Académique Documents
Professionnel Documents
Culture Documents
Mode of Action: Insulin
Transféré par
manus7777Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Mode of Action: Insulin
Transféré par
manus7777Droits d'auteur :
Formats disponibles
Mode of Action: Insulin
Insulin:
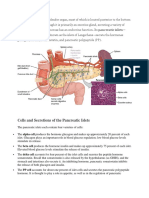
Insulin is a hormone. And like many hormones, insulin is a protein. Insulin is secreted by groups of cells within the pancreas called islet cells. The pancreas is an organ that sits behind the stomach and has many functions in addition to insulin production. The pancreas also produces digestive enzymes and other hormones. Carbohydrates (or sugars) are absorbed from the intestines into the bloodstream after a meal. Insulin is then secreted by the pancreas in response to this detected increase in blood sugar. Most cells of the body have insulin receptors which bind the insulin which is in the circulation. When a cell has insulin attached to its surface, the cell activates other receptors designed to absorb glucose (sugar) from the blood stream into the inside of the cell.
Chemistry:
Insulin is a rather small protein, with a molecular weight of about 6000 Daltons. It is composed of two chains held together by disulfide bonds. Insulin is composed of two peptide chains referred to as the A chain and B chain. A and B chains are linked together by two disulfide bonds, and an additional disulfide is formed within the A chain. In most species, the A chain consists of 21 amino acids and the B chain of 30 amino acids. The amino acid sequence is highly conserved among vertebrates, and insulin from one mammal almost certainly is biologically active in another. Even today, many diabetic patients are treated with insulin extracted from pig pancreas. Insulin is a small protein with a molecular weight in humans of 5808. It contains 51 amino acids arranged in two chains (A and B) linked by disulfide bridges; there are species differences in the amino acids of both chains. Proinsulin, a long single-chain protein molecule, is processed within the Golgi apparatus and packaged into granules, where it is hydrolyzed into insulin and
a residual connecting segment called C-peptide by removal of four amino acids.
Insulin and C-peptide are secreted in equimolar amounts in response to all insulin secretagogues; a small quantity of unprocessed or partially hydrolyzed proinsulin is released as well. Although proinsulin may have some mild hypoglycemic action, C-peptide has no known physiologic function. Granules within the B cells store the insulin in the form of crystals consisting of two atoms of zinc and six molecules of insulin. The entire human pancreas contains up to 8 mg of insulin, representing approximately 200 biologic units. Originally, the unit was defined on the basis of the hypoglycemic activity of insulin in rabbits. With improved purification techniques, the unit is presently defined on the basis of weight, and present insulin standards used for assay purposes contain 28 units per milligram.
Mode of Action
Insulin is the major hormone controlling critical energy functions such as glucose and lipid metabolism. Insulin elicits a diverse array of biological responses by binding to its specific
receptor. The insulin receptor belongs to a subfamily of receptor tyrosine kinases that includes the IGF (Insulin-like Growth Factor) receptor and the IRR (Insulin Receptor-Related Receptor). These receptors are tetrameric proteins consisting of two alpha and two beta subunits that function as allosteric enzymes in which the alpha subunit inhibits the tyrosine kinase activity of the beta subunit. Insulin has diverse effects on cells including stimulation of glucose transport, gene expression and alterations of cell morphology. The hormone mediates these effects by activation of signaling pathways which utilize, i) ii) iii) Adaptor molecules such the IRS (Insulin Receptor Substrates), the SHC (Src and Collagen Homologues) and the GRB2 (Growth Factor Receptor Binding protein-2), Lipid kinases such as PI3K (Phosphatidylinositol 3-Kinase), Small G-proteins like Rac, and iv) serine, threonine and tyrosine kinases (Ref.2).
