Académique Documents
Professionnel Documents
Culture Documents
Lab Report
Transféré par
they12Description originale:
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Lab Report
Transféré par
they12Droits d'auteur :
Formats disponibles
Examination of light spectra and leaf pigments with reference to photosynthesis ABSTRACT This study examined properties, including
polarity, of different pigments that make up a plant leaf and the role each pigment plays in photosynthesis when coupled with certain wavelengths of light. Since pigments reflect light of the color that is seen and absorb all others, a green pigment such as chlorophyll should absorb red and blue light and utilize it in photosynthesis to eventually produce starch while reflecting green light and making no use of it. In testing different colors of filtered light, factors such as the poor strength of sunlight as well as the disadvantageous location in which the plants were positioned did not allow them to absorb any color of light and consequently they produced no starch. Due to the fact that green chlorophyll contributes to photosynthesis while blue and pink anthocyanins do not, it was believed and shown with the reagent that pink regions, after being given sunlight for days, will test negative for starch and that green regions and purple regions (being a combination of green and pink) will test positive because some chlorophyll is present in each. Through paper chromatography, it was verified that, due to the number of polar oxygen atoms present in each molecule, of the following four pigments-chlorophyll a, chlorophyll b, beta carotene, and xanthophylls- chlorophyll b is the most polar while carotene is the least polar. With the use of a spectrophotometer, the absorption spectrum of each of the four previously mentioned pigments was obtained and it was found that chlorophyll a gives the highest absorption therefore being the most important and contributing the most to photosynthesis while the other three serve as accessory pigments and broaden the spectrum of light available to use. INTRODUCTION The energy that drives photosynthesis and consequently all of life on earth comes from light energy emitted from the sun in the form of photons. The energy of these photons can be expressed in terms of their frequency or inversely, its wavelength , and is given as = = (1) where h is the Planck constant equal to 6.63 10 and c is the speed of light equal to 2.997 10 / . The study of photosynthesis is concerned only with the photons whose wavelengths are within the visible light spectrum, which falls between 380 and 750 nanometers (1 = 10 ). Plants contain substances known as pigments that can absorb photons with wavelengths within this spectrum and thus can absorb some of the energy carried. In photosynthesis, plants convert this absorbed light energy into chemical energy while also utilizing and converting carbon dioxide and water into glucose and oxygen, respectively. This process can be summarized in the following equation: +6 +6 +6 (2) The glucose produced by photosynthesis can then be used by the plants in cellular respiration or passed and utilized in animals that consume the plants. Plants can also connect molecules of glucose to form the polysaccharide starch, enabling it to efficiently store surplus glucose (Campbell & Reece, et. al, 2008). Not all visible light is absorbed by pigments of plants. Photons of green light (wavelength of 520-650 nm) is reflected and transmitted by plants, which is precisely why plants appear green. Violet-blue and red light, which falls outside this reflected wavelength range, is absorbed and used for photosynthesis. One of the goals of this study was to investigate the effect that filtering white light, a mixture of all wavelengths of visible light, would have on photosynthesis. 1
A filter allows only light within certain wavelengths to pass, such as blue light, green light, red light, etc. while blocking all others. Given that light is passed onto different plants either through black construction paper or through green, red, or blue plastic filters, it can be assumed that photosynthesis will occur only in areas where red and blue light are transmitted. Since photosynthesis produces glucose and glucose can be used to form starch, one of the ways to detect for photosynthesis would be to test a plant for starch. The iodine reagent, I KI, changes from a transparent yellow color to a blue-black complex in the presence of starch and so would be useful in detecting for photosynthesis. If photosynthesis will occur in regions where blue and red light are transmitted by the filter, then starch will be produced and the leaf under the blue and red filters will turn dark blue in the presence of I KI, but the leaf areas under black and green filters will experience no effect. Plant leaves possess not only pigments that facilitate photosynthesis but a variety of other, accessory pigments as well. Research has shown that the pigment which contributes the most to photosynthesis is chlorophyll a, which absorbs blue and red light while reflecting green light. The accessory pigment chlorophyll b absorbs light within a very similar range of wavelength as chlorophyll a, while other pigments known as carotenoids have a different absorption spectrum, yet are still photosynthetically useful (Campbell & Reece, et. al). Carotenoids account for yellow, orange, and bright red spots that are found in some areas of leaves because they, unlike chlorophyll, reflect and transmit these colors of