodofinitialrates.html
4/8
4/11/2016
ChemTeam:Kinetics:determineratelawbymethodofinitialrates
Problem#6:2A+B>C+D
Thefollowingdataaboutthereactionabovewereobtainedfromthreeexperiments:
Exp. [A]
[B]
Initialrateof
formationofC
0.6 0.15
6.3x103
0.2
0.6
2.8x103
0.2 0.15
7.0x104
Calculatetherateexpressionintermsof[A]forexperiment1.
Solution:
rate1/rate3=k1[A1]x[B1]y/k3[A3]x[B3]y
k'swillcanceland[B]willcancel
rate1/rate3=[A1]x/[A3]x
0.0063/0.0007=(0.6)x/(0.2)x
9=0.6x/0.2x
9=3x
rate=k[A]2
Comment:notethat,withasecondorder,whentheconcentrationincreasesbyafactorof3,therate
goesupbyafactorof9(whichis32).
Problem#7:Determinetheproperformoftheratelawfor:
CH3CHO(g)>CH4(g)+CO(g)
Exp. [CH3CHO] [CO] Rate(Ms1)
1
0.30
0.20
0.60
0.10
0.30
0.067
0.10
0.20
0.067
Solution:
1)Noteexperiments2and3.The[CH3CHO]remainsconstantwhilethe[CO]changes.
However,nochangeofthereactionrateisobserved.Fromthis,weconcludethatCOis
http://www.chemteam.info/Kinetics/WSKineticsmethodofinitialrates.html
5/8
4/11/2016
ChemTeam:Kinetics:determineratelawbymethodofinitialrates
notpartoftheratelaw.
2)Examineexperiment3comparedtoexperiment1.from3to1,the[CH3CHO]triples
andtherategoesupbyafactorof9(whichis32).Conclusion:thereactionissecond
orderinCH3CHO.
3)Therateexpressionisthis:rate=k[CH3CHO]2
Problem#8:Determinetheratelaw,includingthevaluesoftheordersandratelawconstant,forthe
followingreactionusingtheexperimentaldataprovided.
Q+X>products
Trial
[Q]
[X]
Rate
0.12M 0.10M 1.5x103M/min
0.24M 0.10M 3.0x103M/min
0.12M 0.20M 1.2x102M/min
Solution:
1)Examinetrials1and2.[X]isheldconstantwhile[Q]isdoubled(from1to2).Asa
result,theratedoubles.WeconcludethatthereactionisfirstorderinQ.
2)Examinetrials3and1.TheconcentrationofQdoesnotchangefrom3to1butthe
concentrationforXisdoubled.Whenthishappens,weobserveaneightfoldincreasein
therateofthereaction.WeconcludethatthereactionisthirdorderinX.Thisarisesfrom
thefactthat23=8.Inmoredetail:
rate3/rate1=k3[Q3]x[X3]y/k1[Q1]x[X1]y
k'swillcanceland[Q]willcancel
rate3/rate1=[X3]x/[X1]x
0.012/0.0015=(0.20)x/(0.10)x
8=0.20x/0.10x
8=2x
x=3
Comment:notethat,withathirdorder,whentheconcentrationincreasesbyafactorof2,
therategoesupbyafactorof8(whichis23).
3)valueoftherateconstant:
rate=k[Q][X]3
http://www.chemteam.info/Kinetics/WSKineticsmethodofinitialrates.html
6/8
4/11/2016
ChemTeam:Kinetics:determineratelawbymethodofinitialrates
0.012Mmin1=k(0.12M)(0.20M)3
k=12.5L3mol3min1
Notethatthisreactionisoverallfourthorder.
Problem#9:Withthefollowingdata,usethemethodofinitialratestofindthereactionorderswith
respecttoNOandO2.:
Initialreaction
Trial [NO]o [O2]o
rate,M/s
1
0.020
0.010
0.028
0.020
0.020
0.057
0.040
0.020
0.227
Solution:
1)DoublingO2doublesreactionrate(trials2and1).
