Académique Documents
Professionnel Documents
Culture Documents
HAZMAT Articles - Titanium Tetrachloride
Transféré par
Radko BankrasDescription originale:
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
HAZMAT Articles - Titanium Tetrachloride
Transféré par
Radko BankrasDroits d'auteur :
Formats disponibles
HAZMAT Articles
Titanium Tetrachloride
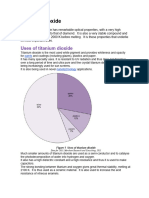
Introduction Titanium tetrachloride (TiCl4) contains four chlorine atoms which are bonded evenly around one titanium atom in the shape of a tetrahedron. It is a colourless to light yellow liquid, with irritating acidic vapours, which fume in air to give off a dense white smoke. It is often loosely called tickle. It is formed, as part of the process, to purify titanium dioxide, which has been extracted from mineral sands. So what is so important about titanium dioxide, that requires the manufacture of tickle? Titanium dioxide Titanium dioxide is an extremely stable, unreactive powder, that interacts with light in a way that makes it one of the whitest white materials available. It is ideal to use as a white pigment in both oil and water-based paints, as well as in paper, plastics, inks, and cosmetics. It is even allowed to be used this way in food and medicine. It is also used as a UV blocker in sunscreens, and in a range of high tech applications. Titanium dioxide has replaced the white pigments based on lead carbonate, that were once widely used. Properties of titanium dioxide Chemical name Titanium dioxide Formula TiO2 CAS 13463-67-7 Form White powder Specific gravity 4.23 Melting point 1870 deg C Solubility Insoluble Mineral sands Titanium dioxide as it is found in mineral sands, is anything but white. These sands are widespread throughout Western Australia,1 and they contain a range of minerals which are of value. They are:2 Rutile (TiO2) Impure titanium dioxide as brown red crystals. Specific gravity 4.3. Ilmenite (FeTiO3) Iron titanium oxide as black/grey crystals. Slightly magnetic with a specific gravity 4.5 to 5.0. Zircon (ZrSiO4) Zirconium silicate, usually off-white to cream in colour. Specific gravity 4.6 to 4.7. Monazite [(Ce,La,Nd,Th)PO4] Rare earth minerals and thorium as a phosphate, as brownish red crystals. Mildly radioactive due to thorium content (30%), and specific gravity of 4.6 to 5.4. The mineral content of these sands make them relatively heavy, and this enables these minerals to be separated from the lighter quartz fraction of sand, by a series of centrifuges and the use of magnets. Zircon and monazite are exported overseas for processing, while the rutile and ilmenite are processed to form ultra pure titanium dioxide. Manufacture of titanium tetrachloride To prepare titanium tetrachloride, rutile or ilmenite are heated with carbon, under a flowing stream of chlorine, that is heated to 900 deg C. TiO2 + 2Cl2 + 2C or 2FeTiO3 + 7Cl2 + 6C 2TiCl4 + 2FeCl3 + 6CO TiCl4 + 2CO
Titanium dioxide is formed again, either by reaction with water, or with pure oxygen. TiCl4 + H2O + TiO2 + 4HCl or 2TiCl4 + O2 TiO2 + Cl2 The second option is preferred, since it allows chlorine to be regenerated and this reduces costs. This process is also used worldwide to produce not only titanium dioxide, but also titanium metal and titanium sponge. Dangerous Goods Titanium tetrachloride is classified for the purposes of transport as Dangerous Goods: UN 1838 Class 8 Corrosive Packing Group II Hazchem Code 4WE Titanium tetrachloride however, is not transported in Western Australia, rather it is manufactured and consumed on-site in Kwinana, and in the Kemerton Industrial Park near Bunbury.
Page 1
Titanium Tetrachloride, 19th May 2009
Properties Chemical name: Formula Molecular weight CAS number Solubility Melting Point Boiling Point Specific gravity Vapour pressure Vapour density Flammability Conversion
Titanium tetrachloride TiCl4 189.7 7550-45-0 Reacts (extremely exothermic) -24 deg C 136.4 deg C 1.73 (water = 1.00) 1.3 kPa (21.3 deg C) 6.5 (air = 1.00) Does not burn 3 1 mg/m = 7.76 ppm 1 ppm = 0.129 mg/m3
There has been at least one recorded fatality from the effects of inhalation, after titanium tetrachloride splashed onto the face of a worker.5 Exposure hazards skin contact In another accident, three workers were splashed with titanium tetrachloride. However the situation was made worse when they were washed down with water. Titanium tetrachloride reacts so vigorously with water and in this case sufficient heat was generated to cause thermal burns. This enabled the corrosive effects of hydrochloric acid to penetrate more deeply, particularly in the areas covered by belts and boots. The consequences of both an acid and thermal burn, were far worse than if it had only been one or the other. Any approach to titanium tetrachloride vapours, that may require entry into white clouds requires breathing protection. And if for any reason, contact with the liquid occurs, it must first be wiped off, and then washed down under a decontamination shower.
