Académique Documents
Professionnel Documents
Culture Documents
Surface Modifications of High Density Polycarbonate by Argon Plasma
Transféré par
IJRRRCopyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Surface Modifications of High Density Polycarbonate by Argon Plasma
Transféré par
IJRRRDroits d'auteur :
Formats disponibles
International Journal of Recent Research and Review, Vol.
III, September 2012
ISSN 2277 8322
Surface Modifications of High Density Polycarbonate by Argon Plasma
Khalaf Ibrahim Khaleel1, Awatif Sabir Jasim1, Mohamad Abdul Kareem Ahmed 1, Y. K. Vijay2, Subodh Srivastava2
Department of Physics, College of Education, Tikrit University, Iraq Thin Film and Membrane Science Laboratory, University of Rajasthan, Jaipur, India Email: muhamed68@ymail.com
Abstract The polycarbonate (PC) membranes were implanted to 16 W argon ions plasma. The samples were treated for different exposure time 10 min, 30 min and 60 min. The effect of argon ions implantation on optical and chemical properties of PC membrane has been investigated. The observed changes have been correlated with the induced structural changes in the implanted layer using UV-Visible spectroscopy. The Atomic Force Microscopy (AFM) and Scanning Electron Microscopy (SEM) were employed to study the surface characteristics of the membrane. The AFM results indicate significant changes in the surface morphology of the polymer membranes before and after plasma treatment. Keywords Polycarbonate (PC), Plasma treatment, AFM, SEM, UV-Visible spectroscopy.
I. INTRODUCTION For several past decades, polymers have been used in different fields such as adhesion, biomaterials, protective coatings, friction and wear, composites, microelectronic devices, and thin-film technology. In general, special surface properties with regard to chemical composition, hydrophilicity, roughness, crystallinity, conductivity, lubricity, and cross-linking density are required for successful applications in various fields. Most Polymers do not have the surface properties required for these applications. They have excellent bulk physical and chemical properties, and are inexpensive and easy to process. For these reasons, surface modification techniques which can transform these advantageous materials into highly
valuable finished products have become an important part of the plastics industry [1,2]. Surface modifications, such as plasma treatment, corona discharge and flame treatment, are frequently employed to enhance the surface properties of thin films. Actually, a simple way to modify the chemical and physical states of the polymer surface, without altering the bulk properties, is by plasma treatment. The plasma treatment of polymers leads to the creation of new chemical groups, cross-linking, degradation, branching of the macromolecules and formation of low-molecular weight oxidized structures like free radicals [3-8]. The rates of these processes being a function of the plasma reactivity which depends strongly upon the structure of polymers and deposited energy by the ion irradiation. Therefore, appropriate plasma treatment can be applied to improve the modifications occur in surface layer only and polymer bulk saves the same unchanged mechanical properties. Furthermore, the modified surface layer can be affected by composition of ambient atmosphere, and the modified surface layer will able to have a good homogeneity. Argon plasma treatment can enhance sputtering of the PC surface layer, which increased surface roughness, and the sputtering depth is up to tens of nanometers [9]. Furthermore, argon plasma causing a cross-linking of polymer matrices which can improve the chemical resistance, optical density, hardness, cohesive strength of the surface and other surface properties[10,11]. The argon plasma treatment can
also induce the direct energy transfer of the surface, due to vacuumed- ultra violet radiation (VUV) emitted by plasma glow [12]. The kinetic energy of argon ions, fast neutrals, and UV-radiation from DCdischarge also causing a significant rearrangement of the surface structure. Surface roughness can be depending on ion implementation, gas composition and treatment conditions such as using an ions, electrons, fast neutrals and radicals. These species can contribute to the polymer treatment, resulting in etching, activation and /or cross-linking [13,14]. A synthetic polymer like Polycarbonate (PC) is considered an important polymer due to its various desirable properties. It is transparent about 90% in the visible range of the electromagnetic spectrum, which make it an excellent substitute of glass substrate in different optical applications like solar cells, antireflective coatings, optical lenses, etc. The monomer structure of PC is shown in Figure 1. The surface properties like etching, roughness, transmission and absorption so as mechanical properties can be modified depending on type of gas used [15]. Consequently, ions flow current i.e. the number of ions passing through plasma discharge channel per unit time, or the energy deposited in molecules matrix, depend basically on the accelerated molecular charges of specific gas used in plasma treatment. Furthermore, surface treatment properties can be chosen according to type of required