2)DoublingNOincreasesreactionrate8x(trials2and3).
3)Conclusion:firstorderO2,thirdorderNO.
Problem#10:Forthefollowingreaction:
A+B>2C
itisfoundthatdoublingtheamountofAcausesthereactionratetodoublewhiledoublingtheamount
ofBcausesthereactionratetoquadruple.Whatisthebestratelawequationforthisreaction?
(a)rate=k[A]2[B]
(b)rate=k[A][B]
(c)rate=k[A][B]2
(d)rate=k[A]1/2[B]
Theratedoublingwhentheconcentrationisdoubledisahallmarkoffirstorder.Therategoingupby
afactorof4(withistwosquared)whentheconcentrationisdoubledisahallmarkofsecondorder.
Answerchoice(c)isthecorrectanswer.
Here'sanansweronYahooAnswerstoaproblemliketheonesabove.
BonusProblem:Aratelawis1/2orderwithrespecttoareactant.Whatistheeffectontheratewhen
theconcentrationofthisreactantisdoubled?
Solution:
Letusexaminetheeffectontherate(symbolizedasR)aswedoubletheconcentrationfromstepto
http://www.chemteam.info/Kinetics/WSKineticsmethodofinitialrates.html
7/8
4/11/2016
ChemTeam:Kinetics:determineratelawbymethodofinitialrates
step:
R=k[1]0.5=1
R=k[2]0.5=1.4
R=k[4]0.5=2
R=k[8]0.5=2.8
R=k[16]0.5=4
OneanswerIfoundonYahooAnswerswasthis:
Thereactionrateisnotdoubledwhenyoudoubletheconcentration.Noticethatwhenthe
concentrationchangedfrom2to8,theRwentfrom1.4to2.8.Sowhentheconcentration
isquadrupled,theRdoubles.
Iwouldaddtotheabovebysaying:
Whentheconcentrationdoubles,therategoesupbyafactorwhichisthesquarerootof
two.
ReturntoKineticsMenu
http://www.chemteam.info/Kinetics/WSKineticsmethodofinitialrates.html
8/8
Vous aimerez peut-être aussi
- KINETICS Practice Problems and SolutionsDocument9 pagesKINETICS Practice Problems and SolutionsnairdanipsoPas encore d'évaluation
- Chem 16 Long Exam 1 ReviewerDocument4 pagesChem 16 Long Exam 1 Reviewerdesperateboy100% (1)
- 3.1 Notes - Avogadro & The MoleDocument5 pages3.1 Notes - Avogadro & The MoleRoddyPas encore d'évaluation
- CH 14-VP-Stoichiometry - 2020Document37 pagesCH 14-VP-Stoichiometry - 2020Mlamuli MlarhPas encore d'évaluation
- 1 - Intro + Basic ConceptsDocument48 pages1 - Intro + Basic ConceptsShawki BsatPas encore d'évaluation
- Z-8000-BB-4076 - Water Injection SystemDocument158 pagesZ-8000-BB-4076 - Water Injection Systembhuvanchaudhari100% (2)
- CHE1401 LabManual JULY2015Document76 pagesCHE1401 LabManual JULY2015Adrian Prashantha WeerakkodyPas encore d'évaluation
- Curso Ipims CompletionDocument951 pagesCurso Ipims CompletionpietrokiPas encore d'évaluation
- L1 Nature of ReadingDocument15 pagesL1 Nature of ReadingTinni Tapawan100% (1)
- Gold Extraction With Halogens: J.-M. Lalancette, B. Dubreuil, D. Lemieux and C. ChouinardDocument16 pagesGold Extraction With Halogens: J.-M. Lalancette, B. Dubreuil, D. Lemieux and C. ChouinardLudwig Kommer100% (2)
- SCIENTIFIC PAPER (Chemistry Lab) - Determination of DensitiesDocument4 pagesSCIENTIFIC PAPER (Chemistry Lab) - Determination of DensitiesMi Rivera75% (4)
- Experiment 6 FinalDocument13 pagesExperiment 6 FinalFroileth Pulido100% (1)
- Exam I - Rate Law ProblemsDocument26 pagesExam I - Rate Law ProblemsPeachYpeachasPas encore d'évaluation
- GX Operating Manual v0.4 EDocument38 pagesGX Operating Manual v0.4 EBadmaarag JlssPas encore d'évaluation