Effect of spillages Whenever there is a spillage of titanium tetrachloride, the single most outstanding feature, is the production of dense, thick white clouds. Titanium tetrachloride vapour immediately reacts with moisture in the air, and once again it is the whiteness of the titanium dioxide that gives it this appearance. In reacting with moisture in air, it goes through a two stage process. First, it forms a range of compounds known as the titanium oxychlorides and hydrogen chloride.3 It can be described as follows: TiCl4 + H2O + TiOCl2 + 2HCl or TiCl4 + H2O + Ti(OH)nClx + HCl These oxychlorides are also particles, and they will continue to react with water to form hydrogen chloride. TiOCl2 + H2O + TiO2 + 2HCl Exposure hazards - inhalation The hazards of inhalation of titanium tetrachloride and its reaction products, is related to the formation of hydrogen chloride. However it has been found on experimental animals, that the toxic effect is much greater than for an equivalent amount of hydrogen chloride.4 Hydrogen chloride is extremely soluble in water, and if inhaled at concentrations within the capability of the body's defence system, it will be captured by the moisture in the upper respiratory tract and penetration into the lungs will be minimised. Hydrogen chloride resulting from the titanium tetrachloride reaction however, can be transported deep into the lungs. It can do this by being absorbed on titanium dioxide particles, or as semi-reacted titanium oxychlorides, or even as titanium tetrachloride vapour. And then once deep in the lungs, it is in a position to do far greater damage.
Incident in Guangzhou On 17 Aug 2006, a leak of titanium tetrachloride occurred in a factory in Guangzhou in China as 6 workers were dismantling a container of tickle. Look up the web-site while it is available for some interesting pictures.
1
5 6
Fact Sheet 10, http://www.dmp.wa.gov.au/documents/G SWA_MineralSandsPamphlet.pdf (15th April 2009) http://www.australianminesatlas.gov.au/education/down_ under/minerals_sands/Australia.html (15th April 2009) Hydrogen chloride once it is dissolved in water becomes hydrochloric acid. This will be its form once in the atmosphere, however for consistency, the term hydrogen chloride will continue to be used.. Toxicological Profile for Titanium Tetrachloride, US Department of Health and Human Services, Sept 1997, http://www.atsdr.cdc.gov/toxprofiles/tp101.pdf (15th April 2009) Ibid http://www.lifeofguangzhou.com/node_10/node_37/node_85/20 06/08/18/11558733167091.shtml (10th May 2009)
Prepared by Leith Higgins, Principal Scientific Officer, FESA Any comments 08 93239311 or Leith.Higgins@fesa.wa.gov.au Dec, 2008 by the Fire & Emergency Services Authority of Western Australia (FESA) This material is copyright and provided courtesy of FESA and published in the members only area of the AFAC Knowledge Web. The material is subject to the operation of the Copyright Act 1968 and its subsequent amendments. Material may be downloaded, displayed, printed and reproduced in unaltered form only for the sole use of AFAC members or partner organisations. This material may not be used for commercial purposes. Distribution of material from the members only area of the Knowledge Website to personnel outside of AFAC members and partner agencies is not permitted unless written authorisation is obtained from the content owner.