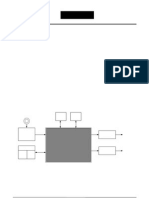
application, and can very well optimized by selecting type of gas or the ions current [16]. In the present work the effect of Ar-ion plasma treatment on surface properties for PC membranes under different treatment time was studied at discharge power of 16W. II. MATERIALS AND METHOD A. Sample preparation The 30m thick PC membranes were prepared by solution cast method. The PC granular has dissolved in dichloromethane (CH2C12) solution using magnetic stirrer for 10 hours. The solution was then pursed into flat-bottomed petrie-dish floated on mercury to ensure the uniformity in the thickness. The solvent was allowed to evaporate slowly over a period of 12-14 hours in the dry atmosphere. The films so obtained were peeled off and dried in vacuum at 50 C, for 2 hours in order to ensure the removal of the solvent [17]. B. Plasma Treatment The schematic diagram of set up for plasma treatment is shown in Figure 2.Typical configuration of a complete plasma processing system is constituted by a stainless-steel cylinder with 40 cm length and 30 cm diameter, electromagnetic energy (DC generator). The vacuum chamber connected to vacuum pumps consist of rotary and diffusion pumps, needle valve to control gas pressure, and process gas sources with gas regulators. The 1x1 mm metal mesh shown in Figure (2) were used to repel the electrons and accelerates the positive ions to impact PC membrane. The plasma chamber was evacuated to 10-4 mbar pressure. Samples of PC were treated by Ar-plasma at various exposure times and 16 W discharge power. The PC samples were located at the midpoint of the chamber using a glass support.
Fig. 1 Monomer structure of polycarbonate (PC)
Fig. 2. Experimental set-up for plasma treatment system
C. Characterizations The Atomic Force Microscope (AFM) type (Veeco,diCp-II) was used for surface morphology. The Scanning Electron Microscope (SEM) manufactured by ZEISS, EVD 18 to study the surface properties. We use the UV-visible spectroscopy for optical properties manufactured by HITACHI model U-2900 spectrophotometer in the wavelength range of 200 to 500 nm. The Fourier Transform Infrared Spectroscopy (FTIR) model IRAffinity-1 (SHIMADZU) was used to study the chemical structures of the polymeric membrane surface. III. RESULTS AND DISCISSION A. Atomic Force Microscope Fig. 3(a) shows the corresponding AFM topographical images of the untreated membrane, and Fig. 3(b), (c) and (d) for treated membranes by argon plasma at 10 min., 30 min. and 60 min. respectively. It is well known, that the plasma treatment is slightly change the morphology of the polymer surface and can depend on the gas composition and pressure. Argon Plasma treatment leads to the ablation of the polymer surface layers, the ablation rate being different for different polymers. As expected, the thickness of the ablated layer is an increasing function of the plasma exposure time [18]. The AFM measurements show clearly, that the short time argon plasma treatment does not influence the surface
morphology as in Fig. 3(b), whereas the root mean square value of roughness (Rrms) for treated film within 10 min time is 9.58 nm. The Higher values of roughness were observed only for over-expositions (30 min. or more) as in Fig. 3(c) and 3(d), whereas the Rrms increased up to 15.33 nm and 29.79 nm for treatment time of 30 min and 60 min, et.at reported the direct dependence between the degree of crystallinity of polymer and surface roughness after the plasma treatment [19-20]. Plasma predominantly etches the amorphous regions than the crystalline ones which are bonded with higher energy. Hence, after plasma treatment, these crystalline regions will remain present, while amorphous are etched away which is leading to rougher surface, because our samples were amorphous. These results have been corroborated by SEM measurements as described in next section. B. Scanning Electron Microscope (SEM) The results discussed in the preceding paragraph were further confirmed by SEM analysis. The SEM images, Fig.4 shows that the image of the pristine PC film has smooth surface structure without any changes on surface morphology. It can be observed from Fig.4 (b) that after a treatment time of 10 min, some uneven granular like defect spots began to appear on the surface. It seems that the PC film begins rough due to the nucleation process during the plasma treatment. It was observed that defective spots increased in density
as well as became larger with longer treatment time as shown in Fig.4(c) and (d).