light. Both carotenoids and chlorophyll pigments are found in the chloroplasts of plants. There are also pigments located in the cell vacuole called anthocyanins that reflect blue, purple, and pink colors and do not contribute to photosynthesis. A mixture of the pigments in cells can produce additional colors (Morgan & Carter, 2008). It can be inferred that, looking at a leaf with green, purple, and pink regions, photosynthesis will occur only in the green area and possibly the purple area (if purple is a combination of chlorophyll and anthocyanin pigments). Testing for photosynthesis in a similar manner described in the previous paragraph, if photosynthesis occurs in the presence of green chlorophyll pigment, then an iodine (I KI) test will give a black stain only in the green and purple areas of a leaf. Any white regions on a leaf can be disregarded because white areas transmit and reflect all light, absorbing nothing that can be used for photosynthesis. It is possible to separate plant pigments in solution by exploiting the polarity of each molecule. Chlorophyll a, chlorophyll b, beta carotene, and xanthophyll all possess certain degrees of polarity based on the number of electronegative elements they possess- in this case, the number of oxygen atoms. It is clear that, with its two pairs of valence electrons, oxygen is electronegative. It tends to create a dipole by having one partially negative area due to the oxygen and one partially positive one due to some relatively neutral element such as carbon, which possesses no valence electron pairs. The more of these regions that are present in a molecule, the greater its polarity will be. By counting the number of oxygens present in different molecules, their relative polarities can be determined. In this study, by examining the molecular structure of each pigment, it was believed that the order of polarity of the each, from most polar to least polar, was chlorophyll b, chlorophyll a, xanthophyll, and beta carotene. Crucial to understanding how molecules can be separated based on polarity is to note that polar molecules are attracted to or tend to dissolve in substances that are polar, while nonpolar molecules are attracted to or tend to dissolve in nonpolar substances (Morgan & Carter, 2008). In a separation technique called paper chromatography, a small amount of a mixture of substances with varying polarity is placed on a piece of chromatography paper, which is a polar substance, and a nonpolar solvent is run up the paper until it reaches a certain point. The substances in the mixture that are 2
least polar tend to dissolve best in the nonpolar solvent and therefore tend to travel farther up the chromatography paper, while the more polar substances tend to remain attached to the polar paper, not dissolving well in the solvent and not traveling far. Given a mixture of chlorophyll a, chlorophyll b, xanthophyll, and beta carotene to use in paper chromatography and knowing the molecular structure of each molecule, it can be hypothesized that carotene is the least polar pigment and will be the most soluble in the solvent, followed by xanthophyll, chlorophyll a, and chlorophyll b, which is the most polar and will be the least soluble. If this assumption is true, then carotene will travel the greatest distance on the paper, followed by xanthophyll, chlorophyll a, and chlorophyll b, which will travel the least distance. The absorption spectrum, defined as the absorption pattern for a particular pigment, shows the relative absorbance at different wavelengths of light and can be determined through a process called spectrophotometry. Spectrophotometry uses a device called a spectrophotometer or calorimeter which holds a sample of the material to be studied as light of different wavelengths is shined through it. A spectrophotometer is able to show the percent transmittance of light of a particular wavelength through the material and from this it is possible to calculate the absorbance. It is important to know the range of wavelengths of light that are best absorbed by plant pigments because these wavelengths are important to photosynthesis. When people look at an object and see its color, what they are really seeing is the color of light reflected by that object. It can then be said that pigments, including chlorophyll, carotene, and xanophyll, will reflect and transmit light of the color seen and absorb all others. If pigments reflect the light of the color seen and absorb all others, then it can be predicted that chlorophyll a and b should absorb red and blue, xanophyll should absorb blue, and carotene should absorb green. Converting these colors to wavelengths, chlorophyll a will absorb light with wavelengths of , chlorophyll b will absorb those around 450 and 640 , and roughly around 430 and 660 carotene and xanophyll will absorb those between approximately 450-550 . MATERIALS AND METHODS Four days before any experiments were performed, several pieces of red, blue, and green plastic filter and black construction paper were cut out. Each piece was rectangular in shape and by 4.8-5.0 . Several leaves of roughly the same shape and size were roughly 2.5-2.7 selected from a geranium plant. One piece of filter or black construction paper was folded onto each leaf and secured with a paper clip, so that there was one leaf for each different color. The geranium plant was placed on a windowsill and exposed to light. After four days, leaves possessing the attachments were taken from the geranium plants and sketched, with attention