- CHM 101 Complete - LNDocument80 pagesCHM 101 Complete - LNSimon Adediran100% (1)
- H 3159Document58 pagesH 3159Alex GigenaPas encore d'évaluation
- 102 MSJC 13Document11 pages102 MSJC 13noelPas encore d'évaluation
- Analytical Chemistry Notes IiDocument9 pagesAnalytical Chemistry Notes IiJabez MatigaPas encore d'évaluation
- Rate LawsDocument20 pagesRate LawsReginal MoralesPas encore d'évaluation
- Chapter 16 ADocument30 pagesChapter 16 AAbhishek Isaac MathewPas encore d'évaluation
- Solved Problem Question (Gas Ab)Document2 pagesSolved Problem Question (Gas Ab)Seruzna IshxPas encore d'évaluation
- AP Chemistry - Hess's Law LabDocument3 pagesAP Chemistry - Hess's Law LabJonathan Chen83% (12)
- Chemical Engineering Calculations: Input Output AccumulationDocument16 pagesChemical Engineering Calculations: Input Output AccumulationRose Dane Escobedo DiestaPas encore d'évaluation
- Calculation of Vapor-Liquid Equilibria From Infinite-Dilution Excess Enthalpy Data Using The Wilson or NRTL EquationDocument12 pagesCalculation of Vapor-Liquid Equilibria From Infinite-Dilution Excess Enthalpy Data Using The Wilson or NRTL EquationneftPas encore d'évaluation
- Flow Through PipesDocument6 pagesFlow Through PipesammuvijjiPas encore d'évaluation
- Ap Chemistry Lab RubricDocument3 pagesAp Chemistry Lab Rubricapi-258145192Pas encore d'évaluation
- Drying 01Document12 pagesDrying 01Charisse MadiaPas encore d'évaluation
- Palaganas Hw2 Che 197Document5 pagesPalaganas Hw2 Che 197Elah Palaganas100% (1)
- Applied Chemical Engineering CalculationsDocument7 pagesApplied Chemical Engineering Calculationsmbolantenaina100% (1)
- Experiment No 18Document4 pagesExperiment No 18Suvrasoumya Mohanty100% (2)
- C H O + A O + B NH C C H NO + D H O+eCO: InstructionsDocument4 pagesC H O + A O + B NH C C H NO + D H O+eCO: InstructionsJohn Paul Jandayan33% (3)
- SCH 2102Document4 pagesSCH 2102Clare Mueni Makaa100% (1)
- 474 - CHM 703Document25 pages474 - CHM 703permata100% (1)
- Chemical Kinetics of Complex ReactionsDocument3 pagesChemical Kinetics of Complex ReactionsTrung VõPas encore d'évaluation
- Stoichiometry of Cell Growth and Product FormationDocument2 pagesStoichiometry of Cell Growth and Product Formationmneilg0% (1)
- Chemical Reaction EngineeringDocument101 pagesChemical Reaction EngineeringGerard Toby CalixtoPas encore d'évaluation
- Transient Growth KineticsDocument14 pagesTransient Growth KineticsCeeEeePas encore d'évaluation
- Experiment 10-Spectrophotometric MethodsDocument6 pagesExperiment 10-Spectrophotometric MethodsMarc DiongcoPas encore d'évaluation
- Isothermal Reactor Design: 1. Batch OperationDocument3 pagesIsothermal Reactor Design: 1. Batch Operationنزار الدهاميPas encore d'évaluation
- Lab 3 Le Chatelier's Principle - and Chemical Equilibrium BCCDocument9 pagesLab 3 Le Chatelier's Principle - and Chemical Equilibrium BCCXavier BuenoPas encore d'évaluation
- Half LifeDocument2 pagesHalf LifeawesomearlenePas encore d'évaluation
- Expt5 G2 MWDocument39 pagesExpt5 G2 MWayraPas encore d'évaluation