Fig.3. AFM images of Polycarbonate film with different plasma treatment time (a) untreated, (b) 10 min, (c) 30 min, and (d) 60 min.
Fig. 4. SEM micrographs of polycarbonate film (a) untreated, (b) 10 min, (c) 30 min and (d) 60 min.
C. UV-Visible Spectroscopy The optical transparency for treated PC films was evaluated by recording the UV-visible transmission spectra for various argon plasma glowing duration as shown in Fig.5, the reference used for transmission measurements is untreated PC film. It is well known that the transparency of a bulk film strongly depends on the surface roughness and an increase in the
average roughness will result in a decrease in transmittance due to light scattering effects, but the action of some species present in the plasma promotes chain scission (as described in introduction), and this could lead to etching and material removal, thus promoting changes in surface roughness which in most cases positively contributes to a transparency decrease. The increased roughness may be envisioned as the increase in the concentration and sizes of the
degradations on surface, which leads to higher light scattering [20]. This effect shown clearly in PC membrane treated with argon gas for higher treatment time such as 30 min. and 60 min. as in Fig.5 and also corroborated by Atomic Force Microscopy images shown in Fig.3.
Fig. 5. UV-Visible spectrum for polycarbonate treated membrane for different irradiation time (a) 10 min (b) 30 min (c) 60 min.
Fig. 6. FTIR spectrum for polycarbonate film (a) untreated, (b) 10 min, (c) 30 min and (d) 60 min.
D. Fourier Transform Infrared Spectroscopy (FTIR) Plasma treatment by argon ions can break chemical bonds in PC matrix like C-C and C-H, forming free radicals at or near the surface. These radicals tend to be stable by reaction with other radicals by chain-scissioning [21]. Consequently, recombination process or cross-linking can occur when these free radicals start moving, this interaction can produce high-molecular weight structures. The absorption bands as obtained from the untreated film are classified as (A) 1030 cm-1 ,C-O stretching vibration (B) 1645 cm-1 ,C=C unsaturated (C) 1770cm-1 , C=O stretching vibration (D)2890 cm-1, CH3 stretching vibration and (E) 3070 cm-1 C-H stretching vibration of aromatic compound. The enhancement in the absorption bands of C-O and C=O at 1030 cm-1 and 1770 cm-1 for 10 min,30 min and 60 min treatment duration has been attributed to the creation of unsaturated C=C bonds at 1645 cm1 . It is also observed from FTIR spectra that there is decrease in C-C and C-H bands after plasma treatment. It indicates that cross linking phenomenon enhance during plasma treatment. IV.CONCLUSIONS Polymer membrane of PC were prepared by solution cast method and the effect of argon ions, neutrals, free radicals and other plasma species were studied after plasma treatment by argon gas for different treatment time. It was observed that an Ar-plasma treatment can change surface properties of PC membrane like: optical transmittance, surface roughness and surface energy due to surface cross-linking, degradation, functionalization and free radical formation. The AFM and SEM images shows enhancement in surface roughness related to longer argon plasma treatment time. The plasma treatment by argon ions leads to decrease the optical transparency in PC due to high surface roughness and consequently high light scattering effect. The FTIR spectroscopy results indicate to decrease in C-C and C-H absorption bands after argon plasma treatment. In other hand, a creation of new functional groups like unsaturated C=C bonds has been observed.