given to the location of the papers and filters. The attachments were removed and the leaves were placed in a beaker containing an ethanol solution. This beaker was then placed in a larger beaker containing water and located on a hot plate. The hot plate was turned on and the solution was allowed to heat until boiling, effectively removing pigments from the leaves until they lost their color. The leaves were taken from the beaker and placed in a Petri dish, where they were treated with water and then . The leaves were allowed to sit for 6 minutes as they absorbed the iodine reagent. Finally, the colors of each leaf were recorded, noting which leaves and which areas on each leaf were particularly black, indicating large amounts of starch. In order to explore which pigments support photosynthesis, Coleus leaves were given a treatment identical to the one given to the geranium leaves. A multicolored leaf was taken from a Coleus plant and sketched, labeling the colors of each region of the plant. The leaf was placed in a beaker of ethanol solution, which was then placed in another beaker of water on a hot plate. 3
The solution was boiled until the color of the leaf disappeared and the pigments were extracted, then the leaf was removed from the beaker and placed in a Petri dish. The leaf was treated with water and then iodine reagent until the water was amber in color. After leaving the leaf undisturbed for about 7 minutes, its color was examined and recorded, making note of which areas were blacker than others and therefore possessed more starch. The final results were compared with the initial sketch of the leaf possessing the different colored pigments. A mixture of chlorophyll a, chlorophyll b, beta carotene, and xanthophyll pigments was obtained by extracting it from fresh spinach. Chromatography paper was also obtained. A light pencil line was drawn roughly 1.5 from the bottom of the paper. Then a capillary pipette was used to draw some pigment mixture and streak it across the drawn pencil line. After the mixture was allowed to dry, the paper was rolled into a cylinder and fixed with a paper clip. The paper was placed in a quart chamber containing a nonpolar organic solvent made up of acetone and petroleum ether. The lid was carefully placed on top of the jar and the solvent was allowed to run up the paper, carrying the pigments with it. When the leading edge, or front, of the solvent reached the top of the paper, the paper was taken from the chamber. The paper clip was removed and the paper was allowed to dry. The pigments were located on the paper and their distance traveled from the origin was recorded. The final procedure made use of the separated pigments obtained from the previous one. Each pigment was cut out of the chromatography paper and all were placed in separate small beakers with acetone. The beakers were swirled. A separate chromatography paper possessing separated pigments was also used, as all pigments were cut out and all were placed in a single beaker filled with acetone. The contents of all beakers were poured into separate cuvettes. Pure acetone was also poured into a separate cuvette to serve as the reference material (control) in the experiment. After the spectrophotometer was zeroed and calibrated, readings were taken for each pigment at different wavelengths. The percent transmittance for each pigment, including the and increased in mixture of pigments, was recorded for each wavelength beginning at 400 increments of 20 until 720 was reached, making sure to recalibrate the instrument every time the wavelength was increased. Using a conversion table, the percent transmittance was able to be converted to absorption density. RESULTS All leaves with any sort of cover, including red and blue filters, did not turn black when treated with the iodine reagent and therefore tested negative for starch, indicating that little or no photosynthetic activity had taken place in the days when the leaves were exposed to sunlight. Color Green Purple Pigments Starch Present (Predicted) Yes Yes Starch present (actual results) Yes Yes
Chlorophyll a &b Chlorophylls, anthocyanin Pink Anthocyanin No No White None No No Table 1 Table 1, shown above, gives a summary of the results found by applying iodine reagent to different colored regions of a Coleus leaf after the pigments had been removed. Referring to table 1, it is clear that chlorophyll a & b supported photosynthesis and the green regions as well 4
as the purple regions both contained starch. Before the Coleus leaf was tested, a sketch was made that recorded the different colored regions, giving particular emphasis to the green and purple areas because those were believed to contain pigments that would contribute to photosynthesis and produce starch. After the test, those emphasized regions were black. When the chromatography paper was examined after removing it from the chamber, it was found that carotene, a golden yellow pigment, had traveled the farthest distance up the paper. This indicated that carotene was, in fact, the least polar of the four pigments. Below carotene was the pale yellow pigment xanthophyll, and below xanthophyll were chlorophyll a and then chlorophyll b. Chlorophyll a and b were difficult to distinguish because both were very similar in color, the only difference being that chlorophyll a was more of a grass green color while chlorophyll b was more yellow-green. Finding that chlorophyll b traveled the least distance indicated that it was the most polar of the four pigments.