Titanium Tetrachloride, 19th May 2009
Page 2
Vous aimerez peut-être aussi
- Powerpoint Presentation On Extraction of TitaniumDocument31 pagesPowerpoint Presentation On Extraction of TitaniumLuis Sahoo90% (10)
- Kroll ProcessDocument5 pagesKroll ProcessNavarro SalgadoPas encore d'évaluation
- The Chemistry of Iron, Cobalt and Nickel: Comprehensive Inorganic ChemistryD'EverandThe Chemistry of Iron, Cobalt and Nickel: Comprehensive Inorganic ChemistryPas encore d'évaluation
- Nano Titania PowerPoint Presentationد رضويDocument40 pagesNano Titania PowerPoint Presentationد رضويArilu2010Pas encore d'évaluation
- Chapter 57 - Titanium - 2015 - Handbook On The Toxicology of MetalsDocument10 pagesChapter 57 - Titanium - 2015 - Handbook On The Toxicology of MetalsChanWingSanPas encore d'évaluation
- Review Extractive Metallurgy Titanium - Daffa HandyanDocument6 pagesReview Extractive Metallurgy Titanium - Daffa HandyanDaffa HandyanPas encore d'évaluation
- TiO2 Titanium Dioxide Extraction Production Project PresentationDocument48 pagesTiO2 Titanium Dioxide Extraction Production Project Presentationkaranved780% (5)
- Production of Titanium Tetrachloride (TiCl4) From Titanium Ores - A ReviewDocument49 pagesProduction of Titanium Tetrachloride (TiCl4) From Titanium Ores - A Reviewrazor75apPas encore d'évaluation
- Manufacture of Titanium DioxideDocument52 pagesManufacture of Titanium DioxideJayraj Dayma100% (1)
- Production of Titanium DioxideDocument16 pagesProduction of Titanium Dioxidehaisamdo100% (1)
- Titanium DioxideDocument18 pagesTitanium DioxidePrrinisha KanabathyPas encore d'évaluation
- Titanium : Karan Saxena Class Xi-A ROLL NO.-22Document18 pagesTitanium : Karan Saxena Class Xi-A ROLL NO.-22Karan SaxenaPas encore d'évaluation
- Uses of Titanium DioxideDocument5 pagesUses of Titanium DioxideAbdul RazzaquePas encore d'évaluation
- TITANIUM DIOXIDE Chemical and Technical AssessmentDocument8 pagesTITANIUM DIOXIDE Chemical and Technical AssessmentDi Stovall100% (1)
- Golongan Ivb - English VersionDocument20 pagesGolongan Ivb - English VersionAriska AndrainiPas encore d'évaluation
- Piru Seminar ReportDocument52 pagesPiru Seminar Reportsujit_sekharPas encore d'évaluation
- TitaniumDocument2 pagesTitaniumpotterhead1DPas encore d'évaluation
- TICL4Document7 pagesTICL4Chandra SekarPas encore d'évaluation
- Titanium Corrosion PDFDocument3 pagesTitanium Corrosion PDFSellappan MuthusamyPas encore d'évaluation
- Titaniun Dioxide Basic InformationDocument4 pagesTitaniun Dioxide Basic Informationalexandria.padilla11Pas encore d'évaluation
- New Microsoft PowerPoint PresentationDocument31 pagesNew Microsoft PowerPoint PresentationASHRAFUL KABIRPas encore d'évaluation
- History of TitaniumDocument12 pagesHistory of TitaniumjagadeesanbPas encore d'évaluation
- Recent Progress in Titanium Extraction and RecyclingDocument14 pagesRecent Progress in Titanium Extraction and Recyclingraihan dzakyPas encore d'évaluation
- 16-04-2020-1587032337-6 - Ijce-2. Ijce - Processing of Rutile Concentrates by FluorinationDocument18 pages16-04-2020-1587032337-6 - Ijce-2. Ijce - Processing of Rutile Concentrates by Fluorinationiaset123Pas encore d'évaluation
- Applications of Titanium TitaniumDocument1 pageApplications of Titanium TitaniumRicardo Rovira ChalerPas encore d'évaluation
- TitaniumDocument16 pagesTitaniumrifaldy100% (1)
- Preparation of Highly Pure Thorium Nitrate Via Thorium Sulfate and Thorium PeroxideDocument4 pagesPreparation of Highly Pure Thorium Nitrate Via Thorium Sulfate and Thorium PeroxideGyan PrameswaraPas encore d'évaluation
- Titanium Tetrachloride Production by The ChlorideDocument8 pagesTitanium Tetrachloride Production by The ChlorideMargarita CaceresPas encore d'évaluation
- Formation and Characterization of Nano Sized Tio Powder by Sol-Gel MethodDocument9 pagesFormation and Characterization of Nano Sized Tio Powder by Sol-Gel MethoddmaheswariPas encore d'évaluation