Tyrosine-phosphorylated IRS then displays binding sites for numerous signaling partners. PI3K has a major role in insulin functions. It regulates three main classes of signaling molecules: the AGC family of serine/threonine protein kinases, guanine nucleotide-exchange proteins of the Rho family of GTPases, and the Tec family of tyrosine kinases. The best characterized of the AGC kinases is PDK-1 (Phosphoinositide-Dependent Kinase-1), one of the serine kinases that phosphorylates and activates the serine/threonine kinase Akt/PKB (Protein Kinase-B). Akt possesses a PH domain that also interacts directly with PIP3 (Phosphatidylinositol -3, 4, 5Triphosphate), promoting membrane targeting of the protein and catalytic activation. Akt has been suggested to be important in transmission of the insulin signal, by phosphorylation of the enzyme GSK3 (Glycogen Synthase Kinase-3), the FKHRL1 (Forkhead-Related Family of Mammalian Transcription Factor) and cAMP (Cyclic Adenosine Monophosphate) response element-binding protein. Akt inhibits apoptosis by phosphorylating the BAD (BCL2 Antagonist of Cell Death) component of the BAD/BCLXL complex. Phosphorylated BAD binds to 14-3-3 causing dissociation of the BAD/BCLXL complex and allowing cell survival, and Akt activates IKK, which ultimately leads to NF-KappaB (Nuclear Factor-KappaB) activation and cell survival. Akt also activates the mTOR (Mammalian Target of Rapamycin)/FRAP pathway. Activation of mTOR results in the phosphorylation of ribosomal protein S6 kinase, p70S6K, which is also regulated by phosphorylation by PDK-1. Rapamycin (FRAP) interactions with mTOR also regulate the activity of p70S6K, the kinase that phosphorylates the 40S ribosomal protein S6. S6 is thought to be the only p70S6K substrate, and by controlling S6 phosphorylation, p70S6K regulates the translation of an essential family of mRNAs that contain an oligopyrimidine tract at their transcriptional start site. Activation of mTOR also results in phosphorylation and inactivation of eIF4EBP (Eukaryotic Initiation Factor 4EBinding Protein), also known as PHAS, an inhibitor of the translation initiation factor eIF4E (Eukaryotic Initiation Factor-4E). Insulin induces dephosphorylation of eEF2 (Eukaryotic Elongation Factor-2) and inactivation of eEF2K (Eukaryotic Elongation Factor-2 Kinase), and these effects are blocked by rapamycin, which inhibits the mammalian target of rapamycin, mTOR. Akt and/or the atypical PKCs (Protein Kinase-C) seem to be required for insulin-stimulated glucose transport. The ability of insulin to stimulate glucose uptake relies on a complex signaling cascade that leads to the translocation of GLUT4 (Glucose Transporter Protein-4) from an intracellular compartment to the plasma membrane, which results in increased glucose uptake. While the PI3K/Akt cascade participates in this process, another major pathway leading to GLUT4
translocation involves the insulin receptor-mediated phosphorylation of CAP (c-Cbl Associated Protein) and formation of the CAP:Cbl:CrkII complex. This complex, through its interaction with flotillin, localizes to lipid rafts facilitating GLUT4 translocation, using in the final step a Synipcontaining specialized Snare complex (Ref.3). The signaling contributions of other proteins bound by phosphorylated IRSs, including the phosphotyrosine phosphatase SHP2, Fyn, and the SH3containing adaptor Nck, are yet to be clearly defined. Another important protein involved in insulin signaling is GRB10 (Growth Factor Receptor-Bound Protein 10). GRB10 interacts directly with IR (Insulin Receptor). IR does not phosphorylate GRB10, but phosphorylation by Tyrosine