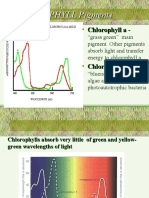
Absorption Spectrum for Leaf Pigments
2.5 2 1.5 Absorbance 1 0.5 0 380 480 580 Wavelength (nm) 680 780 Chlorophyll a Chlorophyll b Xanthophyll Carotene Total Pigment
Figure 1. Absorbance of pigments vs. wavelength of light shined through them. Figure 1, shown above, gives the absorption spectrum plotted for each pigment, including total pigment. Chlorophyll a, shown in the blue legend, gives the highest absorption out of any of the pigments, peaking at around 2.3 at 430 . Chlorophyll b gives the second highest absorption, peaking at slightly higher wavelengths than for chlorophyll a. The total pigment also gives appreciable absorption, particularly at around 1.7 at 440 . The other two lone pigments, xanthophyll and carotene, dont really absorb light very well at any wavelengths. DISCUSSION Of all the experiments performed, the one that gave the poorest results was the geranium leaf covered with filters. By obtaining a negative test for starch regardless of the filter covering the leaf essentially suggests that no light is absorbed by leaves and that photosynthesis never occurs. It is thought that the only leaves that should give a negative test for starch are those covered with either black construction paper or a green filter. Black construction paper would absorb all wavelengths of light, transmitting nothing. A green filter would pass only green light, 5
which is not absorbed well by pigments. Although the hypothesis that red and blue light is absorbed and contributes to photosynthesis is not supported, it should not be dismissed. The experiment took place in the middle of autumn when the sun was becoming weaker and thus not giving strong light, which could account for why results were so poor. Additionally, the geranium plant was not placed in an optimal position to receive light, so it could also be believed that perhaps it was not a matter of the sun not being strong enough, but rather that not enough light was given. If the experiment was performed again at a more favorable time of the year, like the summer or late spring, in conditions that ensured the plant would be given a copious amount of sunlight, results would likely be different. Results for the Coleus leaf were positive. As hypothesized, photosynthesis took place in the green and purple regions containing chlorophyll. The pink and white areas, which lacked photosynthetic pigments, did not support photosynthesis. These results correct for the common misconception that only the green areas on a leaf play a role in photosynthesis. Based on the order of the pigments migrating up the chromatography paper, the hypothesis that chlorophyll b is the most polar and carotene is the least polar is supported. In examining the molecular structure of chlorophyll a and b, it is found that the two molecules are identical except that chlorophyll a holds an extra methyl group, whereas chlorophyll b holds an extra, more polar aldehyde group. This small difference is reflected in the results, where the two chlorophylls are so tightly packed together that it is difficult to tell which of the two has moved the farthest. Beta carotene and xanthophyll are mostly hydrocarbons, which are naturally nonpolar, and their structure is also reflected in the results, where both have traveled considerably farther up the paper than chlorophyll. From the absorption spectrum, it can be concluded that chlorophyll a absorbs the greatest amount of visible light and therefore is the most important in the process of photosynthesis. Chlorophyll a, being green in color, is shown to reflect green, which is in the range of 520-560 . This is precisely the range where absorption was lowest for chlorophyll a. Therefore, the hypothesis that pigments will reflect and transmit light of their own color while absorbing all others is supported. It is shown that the optimal wavelengths for chlorophyll a are roughly 430 and 660 , which are close to the predicted values. For chlorophyll b, the best wavelengths , deviating slightly from the predictions. The other two appear to be around 500 and 700 pigments, particularly carotene, appear to absorb light very poorly. It was predicted that both would absorb light best around 450-550 . Xanthophyll only partially follows this prediction; carotene doesnt absorb light well for any wavelength range. Any deviations from the predicted results could have come from an error in the spectrophotometer. Perhaps for some parts of the data collection it was not calibrated properly. The findings of this study, especially the results taken from the last experiment regarding the absorption spectrum, help to answer a key question of why there exist pigments in plants in addition to chlorophyll a. Clearly chlorophyll a is the best pigment for photosynthesis and so it might be thought that the others are unnecessary. Chlorophyll b, xanthophyll, and beta carotene are often called accessory pigments because, while they are not as strong as chlorophyll a, they increase the spectrum of light available for the plant to use for photosynthesis. When these pigments absorb light and energy, it is eventually passed to chlorophyll a via a light harvesting complex (Campbell & Reece, et. al, 2008). Though it appears that carotene, a carotenoid, is useless because it does not absorb light well, as can be seen from figure 1, it has been proven in other studies that this pigment is responsible for photoprotection. Carotene helps to absorb and dissipate excessive light energy that would otherwise damage chlorophyll a (Campbell & Reece, 6
et. al, 2008). The pigments in a plant all serve a function and all ultimately work together in order to carry out photosynthesis, one of lifes essential processes.
