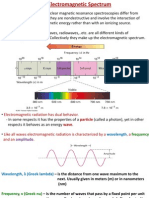
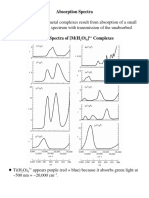
Académique Documents
Professionnel Documents
Culture Documents
Atomic Structure Lab 3
Transféré par
08090311Description originale:
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Atomic Structure Lab 3
Transféré par
08090311Droits d'auteur :
Formats disponibles
Name: Lab Partner: Atomic Structure A Journey into the Atom
Period: Date:
Introduction: Atoms are composed of subatomic particles, such as the protons and the neutrons, which make up the nucleus of the atom and are similar in mass, and electrons, which are found orbiting the nucleus in an electron, cloud and have a negligible mass. All atoms contain the same kinds of particles but may differ in the number of each particle. This accounts for the presence of isotopes and ions for the different elements. This activity will allow you to use what you know about the composition of the atom, as well as isotopes and ions, to describe sixteen atoms. The atoms are contained in Ziploc bags and the subatomic particles are coded as follows. Protons black beans Neutrons white beans Electrons popcorn Purpose: Students will collect data and relate number of subatomic particles to atomic number, mass number, electrical charge, atomic symbol, and name of element. New Terms: isotope, ion Equipment: Materials: Ziploc bags representing atoms
Procedure: Analyze each Ziploc bag (atom) and record its vital statistics in the data table provided. Data Analysis: 1. List all sets of isotopes. How do you know they are isotopes? 2. List all sets of ions. How do you know they are ions? Conclusions: A nuclear reactor generates a very large amount of energy by splitting a uranium235 atom to produce Barium-139 and Krypton-94. How would each of these atoms be represented using the coding system used for atoms #1 - 16?
Atomic Structure A Journey into the Atom Bag # # of # of # of Atomic Mass Electrical Chemical Protons Neutrons Electrons Number Number Charge Symbol
Name
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16
Vous aimerez peut-être aussi
- Radioactivity RevisionDocument137 pagesRadioactivity RevisiongayboiPas encore d'évaluation
- Evolution Vocab ChartDocument8 pagesEvolution Vocab ChartsarfarazPas encore d'évaluation
- L1 Universe and Solar SystemDocument36 pagesL1 Universe and Solar Systemrollyn_poncePas encore d'évaluation
- 1f - Plate TectonicsDocument19 pages1f - Plate TectonicsPat Bautista100% (1)
- Module 6 KISS QuestionsDocument13 pagesModule 6 KISS QuestionsHewadPas encore d'évaluation
- Atoms Molecules and IonsDocument12 pagesAtoms Molecules and IonsI am stupdPas encore d'évaluation
- Quiz, Quiz, Trade Atom and Periodic TableDocument5 pagesQuiz, Quiz, Trade Atom and Periodic TablefatraxiiPas encore d'évaluation
- IGCSE Geography Course Notes: TectonicsDocument42 pagesIGCSE Geography Course Notes: TectonicsxerxesPas encore d'évaluation
- Chapter17-Lesson 2Document23 pagesChapter17-Lesson 2api-185034533Pas encore d'évaluation
- Atoms and Molecules WorksheetDocument2 pagesAtoms and Molecules Worksheet23_11_1993Pas encore d'évaluation
- Yr8 Unit PlanDocument8 pagesYr8 Unit Planapi-334786948Pas encore d'évaluation
- Rs1088 - Final Optional Science Grade 9Document378 pagesRs1088 - Final Optional Science Grade 9deepak subediPas encore d'évaluation
- Earthquake Epicenter LabDocument15 pagesEarthquake Epicenter LabRahman Atthariq0% (1)
- Determine The Epicenter of An Earthquake LabDocument7 pagesDetermine The Epicenter of An Earthquake Labapi-251355123Pas encore d'évaluation
- Second Term: Grade 9 ScienceDocument70 pagesSecond Term: Grade 9 Sciencecaeameko:)Pas encore d'évaluation
- Advanced Periodic TrendsDocument4 pagesAdvanced Periodic TrendsBianca RolstonPas encore d'évaluation
- Electricity Worksheet Page 2Document1 pageElectricity Worksheet Page 2Laura LucumiPas encore d'évaluation
- Human Respiratory SystemDocument80 pagesHuman Respiratory SystemAj MirandaPas encore d'évaluation
- Balancing Equations PDFDocument6 pagesBalancing Equations PDFFeli CiaPas encore d'évaluation