Kinases of the Src family negatively regulates binding to IR. GRB10 is believed to interact with MEK and play a role in signaling. As is the case for other growth factors, insulin stimulates the MAPK (Mitogen-Activated Protein) ERK (Extracellular Signal Regulated Kinase). This pathway involves the tyrosine phosphorylation of IRS proteins and/or SHC, which in turn interact with the adapter protein GRB2, recruiting the SOS (Son of Sevenless) exchange protein to the plasma membrane for activation of Ras. The activation of Ras also requires stimulation of the tyrosine phosphatase SHP2, through its interaction with receptor substrates such as GAB1 (GRB2 Associated Binding Protein-1) or IRS1/2. Once activated, Ras operates as a molecular switch, stimulating a serine kinase cascade through the stepwise activation of Raf, MEK and ERK. Activated ERK can translocate into the nucleus, where it catalyses the phosphorylation of transcription factors such as p62TCF, initiating a transcriptional programme that leads to cellular proliferation or differentiation (Ref.4). Signal transduction by the insulin receptor is not limited to its activation at the cell surface. The activated ligand receptor complex, initially at the cell surface, is internalized into endosomes, and this process is dependent on tyrosine autophosphorylation. Endocytosis of activated receptors has the dual effect of concentrating receptors within endosomes and allowing the insulin receptor tyrosine kinase to phosphorylate substrates that are spatially distinct from those accessible at the plasma membrane. Acidification of the endosomal lumen, due to the presence of proton pumps, results in dissociation of insulin from its receptor. The endosome constitutes the major site of insulin degradation by the EAI (Endosomal Acidic Insulinase). This loss of the ligand-receptor complex attenuates any further insulin-driven receptor rephosphorylation events and leads to receptor dephosphorylation by extra lumenal endosomally associated PTPs. PTPase (Protein Tyrosine Phosphatases) catalyze the dephosphorylation of insulin receptor and its substrates, leading to attenuation of insulin action. A number of PTPases have been implicated as the negative regulator of insulin signaling. Among them, the intracellular PTPase, PTP1B, has been shown to function as the insulin receptor phosphatase. PTEN (Phosphatase and Tensin Homolog Deleted On Chromosome-10) negatively regulates insulin signaling. SHIP2 (SH2containing Inositol Phosphatase-2) is another negative regulator of insulin signaling and such negative regulation depends on its 5'-phopshatase activity. Overexpression of SHIP2 protein decreases Insulin-dependent PIP3 production as well as insulin-stimulated Akt activation, GSK3 inactivation, and glycogen synthetase activation. Insulin increases glucose uptake in muscle and fat, and inhibits hepatic glucose production, thus serving as the primary regulator of blood glucose concentration. Insulin also stimulates cell growth and differentiation, and promotes the storage of substrates in fat, liver and muscle by stimulating lipogenesis, glycogen and protein synthesis, and inhibiting lipolysis, glycogenolysis and protein breakdown. Insulin resistance or deficiency results
in profound dysregulation of these processes, and produces elevations in fasting and postprandial glucose and lipid levels.
Reference: 1. insulin 2. http://www.vivo.colostate.edu/hbooks/pathphys/endocrine/pancreas/insu lin.html http://www.endocrineweb.com/conditions/diabetes/diabetes-what-
Vous aimerez peut-être aussi