- Hydrometallurgy: Scott Middlemas, Z. Zak Fang, Peng FanDocument7 pagesHydrometallurgy: Scott Middlemas, Z. Zak Fang, Peng FanmonisalesPas encore d'évaluation
- Schm312 NotesDocument97 pagesSchm312 NotesSandile SynthaxError Mabika100% (1)
- Titanium Extraction Metallurgy Developments and Control of Impurity ElementsDocument18 pagesTitanium Extraction Metallurgy Developments and Control of Impurity Elementsgovind_galamPas encore d'évaluation
- Case Study: Testing of PetroGuard For Use As Hazardous Chemical Spill ControlDocument2 pagesCase Study: Testing of PetroGuard For Use As Hazardous Chemical Spill ControlGuardian Environmental TechnologiesPas encore d'évaluation
- Powder Technology: Juan A. Torres-Luna, Nancy R. Sanabria, José G. CarriazoDocument7 pagesPowder Technology: Juan A. Torres-Luna, Nancy R. Sanabria, José G. CarriazoClaudia Retamoso LlamasPas encore d'évaluation
- Life Cycle Assessment Comparison of Emerging and Traditional Titanium Dioxide Manufacturing ProcessesDocument11 pagesLife Cycle Assessment Comparison of Emerging and Traditional Titanium Dioxide Manufacturing ProcessesFelipe TavaresPas encore d'évaluation
- Titanium T1Document2 pagesTitanium T1ThunderkickPas encore d'évaluation
- KMML IV ReportDocument24 pagesKMML IV ReportSajeer Savad100% (1)
- Travancore Titanium Products Industrial Training ReportDocument75 pagesTravancore Titanium Products Industrial Training ReportKailas Sree Chandran86% (7)
- Anor TitaniumDocument16 pagesAnor Titaniumcahya larasatiPas encore d'évaluation
- Kang 2016Document8 pagesKang 2016mylover huPas encore d'évaluation
- Synthesis of Au/Tio2 Core-Shell Nanoparticles From Titanium Isopropoxide and Thermal Resistance Effect of Tio2 ShellDocument5 pagesSynthesis of Au/Tio2 Core-Shell Nanoparticles From Titanium Isopropoxide and Thermal Resistance Effect of Tio2 ShellSilvia AzabachePas encore d'évaluation
- Chloride: HZ+ H Tic144 Tic13 Hci Ti 3tic14 4tic13 Tic13 Hc1-Tic14 +HZDocument5 pagesChloride: HZ+ H Tic144 Tic13 Hci Ti 3tic14 4tic13 Tic13 Hc1-Tic14 +HZshruti JadhavPas encore d'évaluation
- Tannic Acid Selectively Extracting Titanium From Ilmenite Experimental and Theoretical Investigation 2168 9806 1000183Document5 pagesTannic Acid Selectively Extracting Titanium From Ilmenite Experimental and Theoretical Investigation 2168 9806 1000183Daniel MayorgaPas encore d'évaluation
- Cutting Tools Materials PDFDocument27 pagesCutting Tools Materials PDFAhmad DanielPas encore d'évaluation
- Processes For Recycling: 4.4.1.2.1 Conventional Kroll ProcessDocument14 pagesProcesses For Recycling: 4.4.1.2.1 Conventional Kroll Processelma watPas encore d'évaluation
- ChlorineProductionProcessSafetyPart IDocument48 pagesChlorineProductionProcessSafetyPart IVipin SomasekharanPas encore d'évaluation
- Mini Talk ChemistryDocument11 pagesMini Talk ChemistryMaria Alison SwiftPas encore d'évaluation
- A Toxic Hot Spot in KeralaDocument7 pagesA Toxic Hot Spot in KeralaVishnu BalachandranPas encore d'évaluation
- Trichloroethylene ThesisDocument62 pagesTrichloroethylene Thesisessakkirajm19902116100% (1)
- A Literature Review of Titanium Metallurgical ProcessesDocument12 pagesA Literature Review of Titanium Metallurgical ProcessesMargarita CaceresPas encore d'évaluation
- Value-In-Use Model For Chlorination of Titania FeedstocksDocument10 pagesValue-In-Use Model For Chlorination of Titania FeedstocksFelipe TavaresPas encore d'évaluation
- Photocatalytic Degradation of Chromium (Vi) From Wastewater Using Nanomaterials Like Tio, Zno, and CdsDocument9 pagesPhotocatalytic Degradation of Chromium (Vi) From Wastewater Using Nanomaterials Like Tio, Zno, and Cdsahmet yavaşPas encore d'évaluation
- 2011 MTDocument9 pages2011 MTSalim ChahidPas encore d'évaluation
- Advance in Titanium ProductionDocument9 pagesAdvance in Titanium ProductionAde SatriaPas encore d'évaluation