- Intro To Science ObjectivesDocument1 pageIntro To Science Objectivesapi-256196103Pas encore d'évaluation
- Green Science and Environment 7 Final 2076Document296 pagesGreen Science and Environment 7 Final 2076Purnima RautPas encore d'évaluation
- Chemistry Unit 2: ST ND RD THDocument24 pagesChemistry Unit 2: ST ND RD THjontstufPas encore d'évaluation
- Physicsskill and Practice Physical Science Worksheets1Document387 pagesPhysicsskill and Practice Physical Science Worksheets1Jan Lloyd OdruniaPas encore d'évaluation
- Plate Tectonic UnitDocument95 pagesPlate Tectonic Unitapi-227272561Pas encore d'évaluation
- Chap 19 No 4Document2 pagesChap 19 No 4api-249777358100% (1)
- Atom WorksheetsDocument4 pagesAtom Worksheetsapi-271960049Pas encore d'évaluation
- Scotch College Science: The Universe & Our Changing EarthDocument37 pagesScotch College Science: The Universe & Our Changing EarthDavid WangPas encore d'évaluation
- Worksheet: Qualitative vs. Quantitative Observations w8.SILN.1Document2 pagesWorksheet: Qualitative vs. Quantitative Observations w8.SILN.1Aisaka TaigaPas encore d'évaluation
- DNA Molecule & Central DogmaDocument43 pagesDNA Molecule & Central DogmaJoanna Ruth SeproPas encore d'évaluation
- Instapdf - in 118 Elements List 216Document5 pagesInstapdf - in 118 Elements List 216HARISH UPas encore d'évaluation
- Emission SpectraDocument4 pagesEmission SpectraKarla Jara Hidalgo GalarionPas encore d'évaluation
- Solar System InquiryDocument4 pagesSolar System Inquiryapi-232002863Pas encore d'évaluation
- Handbook of Special Edu Tech Research and PracticeDocument4 pagesHandbook of Special Edu Tech Research and PracticeVORTEX666Pas encore d'évaluation
- Development of Space Technology in IndiaDocument19 pagesDevelopment of Space Technology in IndiaRadhikamadhavi KvPas encore d'évaluation
- Chapter 10Document28 pagesChapter 10api-32740845100% (1)
- CSM Findingepicenter Activity1 Worksheetas v2 Tedl DWCDocument5 pagesCSM Findingepicenter Activity1 Worksheetas v2 Tedl DWCJudarlyn Madria0% (1)
- Plant and Animal Science UnitDocument13 pagesPlant and Animal Science Unitapi-251141297Pas encore d'évaluation
- 8th Grade Science Test 3 Earth Science Study Guide - Answer KeyDocument3 pages8th Grade Science Test 3 Earth Science Study Guide - Answer KeyTese Watkins100% (1)
- KISS Chemistry - WaterDocument33 pagesKISS Chemistry - WaterJoannaDGunawan50% (2)
- Biology Holt Chapter 3 PPT 07Document44 pagesBiology Holt Chapter 3 PPT 07Dini HanifaPas encore d'évaluation
- Circulatory Lesson PlansDocument4 pagesCirculatory Lesson Plansapi-281424654Pas encore d'évaluation
- Osmosis Diffusion Lab-1Document7 pagesOsmosis Diffusion Lab-1api-1658690000% (1)
- Characteristics of Living Things Worksheet Answers Key PDFDocument9 pagesCharacteristics of Living Things Worksheet Answers Key PDFJasmine PenniePas encore d'évaluation
- Radioisotopes SeminarDocument23 pagesRadioisotopes SeminarDrVikasPas encore d'évaluation
- The SunDocument16 pagesThe Sun3XTR3M3 ᜰ꙰ꦿ NJRPas encore d'évaluation
- PLANETSDocument2 pagesPLANETSIan JadePas encore d'évaluation
- High School Earth Science 14-26Document529 pagesHigh School Earth Science 14-26Andrei PopaPas encore d'évaluation
- Earth Structure..Document10 pagesEarth Structure..Maitum Gemark BalazonPas encore d'évaluation
- Physics Grade and 12 BibDocument30 pagesPhysics Grade and 12 Bibmmmkkk_1Pas encore d'évaluation
- Nys 8th Grade Science Test 2012Document32 pagesNys 8th Grade Science Test 2012api-267822960Pas encore d'évaluation
- Foundation &fundamentals of Chemistry Unit: 3 Atomic StructureDocument34 pagesFoundation &fundamentals of Chemistry Unit: 3 Atomic StructurePiyush KumarPas encore d'évaluation
- Solar System Unit Cover PageDocument6 pagesSolar System Unit Cover Pageapi-382675034Pas encore d'évaluation
- Isotopes and IsobarsDocument16 pagesIsotopes and IsobarsAdityakingdomPas encore d'évaluation
- AP Biology Syllabus 2009-2010Document10 pagesAP Biology Syllabus 2009-2010yulianaholicPas encore d'évaluation
- 2020-21 Ap 08 PS Tqa emDocument31 pages2020-21 Ap 08 PS Tqa emsrikanth PosaPas encore d'évaluation
