Académique Documents
Professionnel Documents
Culture Documents
PHY3 CJune 2006
Transféré par
api-3726022Description originale:
Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
PHY3 CJune 2006
Transféré par
api-3726022Droits d'auteur :
Formats disponibles
PHY3 JUNE 2006 - TOPIC C - NUCLEAR & PARTICLE PHYSICS 30 minutes 1
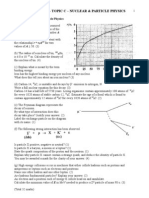
3. (a) During an experiment into the energy spectrum of β- particles, the following graph was
produced.
(i) To a copy of this graph, add suitable labels, with units where appropriate, to each axis. [3]
(ii) State the significance of the figure 0.78. Explain how the results of this experiment led to the
prediction of the existence of an antineutrino. You may be awarded a mark for the clarity of your
answer. [4]
(b) The equation for p decay can be written as:
(i) For each particle, either give its quark composition or state that it is a fundamental particle. [2]
(ii) Write a similar equation for β+ decay. [2]
(iii) Explain fully why these reactions can only be mediated by the weak interaction. [3]
(iv) Name the exchange particle for each one of these decays. [2]
(c) (i) The density of a nucleus of strontium Sr is 2.29 x 1017 kg m-3. Calculate the mass of a nucleus
of radius 5.34 x 10-15m. [3]
(ii) Show that the nucleon number of this isotope is 88. (u = 1.66 x 10-27 kg) [2]
(iii) Hence calculate the radius ro of a single nucleon. [3]
(d) A hydrogen atom consists of one proton and one electron. For each particle choose all the words
that could be used to make a correct statement.
A proton is a {baryon / meson / lepton / hadron}
An electron is a {baryon / meson / lepton / hadron} [2]
(e) In 1995 scientists at CERN created atoms of antihydrogen.
(i) Name the particles that make up antihydrogen. [1]
(ii) Describe these particles in terms of charge and quark structure where relevant. [2]
(iii) State the charge of an atom of antihydrogen. [1]
(iv) Explain why it is not possible to store atoms of antihydrogen. [2]
Total 32 marks
Vous aimerez peut-être aussi
- Physics-Sample-Paper-ICSE-2020 BababababDocument13 pagesPhysics-Sample-Paper-ICSE-2020 BababababKUSHAL LUNKADPas encore d'évaluation
- MSC Chemistry QuestionsDocument277 pagesMSC Chemistry QuestionsChemistry MES100% (1)
- Worksheet 30 PDFDocument4 pagesWorksheet 30 PDFVijay Bhaskar100% (3)
- PHY3 CJune 2004Document1 pagePHY3 CJune 2004api-3726022Pas encore d'évaluation
- SC IT 7 - 10th CBSE P I&II - 07.01.2023 SCIENCEDocument4 pagesSC IT 7 - 10th CBSE P I&II - 07.01.2023 SCIENCEmayukhsarkar100Pas encore d'évaluation
- Questions on Fundamental Particles: Cs Cs Cs Cs β β β βDocument10 pagesQuestions on Fundamental Particles: Cs Cs Cs Cs β β β βjoePas encore d'évaluation
- PHY3 CJune 2002Document1 pagePHY3 CJune 2002api-3726022Pas encore d'évaluation
- Assignment 01 PDFDocument2 pagesAssignment 01 PDFRachit ShahPas encore d'évaluation
- Science Department: Topic 7.1 - Discrete Energy & Radioactivity Topic 7.2 - Nuclear Reactions 12 Grade (Physics SL)Document8 pagesScience Department: Topic 7.1 - Discrete Energy & Radioactivity Topic 7.2 - Nuclear Reactions 12 Grade (Physics SL)ananPas encore d'évaluation
- RadioactivityDocument15 pagesRadioactivityBenjamin Teo0% (2)
- Be - First Year Fe Engineering - Semester 2 - 2019 - November - Engineering Physics Pattern 2015Document4 pagesBe - First Year Fe Engineering - Semester 2 - 2019 - November - Engineering Physics Pattern 2015amkslade101Pas encore d'évaluation
- PHYS 414 Nov 2017Document4 pagesPHYS 414 Nov 2017Joram MuiruriPas encore d'évaluation