LITERATURE CITED Campbell, N., and J. Reece. Biology, 8th ed. San Francisco, CA. Benjamin Cummings, 2008. Morgan, J. and Carter, M. Investigating Biology, 6th ed. San Francisco, CA. Benjamin Cummings, 2008.
Vous aimerez peut-être aussi
- Activity 5 Lab ReportDocument5 pagesActivity 5 Lab ReportJay MarcosPas encore d'évaluation
- PHOTOSYNTHESIS: Pigments of The Chloroplasts: Naira Mitz Ampuan BS Biology StudentDocument5 pagesPHOTOSYNTHESIS: Pigments of The Chloroplasts: Naira Mitz Ampuan BS Biology Studentnaira mitzPas encore d'évaluation
- Color ReflectionsDocument14 pagesColor ReflectionsIan Joshua SanchezPas encore d'évaluation
- BiologyDocument21 pagesBiologyHrituraj banikPas encore d'évaluation
- The Study of Chlorophyll Content in Various PlantsDocument24 pagesThe Study of Chlorophyll Content in Various PlantsShubham Raj72% (54)
- Nishant Mishra: Dav Public School Kalinga NagarDocument24 pagesNishant Mishra: Dav Public School Kalinga NagarNishant MishraPas encore d'évaluation
- Ivestigatory Project On Biology 12Document24 pagesIvestigatory Project On Biology 12Nishant MishraPas encore d'évaluation
- Amount of Chlorophyll in Five LeafDocument24 pagesAmount of Chlorophyll in Five LeafRakshitha MgowdaPas encore d'évaluation
- La Idea Química: Camouflage PigmentationDocument10 pagesLa Idea Química: Camouflage PigmentationCristinaPas encore d'évaluation
- Cholorophyll Content in Five Different Species of PlantDocument14 pagesCholorophyll Content in Five Different Species of Plantlatest tamil moviesPas encore d'évaluation
- AP Lab 4 PhotosynthesisDocument6 pagesAP Lab 4 PhotosynthesisJack LiuPas encore d'évaluation
- The Study of Chlorophyll Content in Various PlantsDocument16 pagesThe Study of Chlorophyll Content in Various PlantssasidharanPas encore d'évaluation
- Biology Project 1Document23 pagesBiology Project 1Aashray KothaPas encore d'évaluation
- General Biology 1 Quarter 2 WEEK 1 Module 1bDocument15 pagesGeneral Biology 1 Quarter 2 WEEK 1 Module 1bNormal Fan100% (1)
- Chromatography LabDocument2 pagesChromatography LabNez SabanogluPas encore d'évaluation
- Laboratory 1. ANALYSIS OF PLANT PIGMENTS USING PAPER CHROMATOGRAPHYDocument8 pagesLaboratory 1. ANALYSIS OF PLANT PIGMENTS USING PAPER CHROMATOGRAPHYGualberto Tampol Jr.Pas encore d'évaluation
- Chemistry of Colours: Jayanthi ChandrasekaranDocument10 pagesChemistry of Colours: Jayanthi ChandrasekaranFisnik Y. LimaniPas encore d'évaluation
- WEEK 2 Color ReflectionsDocument31 pagesWEEK 2 Color Reflectionsjhon achilles dugoPas encore d'évaluation
- Sci PaperDocument16 pagesSci PaperkarinadegomaPas encore d'évaluation
- 436154638-The-Study-of-Chlorophyll-Content-in-Various-Plants 4.docx - 20231113 - 184531 - 0000Document11 pages436154638-The-Study-of-Chlorophyll-Content-in-Various-Plants 4.docx - 20231113 - 184531 - 0000Saran.kPas encore d'évaluation
- Photosynthesis in Higher PlantsDocument22 pagesPhotosynthesis in Higher PlantsSourav WisePas encore d'évaluation
- Cbse BioDocument20 pagesCbse BioHrituraj banikPas encore d'évaluation