- How FastDocument54 pagesHow FastKaushal Silva RanpatabendigePas encore d'évaluation
- Theory of The Chemostat Cambridge Studies in Mathematical BiologyDocument330 pagesTheory of The Chemostat Cambridge Studies in Mathematical BiologyChristian HuertaPas encore d'évaluation
- 11 Fruit JuicesDocument8 pages11 Fruit JuicesthangesspPas encore d'évaluation
- FR Experiment 3Document7 pagesFR Experiment 3m kimPas encore d'évaluation
- Microbial Growth and Product FormationDocument26 pagesMicrobial Growth and Product FormationDP PurwadiPas encore d'évaluation
- Experiment No. 7 Measurement of Reaction ConversionDocument8 pagesExperiment No. 7 Measurement of Reaction ConversionHoneylet Recaña TayactacPas encore d'évaluation
- Mass Transfer Operations II Rr320801Document8 pagesMass Transfer Operations II Rr320801Nagwa MansyPas encore d'évaluation
- Experiment 3 Transference NumberDocument3 pagesExperiment 3 Transference NumberRicky JayPas encore d'évaluation
- Catalyst Characterization TechniquesDocument8 pagesCatalyst Characterization TechniquesDaniel DadziePas encore d'évaluation
- MT 1subbuDocument40 pagesMT 1subbuVikas SachanPas encore d'évaluation
- Analytical ChemistryDocument50 pagesAnalytical ChemistryNguyễn Trịnh Anh MinhPas encore d'évaluation
- Chapter 2 - Data InterpretationDocument24 pagesChapter 2 - Data InterpretationPHƯƠNG ĐẶNG YẾNPas encore d'évaluation
- KineticsOverviewbymongraal PDFDocument128 pagesKineticsOverviewbymongraal PDFMungur Dushyant RAiPas encore d'évaluation
- Semi-Batch Reactor: Chemical Reaction Engineering (CRE) Is TheDocument28 pagesSemi-Batch Reactor: Chemical Reaction Engineering (CRE) Is TheJohn Patrick DaglePas encore d'évaluation
- ChE 401 CRD Assignment-4Document3 pagesChE 401 CRD Assignment-4Husnain GujjarPas encore d'évaluation
- C D D C: Topic 4 - The Collision Theory Example of Solved ProblemsDocument5 pagesC D D C: Topic 4 - The Collision Theory Example of Solved ProblemsesmassPas encore d'évaluation
- Exam Solution Chemical ProcessesDocument9 pagesExam Solution Chemical ProcessesInez Karina TanuPas encore d'évaluation
- BuffersDocument3 pagesBuffersIshak Ika Kovac100% (1)
- Experiment 4 - Conductometry PDFDocument18 pagesExperiment 4 - Conductometry PDFWANGPas encore d'évaluation
- The Vocabulary of Analytical Chemistry: Chapter OverviewDocument22 pagesThe Vocabulary of Analytical Chemistry: Chapter OverviewAwash TinsaePas encore d'évaluation
- Reaction Mechanisms in Environmental Engineering: Analysis and PredictionD'EverandReaction Mechanisms in Environmental Engineering: Analysis and PredictionPas encore d'évaluation
- Determining Reaction Order Initial Rates and The Method of IsolationDocument4 pagesDetermining Reaction Order Initial Rates and The Method of IsolationDeepak PandeyPas encore d'évaluation
- Chem Basic FB Answer Key CH 18 (06.14.16)Document4 pagesChem Basic FB Answer Key CH 18 (06.14.16)Katlin CallahanPas encore d'évaluation
- Big Idea 4 AnswersDocument4 pagesBig Idea 4 AnswersSreeyaPas encore d'évaluation
- Reaction Rates Chemistry)Document14 pagesReaction Rates Chemistry)Nasya TehPas encore d'évaluation