- PHY3 CJanuary 2006Document1 pagePHY3 CJanuary 2006api-3726022Pas encore d'évaluation
- 1i' JN ::x. Ii Ii',: Sub: EEE 307 ofDocument23 pages1i' JN ::x. Ii Ii',: Sub: EEE 307 ofTrisha DasPas encore d'évaluation
- PG, 1 Sem, Apc, CC-1, Question Paper - Jan 23Document3 pagesPG, 1 Sem, Apc, CC-1, Question Paper - Jan 23Pralay MaitiPas encore d'évaluation
- Atoms & NucleiDocument15 pagesAtoms & NucleixkryxxzPas encore d'évaluation
- M.SC (Chemistry) 2019 PatternDocument172 pagesM.SC (Chemistry) 2019 PatternAirtel PrepaidPas encore d'évaluation
- M.SC (CHEMISTRY) 20019 PatternDocument168 pagesM.SC (CHEMISTRY) 20019 Patternpapey42271Pas encore d'évaluation
- Nuclei QB XiiDocument23 pagesNuclei QB XiiToshani GuptaPas encore d'évaluation
- KISA Physics QP 2024 Set2Document6 pagesKISA Physics QP 2024 Set2dashrathrai17Pas encore d'évaluation
- University of Cambridge Examinations Advanced Level Physics Paper 3 1990Document4 pagesUniversity of Cambridge Examinations Advanced Level Physics Paper 3 1990Shae MarkPas encore d'évaluation
- Topic 7 - Test 2017 - KeyDocument8 pagesTopic 7 - Test 2017 - KeyananPas encore d'évaluation
- Mini Mock 15q Atomic Structure Unit 1 As ChemistryDocument10 pagesMini Mock 15q Atomic Structure Unit 1 As ChemistrySherif HishamPas encore d'évaluation
- CHEM0030-2019 2020 Exam PaperDocument6 pagesCHEM0030-2019 2020 Exam PaperjPas encore d'évaluation
- Assignment 1Document3 pagesAssignment 1MainzaPas encore d'évaluation
- You May Not Start To Read The Questions Printed On The Subsequent Pages of This Question Paper Until Instructed That You May Do So by The InvigilatorDocument6 pagesYou May Not Start To Read The Questions Printed On The Subsequent Pages of This Question Paper Until Instructed That You May Do So by The InvigilatorSpringOrchidPas encore d'évaluation
- Nuclei: 29. A nucleus undergoes through α-decay and transforms to thorium. What isDocument3 pagesNuclei: 29. A nucleus undergoes through α-decay and transforms to thorium. What isShrvan SudhakarPas encore d'évaluation
- Section A: Answer All Questions (40 Marks)Document11 pagesSection A: Answer All Questions (40 Marks)c3mutPas encore d'évaluation
- M.SC (Chemistry) 2013 - 2014 PatternDocument133 pagesM.SC (Chemistry) 2013 - 2014 Patternrakesh jadhavPas encore d'évaluation
- AL Essays (Radioactivity, Light & Electrons) - 1Document7 pagesAL Essays (Radioactivity, Light & Electrons) - 1umpc1248Pas encore d'évaluation
- Atomic Structure QuestionsDocument4 pagesAtomic Structure QuestionsHovan Tall Nut TanPas encore d'évaluation
- Sample Paper On Physics Grade 10Document3 pagesSample Paper On Physics Grade 10aadesh_mahatoPas encore d'évaluation
- PH110 2010 08Document3 pagesPH110 2010 08lyon juniorPas encore d'évaluation
- 13122023012411physics Practice Paper 2Document8 pages13122023012411physics Practice Paper 2Srushti ChougulePas encore d'évaluation
- Fe Oct2011Document48 pagesFe Oct2011Adriane TaylorPas encore d'évaluation
- Phys 414 22Document3 pagesPhys 414 22Joram MuiruriPas encore d'évaluation
- 1-1 MechanicsDocument19 pages1-1 MechanicsPurna Suresh PedamalluPas encore d'évaluation
- 6Document1 page6Sathiyaseelan SankarjeePas encore d'évaluation
- 87 EssayDocument4 pages87 EssaySam SamPas encore d'évaluation
- RadioactivityDocument16 pagesRadioactivityEng BahanzaPas encore d'évaluation
- PHY3 DJune 2006Document1 pagePHY3 DJune 2006api-3726022Pas encore d'évaluation
- Phy 441 Question BankDocument6 pagesPhy 441 Question Bankandrew silungwePas encore d'évaluation
- Structure of Atom-hsslive-AnilDocument4 pagesStructure of Atom-hsslive-AnilDhana Aryal100% (1)