- Solar SystemDocument4 pagesSolar SystemrizqiPas encore d'évaluation
- Stratigraphy Handout PDFDocument5 pagesStratigraphy Handout PDFatis100% (1)
- 7th Grade Science Curriculum Map Final DraftDocument5 pages7th Grade Science Curriculum Map Final Draftapi-455790872Pas encore d'évaluation
- States of Matter Kids DiscoverDocument1 pageStates of Matter Kids DiscoveraffodellPas encore d'évaluation
- ForceDocument26 pagesForceShinji100% (1)
- Biology Year at A Glance 2019 - 2020Document1 pageBiology Year at A Glance 2019 - 2020Jamal MorelliPas encore d'évaluation
- Dual Nature and Radiation of Matter MMDocument1 pageDual Nature and Radiation of Matter MMSanjay GuptaPas encore d'évaluation
- Worksheet 2 - Electrons Protons and NeutronsDocument3 pagesWorksheet 2 - Electrons Protons and NeutronsAnonymous APas encore d'évaluation
- St. Joseph Academy San Jose, Batangas SY 2019 - 2020 NAME: - GRADE and SECTIONDocument2 pagesSt. Joseph Academy San Jose, Batangas SY 2019 - 2020 NAME: - GRADE and SECTIONArnold Paombong0% (1)
- Scie q1Document11 pagesScie q1Marc AbhelPas encore d'évaluation
- Chemistry Batch-Intake Star Mole Concept Ipec Practice Sheet-1, DATE-15/05/2021Document2 pagesChemistry Batch-Intake Star Mole Concept Ipec Practice Sheet-1, DATE-15/05/2021khushiPas encore d'évaluation
- 37 Analytical Techniques: Analysis - Cie A-Level ChemistryDocument64 pages37 Analytical Techniques: Analysis - Cie A-Level ChemistryHADFINA DAMIA BINTI SHAHIDINPas encore d'évaluation
- Digital Classroom: Advance Worksheet (Icse - Phase-I & Ii) (Objective) & (Subjective)Document3 pagesDigital Classroom: Advance Worksheet (Icse - Phase-I & Ii) (Objective) & (Subjective)SATHIASEELAN SIVANANDAM, AdvocatePas encore d'évaluation
- Mass Spectra of Elements: Kms TarakkyDocument1 pageMass Spectra of Elements: Kms TarakkyKhondokar TarakkyPas encore d'évaluation
- 2 - Modern Physics Practice Assignment @JEEAdvanced - 2024Document9 pages2 - Modern Physics Practice Assignment @JEEAdvanced - 2024Vineet SierraPas encore d'évaluation
- OCR Chemistry A: Isotopic AbundanceDocument3 pagesOCR Chemistry A: Isotopic AbundanceMasih KhanPas encore d'évaluation
- SUBJECT: Science 9 Time Allotment: 1 Hour GRADE AND SECTION: Grade 9-Kyanite I. ObjectiveDocument5 pagesSUBJECT: Science 9 Time Allotment: 1 Hour GRADE AND SECTION: Grade 9-Kyanite I. Objectiverose ann chavezPas encore d'évaluation
- 2022 Atomic Structure G3 FACEDocument3 pages2022 Atomic Structure G3 FACEo3gce8e2lfPas encore d'évaluation
- 3 Atoms and Elements: Core CurriculumDocument2 pages3 Atoms and Elements: Core CurriculumTagan TaganovPas encore d'évaluation
- Lecture 1 InorganicDocument20 pagesLecture 1 InorganicNaveen KumarPas encore d'évaluation
- Struktur Atom Perkembangan Model STUDENT WORKSHEET-1Document6 pagesStruktur Atom Perkembangan Model STUDENT WORKSHEET-1sanihgholiyahPas encore d'évaluation
- Physics 24 - Radioactive DecayDocument25 pagesPhysics 24 - Radioactive DecayMuhammad Amin SuhaimiPas encore d'évaluation
- Chap 12 - IRDocument30 pagesChap 12 - IRTanvir AhmedPas encore d'évaluation
- Polymer (2001) 42, 3569-3580Document12 pagesPolymer (2001) 42, 3569-3580Bill FuPas encore d'évaluation
- Class 12 - Physics - AtomsDocument17 pagesClass 12 - Physics - AtomsSOUMYA SHOUVIKPas encore d'évaluation
- 03 Quiz 1ADocument2 pages03 Quiz 1AKing CjPas encore d'évaluation
- .Lesson 3 - Background RadiationDocument12 pages.Lesson 3 - Background RadiationMarcus WrightPas encore d'évaluation
- Kuliah NMRDocument92 pagesKuliah NMRDedi saputraPas encore d'évaluation
- Basic FTIR Theory (3) Spectra of Single Beam (Background and Sample), Transmission Factor, AbsorbanceDocument2 pagesBasic FTIR Theory (3) Spectra of Single Beam (Background and Sample), Transmission Factor, AbsorbanceSiva Ok100% (1)
- Phy475 Homework 3Document1 pagePhy475 Homework 3ProfAndré GazotoPas encore d'évaluation
- Advanced - Periodic Table - DPP 1 To 5Document9 pagesAdvanced - Periodic Table - DPP 1 To 5OJAS DwivediPas encore d'évaluation
- Ch11 1 Transition Metals Absorption SpectraDocument29 pagesCh11 1 Transition Metals Absorption SpectraTapas GhatakPas encore d'évaluation