- LAB REPORT CromatoghraphyDocument7 pagesLAB REPORT CromatoghraphySyed Shafiq Syed ZainiPas encore d'évaluation
- Biology Investigatory ProjectDocument18 pagesBiology Investigatory ProjectSekhar Sahoo100% (4)
- Biology Investigatory ProjectDocument18 pagesBiology Investigatory ProjectSekhar SahooPas encore d'évaluation
- Handouts Gen Bio Topic 2Document8 pagesHandouts Gen Bio Topic 2Mark Kenneth CastilloPas encore d'évaluation
- A.P. Biology Lab #4 PhotosynthesisDocument5 pagesA.P. Biology Lab #4 PhotosynthesisLiz Marie NunezPas encore d'évaluation
- 13 Photosynthesis NotesDocument5 pages13 Photosynthesis NotesDr. Thomas GeorgePas encore d'évaluation
- Lab 4 Photosynthesis and Plant PigmentsDocument5 pagesLab 4 Photosynthesis and Plant PigmentszaidaPas encore d'évaluation
- Apolonio, Carangian, Sanchez & Tiglao Bio 150 Lec 2: SLIDE 00: Photosynthesis Slide 2Document9 pagesApolonio, Carangian, Sanchez & Tiglao Bio 150 Lec 2: SLIDE 00: Photosynthesis Slide 2Shirley ApolonioPas encore d'évaluation
- CPH-101-Fundamentals of Crop PhysiologyDocument105 pagesCPH-101-Fundamentals of Crop PhysiologyShivansh GurjarPas encore d'évaluation
- 5A 2 Chloroplasts and ChlorophyllDocument11 pages5A 2 Chloroplasts and Chlorophyllkarima akterPas encore d'évaluation
- 12 - Photosynthesis in Higher PlantsDocument5 pages12 - Photosynthesis in Higher PlantsShruti KalePas encore d'évaluation
- SPECTROPHOTOMETRYDocument3 pagesSPECTROPHOTOMETRYSuçsuz SuçluPas encore d'évaluation
- Corrected Photosynthesis LabDocument4 pagesCorrected Photosynthesis LabAbby Shay GaylePas encore d'évaluation
- Chloroplasts and Chlorophyll T5-3Document4 pagesChloroplasts and Chlorophyll T5-3Kyile FernandoPas encore d'évaluation
- Plant Pigments Gen Bio 1 Q2 Week 2 by Sir Leo JR and Sir Bil RamosDocument52 pagesPlant Pigments Gen Bio 1 Q2 Week 2 by Sir Leo JR and Sir Bil Ramosyukrisha5Pas encore d'évaluation
- Biology - XI - Photosynthesis in Higher Plants - IntroductionDocument47 pagesBiology - XI - Photosynthesis in Higher Plants - IntroductionSDO BSNL NALAGARHPas encore d'évaluation
- 5A PhotosynthesisDocument63 pages5A PhotosynthesisF5A12 JimenaChuPas encore d'évaluation
- Photosynthetic Pigments 2Document2 pagesPhotosynthetic Pigments 2Osent LosPas encore d'évaluation
- 2 Color in FoodDocument44 pages2 Color in FoodAbdul RahmanPas encore d'évaluation
- PhotosynthesisDocument19 pagesPhotosynthesisthushyanthPas encore d'évaluation
- Photosynthetic Processes: Chlorophyll and PigmentsDocument5 pagesPhotosynthetic Processes: Chlorophyll and PigmentsLyka LigsonPas encore d'évaluation
- 105 Lab ReportDocument4 pages105 Lab ReportDan WooPas encore d'évaluation
- Plant Pigments and Photosynthesis: Ap Biology Lab 2 Mr. Bambino Period 1/2 Benjamin Dynkin and Brandon MarvisiDocument4 pagesPlant Pigments and Photosynthesis: Ap Biology Lab 2 Mr. Bambino Period 1/2 Benjamin Dynkin and Brandon Marvisivx99Pas encore d'évaluation
- Lab Reort - Chromatography of Plant PigmentsDocument3 pagesLab Reort - Chromatography of Plant Pigmentsapi-257546392Pas encore d'évaluation