- Benelite Process For Upgradation of IllemniteDocument6 pagesBenelite Process For Upgradation of Illemnitemadangk100% (1)
- 10.1016 J.jallCOM.2005.10.048 Extraction of Titanium From Different Titania Precursors by The FFC Cambridge ProcessDocument9 pages10.1016 J.jallCOM.2005.10.048 Extraction of Titanium From Different Titania Precursors by The FFC Cambridge ProcessHooman BaghbanPas encore d'évaluation
- Oxide Titanium Ti O PigmentDocument10 pagesOxide Titanium Ti O Pigmentapi-27149699Pas encore d'évaluation
- Paper Cloracion y Reduccion Con MagnesioDocument3 pagesPaper Cloracion y Reduccion Con MagnesioBryan Roncal LlajarunaPas encore d'évaluation
- Joget Mini Case Studies TelecommunicationDocument3 pagesJoget Mini Case Studies TelecommunicationavifirmanPas encore d'évaluation
- HOPE 2A MODULE 1 Introduction To SportsDocument11 pagesHOPE 2A MODULE 1 Introduction To SportsChristian Ray Lucnagan ReyesPas encore d'évaluation
- Tucker CarlsonDocument4 pagesTucker CarlsonDai ZPas encore d'évaluation
- сестр главы9 PDFDocument333 pagesсестр главы9 PDFYamikPas encore d'évaluation
- MBA Advantage LRDocument304 pagesMBA Advantage LRAdam WittPas encore d'évaluation
- MacDonald, J.& MacDonald, L. (1974)Document17 pagesMacDonald, J.& MacDonald, L. (1974)Mariuca PopescuPas encore d'évaluation
- Reply Speeches What Are They?Document2 pagesReply Speeches What Are They?Yan Hao Nam89% (9)
- Case Study: Direct Selling ConceptDocument20 pagesCase Study: Direct Selling Conceptbansi2kk0% (1)
- Analyzing Text - Yuli RizkiantiDocument12 pagesAnalyzing Text - Yuli RizkiantiErikaa RahmaPas encore d'évaluation
- Rapid Prototyping and Rapid Tooling TheDocument24 pagesRapid Prototyping and Rapid Tooling TheGopinath GangadhariPas encore d'évaluation
- Cropanzano, Mitchell - Social Exchange Theory PDFDocument28 pagesCropanzano, Mitchell - Social Exchange Theory PDFNikolina B.Pas encore d'évaluation
- Report Web AuditDocument17 pagesReport Web Auditanupprakash36Pas encore d'évaluation
- ILO Report On Disability and Labour India - 2011wcms - 229259Document56 pagesILO Report On Disability and Labour India - 2011wcms - 229259Vaishnavi JayakumarPas encore d'évaluation
- Science 7 Las 1Document4 pagesScience 7 Las 1Paris AtiaganPas encore d'évaluation
- Small Scale Business ReportDocument28 pagesSmall Scale Business ReportJatin PahujaPas encore d'évaluation
- Abnormal Menstrual Cycle - MenorrhagiaDocument5 pagesAbnormal Menstrual Cycle - MenorrhagiaSandhyaPrem100% (1)
- TDS 39987 Easycoat Profile Decor 3MM Euk GBDocument3 pagesTDS 39987 Easycoat Profile Decor 3MM Euk GBp4pubgwalyPas encore d'évaluation
- The Liberal Welfare Reforms 1906Document5 pagesThe Liberal Welfare Reforms 1906Skye G-sPas encore d'évaluation
- Effects of Monetory PolicyDocument5 pagesEffects of Monetory PolicyMoniya SinghPas encore d'évaluation
- C Programming Bit Bank U-1, U-2Document17 pagesC Programming Bit Bank U-1, U-2HariahPas encore d'évaluation
- My Cook BookDocument66 pagesMy Cook BookAkshay KumariPas encore d'évaluation
- India: Labor Market: A Case Study of DelhiDocument4 pagesIndia: Labor Market: A Case Study of DelhiHasnina SaputriPas encore d'évaluation
- Enter Pre NaurDocument82 pagesEnter Pre NaurNeha singhalPas encore d'évaluation
- DS ClozapineDocument3 pagesDS ClozapineMiggsPas encore d'évaluation
- IEEE 802.1adDocument7 pagesIEEE 802.1adLe Viet HaPas encore d'évaluation
- 007-012477-001 SAS Token Guide OTP Hardware Token RevEDocument14 pages007-012477-001 SAS Token Guide OTP Hardware Token RevEBarons ArismatPas encore d'évaluation
- Chapter 1: Introduction Aviation Industry: Mini Project On Vistara AirlinesDocument84 pagesChapter 1: Introduction Aviation Industry: Mini Project On Vistara Airlinesselvaraj rapakaPas encore d'évaluation
- Educational Institutions: Santos, Sofia Anne PDocument11 pagesEducational Institutions: Santos, Sofia Anne PApril ManjaresPas encore d'évaluation