- Brothers: Integration of Metabolism and Metabolic Processes in Fed, Fasting and Starvation StatesDocument14 pagesBrothers: Integration of Metabolism and Metabolic Processes in Fed, Fasting and Starvation StatesNischal DhPas encore d'évaluation
- Lecture-5 Hormonal Regulation of Intestinal MotilityDocument4 pagesLecture-5 Hormonal Regulation of Intestinal Motilityمرتضى حسين عبدPas encore d'évaluation
- CH11-Amino Acid MetabolismDocument106 pagesCH11-Amino Acid MetabolismChatchawinPas encore d'évaluation
- Report EnzymesDocument34 pagesReport Enzymesnestie villavirayPas encore d'évaluation
- 16 Pathology of Lipid MetDocument52 pages16 Pathology of Lipid MetyasingadaPas encore d'évaluation
- Biochem Lec Term Paper HypoglycemiaDocument8 pagesBiochem Lec Term Paper Hypoglycemiaapi-318284296Pas encore d'évaluation
- Grape Seed Extract (Vitis Vinifera) Partially Reverses High Fat Diet-Induced Obesity in C57BL/6J MiceDocument7 pagesGrape Seed Extract (Vitis Vinifera) Partially Reverses High Fat Diet-Induced Obesity in C57BL/6J MiceMohammad SutamiPas encore d'évaluation
- Lipid and Glucose Methodology PDFDocument16 pagesLipid and Glucose Methodology PDFJezzah Mae CañetePas encore d'évaluation
- Tryptophan Metabolism: Dr. Ashok Kumar J International Medical School Management and Science University MalaysiaDocument23 pagesTryptophan Metabolism: Dr. Ashok Kumar J International Medical School Management and Science University MalaysiaShah HuzaifaPas encore d'évaluation
- SDLS 2008 Serum Total Protein and Albumin DeterminationDocument2 pagesSDLS 2008 Serum Total Protein and Albumin DeterminationgenieqtPas encore d'évaluation
- Glycoproteins: Charlotte V. Bañes, MDDocument71 pagesGlycoproteins: Charlotte V. Bañes, MDswitimahalePas encore d'évaluation
- Biochemistry: Ihrevale LIPID META Bolism 1Document92 pagesBiochemistry: Ihrevale LIPID META Bolism 1Roy BelenPas encore d'évaluation
- A Brief Overview of Hemoglobin ElectrophoresisDocument46 pagesA Brief Overview of Hemoglobin Electrophoresisمجاهد إسماعيل حسن حسينPas encore d'évaluation
- Body FluidsDocument24 pagesBody FluidsMohamed MidoPas encore d'évaluation
- B.D.S Course Biochemistry/Syllabus: (With Effect From 2010-11 Onwards)Document4 pagesB.D.S Course Biochemistry/Syllabus: (With Effect From 2010-11 Onwards)AnandPas encore d'évaluation
- Introduction To Biochemistry: by Zaheer Uddin (Pharm-D, M Phil (Pharmacy Practice) MBA (MARKETING)Document63 pagesIntroduction To Biochemistry: by Zaheer Uddin (Pharm-D, M Phil (Pharmacy Practice) MBA (MARKETING)Izat KhanPas encore d'évaluation
- Concise Biochemistry: Fundamental Principles: March 2016Document52 pagesConcise Biochemistry: Fundamental Principles: March 2016Sagar DeshmaniPas encore d'évaluation
- 2 - Lipid Metabolism Lecture For StudentsDocument68 pages2 - Lipid Metabolism Lecture For StudentshwhsgxPas encore d'évaluation
- Written Exam Blue PrintDocument12 pagesWritten Exam Blue PrintMotasim_mPas encore d'évaluation
- Principles of Dna Isolation & PurificationDocument10 pagesPrinciples of Dna Isolation & PurificationRatish Singh50% (2)
- First Professional Mbbs Fourth Semester EXAMINATION SESSION-2007-2008 Subject - BiochemistryDocument9 pagesFirst Professional Mbbs Fourth Semester EXAMINATION SESSION-2007-2008 Subject - BiochemistryIsrasami NaparPas encore d'évaluation
- General Introduction and Basic Biochemical PrinciplesDocument6 pagesGeneral Introduction and Basic Biochemical PrinciplescelecosibPas encore d'évaluation
- Git Biochemistry of GitDocument9 pagesGit Biochemistry of GitSibatPas encore d'évaluation
- Sphingolipids: Dr. Abir Alghanouchi Biochemistry Department Sciences CollegeDocument12 pagesSphingolipids: Dr. Abir Alghanouchi Biochemistry Department Sciences CollegeUzac BenuPas encore d'évaluation