- Ch-13 - Photosynthesis in Higher PlantsDocument11 pagesCh-13 - Photosynthesis in Higher PlantsSam RPas encore d'évaluation
- IB Biology Photosynthesis IADocument12 pagesIB Biology Photosynthesis IAAshwinPas encore d'évaluation
- Photosynthesis - 11Document5 pagesPhotosynthesis - 11smitgangurde1307Pas encore d'évaluation
- Importance of Chlorophyll and Other PigmentsDocument35 pagesImportance of Chlorophyll and Other PigmentsEriPas encore d'évaluation
- Photosynthesis: Pigment Separation, Starch Production and CO2 UptakeDocument10 pagesPhotosynthesis: Pigment Separation, Starch Production and CO2 UptakeJim Goetz100% (3)
- sbl1023 Lab 6 Plant PhysiologyDocument7 pagessbl1023 Lab 6 Plant Physiologyapi-385146128Pas encore d'évaluation
- Chromophore - WikipediaDocument20 pagesChromophore - WikipediaVadivelanPas encore d'évaluation
- Botany LabDocument14 pagesBotany LabHorang HaePas encore d'évaluation
- Advanced Chemistry Final Lab ReportDocument14 pagesAdvanced Chemistry Final Lab Reportapi-644259218Pas encore d'évaluation
- 1 PhotosynthesisDocument22 pages1 PhotosynthesisLisa DentonPas encore d'évaluation
- Autotrophic Photosynthesis HeterotrophicDocument15 pagesAutotrophic Photosynthesis HeterotrophiczahraPas encore d'évaluation
- VerbalDocument8 pagesVerbalthey12Pas encore d'évaluation
- MCAT AAMC 2012 Biological Sciences OulineDocument17 pagesMCAT AAMC 2012 Biological Sciences OulinechaopPas encore d'évaluation
- BiologyDocument58 pagesBiologythey1250% (4)
- Jacob SolutionsDocument2 pagesJacob Solutionsthey12Pas encore d'évaluation
- AAMC SA AnswersDocument3 pagesAAMC SA Answersthey12Pas encore d'évaluation
- Jacob SolutionsDocument2 pagesJacob Solutionsthey12Pas encore d'évaluation
- Calculus NotesDocument2 pagesCalculus Notesthey12Pas encore d'évaluation
- More Stuff You Need To Fuckin' KnowDocument1 pageMore Stuff You Need To Fuckin' Knowthey12Pas encore d'évaluation
- Practice Physics TestDocument7 pagesPractice Physics Testthey12Pas encore d'évaluation
- FOTOSINTESISDocument27 pagesFOTOSINTESISSumiatinPas encore d'évaluation
- Chlorophyll Other PigmentsDocument25 pagesChlorophyll Other Pigmentsjeffrey bacayPas encore d'évaluation
- Photosynthesis - Part 1Document90 pagesPhotosynthesis - Part 1Antonio WilloughbyPas encore d'évaluation
- Non-Cyclic Photophosphorylation Notes 10-26 and 10-28Document18 pagesNon-Cyclic Photophosphorylation Notes 10-26 and 10-28kegalina100% (1)
- BSC1005 - Review List - Exam 2Document5 pagesBSC1005 - Review List - Exam 2mystaceePas encore d'évaluation
- INTRODUCTION CHM 361 Case StudyDocument3 pagesINTRODUCTION CHM 361 Case StudyAnnisaPas encore d'évaluation
- 12 - Photosynthesis in Higher PlantsDocument5 pages12 - Photosynthesis in Higher PlantsShruti KalePas encore d'évaluation
- Raven Biology of Plants: Eighth EditionDocument41 pagesRaven Biology of Plants: Eighth EditionMoath EnnabPas encore d'évaluation
- CHLOROPLASTDocument2 pagesCHLOROPLASTsajidamuhammedPas encore d'évaluation