V.ACKNOWLEDGMENT The authors are thankful to Head, Department of Physic, University of Rajasthan, Jaipur, India, and Director USIC, University of Rajasthan, Jaipur, India for providing the experimental facilities during this work. The authors are also thankful to Mr. Sachin Surve for his help and spent of his valuable time during SEM measurements. VI. REFERENCES
[1] CM. Chan, TM. Ko, H. Hiraoka. Surf Sci Rep 3, 24, 1996. [2] JM. Grace, LJ. Gerenser. J Dispersion Sci Technol 24, 305, 2003. [3] MR. Wertheimer, AC. Fozza, A. Hollander. Nucl Instrum Methods Phys Res Sect B 65, 151, 1999. [4] PK. Chu, JY. Chen, LP. Wang, N. Huang. Mater Sci Eng R 36, 143, 2002. [5] Y. Choi,J. Kim , K. Paek , W. Ju , YS. Hwang. Surf Coat Technol 19, 319, 2005. [6] S. Kihlman Oiseth, A. Krozer, B.Kasemo, J. Lausmaa J Appl Surf Sci 92, 202, 2002. [7] M.Kuzuya,S.Kondo,M.Sugito,T.Yamashiro.Macromole cules31, 3225, 1998. [8] RD. Boyd AM. Kenwright, JPS. Badyal, D. Briggs, Macromolecules 30, 5429, 1997. [9] C.-M. Chan, T.-M. Ko, H. Hiraoka: Surface Science Reports1, 24, 1996. [10] R. H. Hansen, H. Schonborn: J. Polym. Sci. B, 4, 1966. [11] H. Schonborn, R. H. Hansen: J. Appl. Polym. Sci. 11, 1967. [12] M. Hudis: Techniques and Applications of Plasma Chemistry, New York, Wiley Interscience, pp. 11314 (1974). [13] E.m. Liston, L. Martinu, M.R. Wertheimer, J. Adhes. Sci.Technol.7, 1091, 1993. [14] A.C. Fozza, J.E. Klemberg-sapieha, M.R. Wertheimer, Plasmas Polym.4, 183, 1999. [15] R. Radwan, A. Abdul-Kader, A. El-Hag Ali. Nucl Instrum Methods B 266, pp.3588-94, 2008. [16] B.Michael, H. Norman, L. Patric. (Editors), Introduction to complex Plasmas, Springer-Verlag, pp.276-298, 2010. [17] Y.K.Vijay, M.Dhayat, K.Awashti, V.Kulshrestha, N.K.Acarya., J.S.Choi, J.of Bio. Nanotech. 2, pp.1-8, 2006.
[18] A. Reznckov a, Z. Kolsk b, V. Hnatowiczc, P. Stopkad, V. vorc ka, Nuclear Instruments and Methods in Physics Research B 269, pp.8388, 2011. [19] I. Junkar, U. Cvelbar, Vesel,N.Hauptman,M.Mozetic. Plasma Processes and Polymers, 6,667, 2009. Journal of Applied Polymer Science, 94, 2383, 2004.
[20] T. Jacobs, N. De Geyter, R. Morent, S. Van Vlierberghe, P. Dubruel, C. Leys, et al. Surface and Coatings Technology; 2011. [21] H.Yasuda, J. Macromol Sci Chem.10,1976.
Vous aimerez peut-être aussi
- A Review On Bioactive Constituents of Medicinal PlantsDocument14 pagesA Review On Bioactive Constituents of Medicinal PlantsIJRRRPas encore d'évaluation
- Extraction of Ferulic Acid Ester From Piper LongumDocument6 pagesExtraction of Ferulic Acid Ester From Piper LongumIJRRRPas encore d'évaluation
- Synthesis and Characterization of New Complex Salts, of Some Transition and Non-Transition Metals With Isoquinolinium Derivative SaltsDocument7 pagesSynthesis and Characterization of New Complex Salts, of Some Transition and Non-Transition Metals With Isoquinolinium Derivative SaltsIJRRRPas encore d'évaluation
- Modeling and Simulation of Dynamic Voltage Restorer For Voltage Sag Mitigation in Distribution SystemDocument4 pagesModeling and Simulation of Dynamic Voltage Restorer For Voltage Sag Mitigation in Distribution SystemIJRRRPas encore d'évaluation
- Morality, Sin and Redemption in The Selected Works of Graham GreeneDocument5 pagesMorality, Sin and Redemption in The Selected Works of Graham GreeneIJRRRPas encore d'évaluation