- Collec The PassportDocument2 pagesCollec The PassportvijikaPas encore d'évaluation
- Knowledge About Visha - Upavisha & Metalic Preparations - Knowledge of Formulations Containing Visha - Upavisha & Rasa AushadhisDocument154 pagesKnowledge About Visha - Upavisha & Metalic Preparations - Knowledge of Formulations Containing Visha - Upavisha & Rasa AushadhisPRASHNT SINGHPas encore d'évaluation
- ICH Quality Guidelines: An Implementation GuideD'EverandICH Quality Guidelines: An Implementation GuideAndrew TeasdalePas encore d'évaluation
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactD'EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactÉvaluation : 5 sur 5 étoiles5/5 (5)
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincD'EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincÉvaluation : 3.5 sur 5 étoiles3.5/5 (137)
- AP® Chemistry Crash Course, For the 2020 Exam, Book + Online: Get a Higher Score in Less TimeD'EverandAP® Chemistry Crash Course, For the 2020 Exam, Book + Online: Get a Higher Score in Less TimeÉvaluation : 5 sur 5 étoiles5/5 (1)
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsD'EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsÉvaluation : 5 sur 5 étoiles5/5 (3)
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactD'EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactÉvaluation : 5 sur 5 étoiles5/5 (1)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeD'EverandChemistry for Breakfast: The Amazing Science of Everyday LifeÉvaluation : 4.5 sur 5 étoiles4.5/5 (14)
- It's Elemental: The Hidden Chemistry in EverythingD'EverandIt's Elemental: The Hidden Chemistry in EverythingÉvaluation : 4 sur 5 étoiles4/5 (10)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeD'EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeÉvaluation : 4 sur 5 étoiles4/5 (1)
- Taste: Surprising Stories and Science About Why Food Tastes GoodD'EverandTaste: Surprising Stories and Science About Why Food Tastes GoodÉvaluation : 3 sur 5 étoiles3/5 (20)
- Guidelines for Defining Process Safety Competency RequirementsD'EverandGuidelines for Defining Process Safety Competency RequirementsÉvaluation : 3 sur 5 étoiles3/5 (1)
- The Production of Volatile Oils and Perfumery Plants in the United StatesD'EverandThe Production of Volatile Oils and Perfumery Plants in the United StatesPas encore d'évaluation
- Formulating, Packaging, and Marketing of Natural Cosmetic ProductsD'EverandFormulating, Packaging, and Marketing of Natural Cosmetic ProductsPas encore d'évaluation
- AP Chemistry Flashcards, Fourth Edition: Up-to-Date Review and PracticeD'EverandAP Chemistry Flashcards, Fourth Edition: Up-to-Date Review and PracticePas encore d'évaluation
- The Periodic Table: A Very Short IntroductionD'EverandThe Periodic Table: A Very Short IntroductionÉvaluation : 4.5 sur 5 étoiles4.5/5 (3)
- Handbook of Formulating Dermal Applications: A Definitive Practical GuideD'EverandHandbook of Formulating Dermal Applications: A Definitive Practical GuidePas encore d'évaluation
- Chemistry for Breakfast: The Amazing Science of Everyday LifeD'EverandChemistry for Breakfast: The Amazing Science of Everyday LifeÉvaluation : 4.5 sur 5 étoiles4.5/5 (90)
- Tribology: Friction and Wear of Engineering MaterialsD'EverandTribology: Friction and Wear of Engineering MaterialsÉvaluation : 5 sur 5 étoiles5/5 (1)
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolD'EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolPas encore d'évaluation
- The Billion-Dollar Molecule: The Quest for the Perfect DrugD'EverandThe Billion-Dollar Molecule: The Quest for the Perfect DrugÉvaluation : 5 sur 5 étoiles5/5 (2)
- Essential Chemistry for Formulators of Semisolid and Liquid DosagesD'EverandEssential Chemistry for Formulators of Semisolid and Liquid DosagesÉvaluation : 5 sur 5 étoiles5/5 (2)