- 7th Grade Science 2013 Elodea and Photosynthesis Lab Report PROBLEM: How Does The Color of Light (Blue, Red) Affect The Rate of PhotosynthesisDocument4 pages7th Grade Science 2013 Elodea and Photosynthesis Lab Report PROBLEM: How Does The Color of Light (Blue, Red) Affect The Rate of Photosynthesisapi-194649051Pas encore d'évaluation
- Applications of Microalga Chlorella Vulgaris in AquacultureDocument19 pagesApplications of Microalga Chlorella Vulgaris in AquacultureMeryl QuinteroPas encore d'évaluation
- Study of Chlorophyll Content in Various PlantsDocument15 pagesStudy of Chlorophyll Content in Various PlantsKunguma Vignesh57% (7)
- Science11 - Q2 - Mod1 - GeneralBiology1 DAN SIMON P. AQUINODocument47 pagesScience11 - Q2 - Mod1 - GeneralBiology1 DAN SIMON P. AQUINOLyka Mae BenitoPas encore d'évaluation
- Biological Science Canadian 3Rd Edition Freeman Test Bank Full Chapter PDFDocument30 pagesBiological Science Canadian 3Rd Edition Freeman Test Bank Full Chapter PDFkieranthang03m100% (12)
- Raven Biology of Plants 8th Edition Evert Test BankDocument13 pagesRaven Biology of Plants 8th Edition Evert Test Bankwilliamvanrqg100% (28)
- Isolation of Chloroplast and Its ActivityDocument6 pagesIsolation of Chloroplast and Its ActivityEsen Nur ÖzlükPas encore d'évaluation
- General Biology 1: Quarter 2 - Module 1: Energy TransformationDocument37 pagesGeneral Biology 1: Quarter 2 - Module 1: Energy TransformationNikki AlquinoPas encore d'évaluation
- Gen Bio1 Module 8Document23 pagesGen Bio1 Module 8Princess Flora100% (1)
- STPM BIOLOGY PhotosynthesisDocument13 pagesSTPM BIOLOGY Photosynthesiswkwhui100% (6)
- 1 Why Are Plants Not Always GreenDocument4 pages1 Why Are Plants Not Always GreenAngkelova Christina100% (1)
- Amount of Chlorophyll in Five LeafDocument24 pagesAmount of Chlorophyll in Five LeafRakshitha MgowdaPas encore d'évaluation
- YAKEEN 2.0 (Legend) : Photosynthesis in Higher PlantsDocument2 pagesYAKEEN 2.0 (Legend) : Photosynthesis in Higher Plantsgk7936Pas encore d'évaluation
- Photosynthetic Processes: Chlorophyll and PigmentsDocument5 pagesPhotosynthetic Processes: Chlorophyll and PigmentsLyka LigsonPas encore d'évaluation
- U2 c1 AnswersDocument8 pagesU2 c1 AnswersMaiah Phylicia Latoya50% (2)
- Chapter-13: Photosynthesis in Higher PlantsDocument8 pagesChapter-13: Photosynthesis in Higher PlantssujushPas encore d'évaluation
- Chapter 6 - PhotosynthesisDocument113 pagesChapter 6 - PhotosynthesisFebian HenryPas encore d'évaluation
- Rishika Individual InvetigationDocument9 pagesRishika Individual InvetigationAviman Pratap SinghPas encore d'évaluation
- Role of Chlorophyll in PhotosynthesisDocument3 pagesRole of Chlorophyll in PhotosynthesisPrincess Alyssa AbidPas encore d'évaluation
- Effect of Light Intensity On The Photosynthetic Pigment Production of Mung Bean Plants (Vigna Radiata)Document9 pagesEffect of Light Intensity On The Photosynthetic Pigment Production of Mung Bean Plants (Vigna Radiata)Lundgren Arce ReyesPas encore d'évaluation
- Uday Agrawal Biology Extended EssayDocument59 pagesUday Agrawal Biology Extended Essayuday agrawalPas encore d'évaluation