- Complex Carbohydrates - GlycoproteinsDocument28 pagesComplex Carbohydrates - GlycoproteinsJessica SannohPas encore d'évaluation
- Diabetes Mellitus: Overview and TreatmentsDocument36 pagesDiabetes Mellitus: Overview and TreatmentsGlenn Nadine N. Masicampo100% (1)
- Molecular CloningDocument61 pagesMolecular CloningDavi DzikirianPas encore d'évaluation
- Creatine and Creatinine MetabolismDocument108 pagesCreatine and Creatinine MetabolismMae Matira AbeladorPas encore d'évaluation
- Cholesterol - Synthesis, Metabolism, Regulation PDFDocument10 pagesCholesterol - Synthesis, Metabolism, Regulation PDFAdreiTheTripleA100% (1)
- Protein SynthesisDocument18 pagesProtein Synthesisabisantiago6131100% (1)
- 19.7 Membrane Lipids: Phospholipids: Group 3Document36 pages19.7 Membrane Lipids: Phospholipids: Group 3Masbateyeandlaser EyeclinicPas encore d'évaluation
- ChromatographyDocument31 pagesChromatographyarun231187Pas encore d'évaluation
- Metabolism of Carbohydrate: Department of Biochemistry Faculty of Medicine University of YARSI JakartaDocument60 pagesMetabolism of Carbohydrate: Department of Biochemistry Faculty of Medicine University of YARSI JakartaAmanda PutriPas encore d'évaluation
- Hexose Monophosphate ShuntDocument43 pagesHexose Monophosphate ShuntSecret AgentPas encore d'évaluation
- 3dr Year Common Biochemistry MCQsDocument8 pages3dr Year Common Biochemistry MCQsYasmin Amr NounouPas encore d'évaluation
- Cells and Secretions of The Pancreatic IsletsDocument4 pagesCells and Secretions of The Pancreatic IsletsSophia OcayPas encore d'évaluation
- LipidsDocument13 pagesLipidsalianaPas encore d'évaluation
- Biochemistry of GlycoproteinDocument6 pagesBiochemistry of GlycoproteinMahathir Mohmed100% (7)
- s15 Miller Chap 3b LectureDocument25 pagess15 Miller Chap 3b LectureDorice Clement100% (1)
- Fatty Acid Synthesis 11.12.19Document18 pagesFatty Acid Synthesis 11.12.19Sanreet Randhawa100% (1)
- Biochemistry - C4 Proteins Determination of Primary StructureDocument2 pagesBiochemistry - C4 Proteins Determination of Primary StructureKim LlamasPas encore d'évaluation
- Bioactive PeptidesDocument16 pagesBioactive PeptidesOmotoyinbo SegunPas encore d'évaluation
- Purine & Pyrimidine MetabolismDocument22 pagesPurine & Pyrimidine MetabolismManda100% (1)
- MCQ MembranesDocument10 pagesMCQ MembranesMarilyne RizkPas encore d'évaluation
- B Y: - Idr. Megha Gaur BDS IDocument89 pagesB Y: - Idr. Megha Gaur BDS IRishab GaurPas encore d'évaluation
- Glucose, Part1Document33 pagesGlucose, Part1SarahPas encore d'évaluation
- Abnormal Constituents of UrineDocument2 pagesAbnormal Constituents of UrineAries DocPas encore d'évaluation
- Digestive System: Digestion and Absorption of Carbohydrates, Proteins and FatsDocument45 pagesDigestive System: Digestion and Absorption of Carbohydrates, Proteins and FatshiPas encore d'évaluation
- 41 & 42 - Nucleic Acid MetabolismDocument56 pages41 & 42 - Nucleic Acid MetabolismMădă Claws100% (1)
- HMPDocument44 pagesHMPraanja2Pas encore d'évaluation
- Chemistryofproteinswithclinicalapplications 190621192525 PDFDocument181 pagesChemistryofproteinswithclinicalapplications 190621192525 PDFAl-waleed Julkanain100% (1)
- Cardiac MetabolismDocument35 pagesCardiac Metabolismwaqas_xsPas encore d'évaluation
- Hormones and Metabolic RegulationDocument40 pagesHormones and Metabolic RegulationEka HabinaPas encore d'évaluation
- Biochem - (With Answers and Achievement Chart)Document11 pagesBiochem - (With Answers and Achievement Chart)Valine Cysteine MethioninePas encore d'évaluation
- Practical BiochemistryDocument35 pagesPractical BiochemistryMockinjay100% (1)