- PharChem Acid and Bases PDFDocument43 pagesPharChem Acid and Bases PDFTinni TapawanPas encore d'évaluation
- PharChem Expt. 7 and 8Document1 pagePharChem Expt. 7 and 8Tinni TapawanPas encore d'évaluation
- 9Document2 pages9Christine TrinidadPas encore d'évaluation
- Demulsification Kinetics of W/O Emulsion in An A.C. Electric FieldDocument7 pagesDemulsification Kinetics of W/O Emulsion in An A.C. Electric FieldajostosPas encore d'évaluation
- 40 Item Test Science 6with Key To CorrectionDocument5 pages40 Item Test Science 6with Key To CorrectionvinnPas encore d'évaluation
- APIDocument4 pagesAPIAnam Hyat KhanPas encore d'évaluation
- Latihan AmaliDocument14 pagesLatihan Amaliazman94Pas encore d'évaluation
- Bioorganic & Medicinal ChemistryDocument7 pagesBioorganic & Medicinal ChemistryWalid Ebid ElgammalPas encore d'évaluation
- Good PDFDocument3 pagesGood PDFDarshna SoniPas encore d'évaluation
- C Pipe Material Behaviour Didier Ilunga Only)Document24 pagesC Pipe Material Behaviour Didier Ilunga Only)AlexandraOdinevPas encore d'évaluation
- Sci Oly Practice QuestionsDocument5 pagesSci Oly Practice QuestionsJessica JimenezPas encore d'évaluation
- Electro Statics Worksheet CbseDocument19 pagesElectro Statics Worksheet CbseAmanPas encore d'évaluation
- Drinking Water AssesmentDocument9 pagesDrinking Water AssesmentMeenu AgarwalPas encore d'évaluation
- Preca Catalog - Read ViewDocument9 pagesPreca Catalog - Read ViewAnyaWestmorelandPas encore d'évaluation
- GCC SyallbusDocument12 pagesGCC SyallbusAbheek KashyapPas encore d'évaluation
- JEE Main 2023 31 January Shift 2Document13 pagesJEE Main 2023 31 January Shift 2barnwalk367Pas encore d'évaluation
- Difference Between Bonding in Ceramics and MetalsDocument2 pagesDifference Between Bonding in Ceramics and MetalsQamarShafiqPas encore d'évaluation
- 1921 - Timoshenko - On The Correction For Shear of The Diferential Equation For Transverse VibrationsDocument3 pages1921 - Timoshenko - On The Correction For Shear of The Diferential Equation For Transverse VibrationsJuan Karlos Alberca AlfaroPas encore d'évaluation
- Cmo11l Quiz 2Document6 pagesCmo11l Quiz 2Ryan GanabPas encore d'évaluation
- ESA SME Initiative Course D:MaterialsDocument64 pagesESA SME Initiative Course D:MaterialsJoseph JonathanPas encore d'évaluation
- ColaTeric CBSDocument1 pageColaTeric CBSmndmattPas encore d'évaluation
- Nuclear Power StationsDocument17 pagesNuclear Power StationsPrathap VuyyuruPas encore d'évaluation
- Paper Mekanika ReservoirDocument23 pagesPaper Mekanika ReservoirAnonymous FcCosOLJPas encore d'évaluation
- Inconel Alloy 740 H PDFDocument24 pagesInconel Alloy 740 H PDFJosé Juan Jiménez AlejandroPas encore d'évaluation
- Development Length - Chapter 7 - Reinforced Concrete DesignDocument11 pagesDevelopment Length - Chapter 7 - Reinforced Concrete DesignHumam GhazalPas encore d'évaluation
- Activity Sheets For Chem With NamesDocument6 pagesActivity Sheets For Chem With Namesapi-283862617100% (1)
- Slugging in A Flare HeaderDocument34 pagesSlugging in A Flare Headercepong89Pas encore d'évaluation