- 3.-T7-2 T-CuestionesDocument46 pages3.-T7-2 T-CuestionesAnonymous zP1ek3ya5nPas encore d'évaluation
- RAS201Document2 pagesRAS201DR ABHISHEK TIWARIPas encore d'évaluation
- New Microsoft Word DocumentDocument11 pagesNew Microsoft Word DocumentMuhammad WaqasPas encore d'évaluation
- (Three Hours) : Sample Paper - 2011 Class - XII Subject - PhysicsDocument5 pages(Three Hours) : Sample Paper - 2011 Class - XII Subject - PhysicsValay DavePas encore d'évaluation
- Applied PhysicsDocument8 pagesApplied PhysicsRaman BhullarPas encore d'évaluation
- Physics Science 1Document7 pagesPhysics Science 1BarunMondalPas encore d'évaluation
- Question Paper of Winter Session 2021 22Document19 pagesQuestion Paper of Winter Session 2021 22moresachin7040Pas encore d'évaluation
- SECTION A (15 Marks) Answer ALL Questions in This SectionDocument15 pagesSECTION A (15 Marks) Answer ALL Questions in This SectionFazliawati MahayuddinPas encore d'évaluation
- Model Test Paper 1Document3 pagesModel Test Paper 1Aman bansalPas encore d'évaluation
- Instruction For CandidatesDocument4 pagesInstruction For CandidatesAmit PokhariaPas encore d'évaluation
- Cbse 12th Question Bank PhysicsDocument6 pagesCbse 12th Question Bank Physicsramayodi223Pas encore d'évaluation
- Chem 102Document4 pagesChem 102akinpelumikingv23Pas encore d'évaluation
- AEA PHYS PP MayJune 2006 AEA Paper 1342Document16 pagesAEA PHYS PP MayJune 2006 AEA Paper 1342Rowena Fletcher-WoodPas encore d'évaluation
- Model Test Paper 2Document3 pagesModel Test Paper 2Aman bansalPas encore d'évaluation
- Physics Complete SyllabusDocument4 pagesPhysics Complete SyllabusSamridhi JainPas encore d'évaluation
- Universiti Pendidikan Sultan Idris Test SEMESTER 1 SESSION 2020/2021Document12 pagesUniversiti Pendidikan Sultan Idris Test SEMESTER 1 SESSION 2020/2021Sentia NazreenPas encore d'évaluation
- 6102 01 Que 20080109Document20 pages6102 01 Que 20080109Inas ManurungPas encore d'évaluation
- Biojune 2008 U1qDocument16 pagesBiojune 2008 U1qapi-3726022Pas encore d'évaluation
- Biojune 2008 U4aqDocument24 pagesBiojune 2008 U4aqapi-3726022100% (1)
- PHY3 DJune 2005Document1 pagePHY3 DJune 2005api-3726022Pas encore d'évaluation
- Biology Jan 2008 6101 QuestionDocument20 pagesBiology Jan 2008 6101 QuestionAdrees MeahPas encore d'évaluation
- PHY3 DJune 2002Document1 pagePHY3 DJune 2002api-3726022Pas encore d'évaluation
- PHY3 DJune 2006Document1 pagePHY3 DJune 2006api-3726022Pas encore d'évaluation
- PHY3 DJanuary 2002Document1 pagePHY3 DJanuary 2002api-3726022Pas encore d'évaluation
- PHY3 DJanuary 2004Document1 pagePHY3 DJanuary 2004api-3726022Pas encore d'évaluation
- PHY3 DJune 2004Document1 pagePHY3 DJune 2004api-3726022Pas encore d'évaluation
- PHY3 DJanuary 2005Document1 pagePHY3 DJanuary 2005api-3726022Pas encore d'évaluation
- PHY3 AJune 2003Document1 pagePHY3 AJune 2003api-3726022Pas encore d'évaluation
- PHY3 AJune 2006Document1 pagePHY3 AJune 2006api-3726022Pas encore d'évaluation
- PHY3 CJanuary 2006Document1 pagePHY3 CJanuary 2006api-3726022Pas encore d'évaluation
- PHY3 CJanuary 2005Document1 pagePHY3 CJanuary 2005api-3726022Pas encore d'évaluation
- PHY3 CJanuary 2003Document1 pagePHY3 CJanuary 2003api-3726022Pas encore d'évaluation
- PHY3 AJune 2005Document1 pagePHY3 AJune 2005api-3726022Pas encore d'évaluation
- PHY3 AJune 2004Document1 pagePHY3 AJune 2004api-3726022Pas encore d'évaluation
- PHY3 AJanuary 2006Document1 pagePHY3 AJanuary 2006api-3726022Pas encore d'évaluation
- PHY3 AJanuary 2004Document1 pagePHY3 AJanuary 2004api-3726022Pas encore d'évaluation