- Format of Animal Development Practical Report:: Text Book Berbahasa Indonesia Atau Bahasa InggrisDocument14 pagesFormat of Animal Development Practical Report:: Text Book Berbahasa Indonesia Atau Bahasa InggrisSafira Dwi OktavianiPas encore d'évaluation
- Citric Acid Cycle - Pyruvate DehydrogenaseDocument33 pagesCitric Acid Cycle - Pyruvate Dehydrogenasesultan khabeeb100% (1)
- Ion TransportD'EverandIon TransportDavid KeelingPas encore d'évaluation
- Estella Carganilla - Presentation For Chaffee Zoo Animal 2018Document35 pagesEstella Carganilla - Presentation For Chaffee Zoo Animal 2018api-395970440Pas encore d'évaluation
- M220008 Euthaphen Product InsertDocument1 pageM220008 Euthaphen Product InsertnadirhussainbuzdarPas encore d'évaluation
- 1 Eye of Horus and Count Like An Egyptian 2Document6 pages1 Eye of Horus and Count Like An Egyptian 2yin yinPas encore d'évaluation
- Chapter 22 Maternal NotesDocument2 pagesChapter 22 Maternal NotesChin T. OndongPas encore d'évaluation
- Sample Paper 1 ENGLISHDocument22 pagesSample Paper 1 ENGLISHDhawan ChoudharyPas encore d'évaluation
- Jane Goodall: Navigation SearchDocument8 pagesJane Goodall: Navigation SearchVeena HegdePas encore d'évaluation
- NEBDN Dental Charting Book APRIL 2015Document13 pagesNEBDN Dental Charting Book APRIL 2015Avita RathPas encore d'évaluation
- Pedia BulletsDocument2 pagesPedia BulletsDebbiearlPas encore d'évaluation
- Minimal Pairs Pronunciation PracticeDocument2 pagesMinimal Pairs Pronunciation Practiceadrian ariasPas encore d'évaluation
- DanMachi Volume 05 - Chapter 1Document32 pagesDanMachi Volume 05 - Chapter 1Joe ForsaPas encore d'évaluation
- Sexout ModsDocument7 pagesSexout ModsPablo Huerdo100% (2)
- Harga Baru Nestle OkeDocument8 pagesHarga Baru Nestle OkeFitri 'cwett' WijayantiPas encore d'évaluation
- Schleich Knights MagazineDocument16 pagesSchleich Knights Magazinebananica28100% (1)
- Vitamins and Minerals: Nutrient (Vitamins) Needed For Key SourcesDocument4 pagesVitamins and Minerals: Nutrient (Vitamins) Needed For Key SourcesKevin Carl A. CorpuzPas encore d'évaluation
- PublicationDocument56 pagesPublicationAlvarin CsboyPas encore d'évaluation
- BeefDocument12 pagesBeefTheamicable Firstlady MarPas encore d'évaluation
- Algorithms For IV Fluid Therapy in Children and Young People in Hospital Set of 6 PDF 2190274957 PDFDocument6 pagesAlgorithms For IV Fluid Therapy in Children and Young People in Hospital Set of 6 PDF 2190274957 PDFFurqon AfandriPas encore d'évaluation
- Unit 2: Academic ReadingDocument5 pagesUnit 2: Academic ReadingJuliana XavierPas encore d'évaluation
- Major and Minor ConnectorsDocument6 pagesMajor and Minor ConnectorsAntal Barbela67% (3)
- Macros HunterDocument5 pagesMacros HunterikenconstructPas encore d'évaluation
- Connective TissueDocument21 pagesConnective TissueCalvin Lin Jia RongPas encore d'évaluation
- Report Text AboutDocument3 pagesReport Text Aboutawe riantoPas encore d'évaluation
- Zoology BiomesDocument11 pagesZoology BiomesDennie Zody LoganPas encore d'évaluation
- Hematology Basics NewDocument32 pagesHematology Basics Newkimbo23Pas encore d'évaluation
- RFTPS2CULTDocument7 pagesRFTPS2CULTAna Vaquer MontoroPas encore d'évaluation
- Narrative TextDocument5 pagesNarrative TextWinniePas encore d'évaluation
- Uas GenapDocument10 pagesUas GenapDream32apPas encore d'évaluation
- EpisiotomyDocument2 pagesEpisiotomydanur kusuma arini putriPas encore d'évaluation
- Kode TopografiDocument69 pagesKode TopografiMUHAMMAD RASYID RPas encore d'évaluation
- 上海市虹口区2017学年度第一学期期中考试七年级英语学科试卷(PDF版 答案)Document5 pages上海市虹口区2017学年度第一学期期中考试七年级英语学科试卷(PDF版 答案)Lidia HuangPas encore d'évaluation