- Paper4-Phytochemicals Present Inamburana Cearensis (Cumaru) Potentially Active Against Parkinson's Disease A Docking Molecular StudyDocument8 pagesPaper4-Phytochemicals Present Inamburana Cearensis (Cumaru) Potentially Active Against Parkinson's Disease A Docking Molecular StudyIJRRRPas encore d'évaluation
- Protein Structure Determination and Prediction A Review of TechniquesDocument14 pagesProtein Structure Determination and Prediction A Review of TechniquesIJRRRPas encore d'évaluation
- Wind and Solar Mobile ChargerDocument6 pagesWind and Solar Mobile ChargerIJRRRPas encore d'évaluation
- Allicin An Inhibiting Potential of HIV Virus A Molecular Docking Studies Comparative With The Ritonavir® InhibitorDocument5 pagesAllicin An Inhibiting Potential of HIV Virus A Molecular Docking Studies Comparative With The Ritonavir® InhibitorIJRRRPas encore d'évaluation
- Electron Momentum Density of Mos2 Using Compton Scattering TechniqueDocument5 pagesElectron Momentum Density of Mos2 Using Compton Scattering TechniqueIJRRRPas encore d'évaluation
- Elements of Sin and Spirituality in The Selected Works of Graham GreeneDocument5 pagesElements of Sin and Spirituality in The Selected Works of Graham GreeneIJRRRPas encore d'évaluation
- A Review On Grid Connected 100 KW Roof Top Solar PlantDocument5 pagesA Review On Grid Connected 100 KW Roof Top Solar PlantIJRRRPas encore d'évaluation
- Synthesis and Antibacterial Activity of Some NovelDocument5 pagesSynthesis and Antibacterial Activity of Some NovelIJRRRPas encore d'évaluation
- Lean Six Sigma - Process Improvement TechniquesDocument6 pagesLean Six Sigma - Process Improvement TechniquesIJRRRPas encore d'évaluation
- A A Review On Man and ManagementReview On Man and ManagementDocument7 pagesA A Review On Man and ManagementReview On Man and ManagementIJRRRPas encore d'évaluation
- Reduction in Power Losses On Distribution Lines Using Bionic Random Search Plant Growth Simulation AlgorithmDocument7 pagesReduction in Power Losses On Distribution Lines Using Bionic Random Search Plant Growth Simulation AlgorithmIJRRRPas encore d'évaluation
- Biodegradation of Endosulfan Using Microbial CultureDocument5 pagesBiodegradation of Endosulfan Using Microbial CultureIJRRRPas encore d'évaluation
- Paper3.pdfenhancing Computer System Reliability Using Fault Tree AnalysisDocument6 pagesPaper3.pdfenhancing Computer System Reliability Using Fault Tree AnalysisIJRRRPas encore d'évaluation
- Paper3.pdfenhancing Computer System Reliability Using Fault Tree AnalysisDocument6 pagesPaper3.pdfenhancing Computer System Reliability Using Fault Tree AnalysisIJRRRPas encore d'évaluation
- Synthesis and Antibacterial Activity of Some Novel 1,4- Diazepines from β-Diketones/ β -Ketoesters of 4- Acetylaminobenzene Sulphonyl ChlorideDocument5 pagesSynthesis and Antibacterial Activity of Some Novel 1,4- Diazepines from β-Diketones/ β -Ketoesters of 4- Acetylaminobenzene Sulphonyl ChlorideIJRRRPas encore d'évaluation
- Production of Alcohol Through Fermentation in Saccharum Officinarum (Sugarcane) and Jaggery (Gur)Document3 pagesProduction of Alcohol Through Fermentation in Saccharum Officinarum (Sugarcane) and Jaggery (Gur)IJRRR100% (1)
- Strategic Planning For Cloud Computing ArchitectureDocument5 pagesStrategic Planning For Cloud Computing ArchitectureIJRRRPas encore d'évaluation
- Scanning Electron Microscopy of Zn1-xMnxTe Thin-Films Prepared by Thermal Evaporation MethodDocument3 pagesScanning Electron Microscopy of Zn1-xMnxTe Thin-Films Prepared by Thermal Evaporation MethodIJRRRPas encore d'évaluation
- Advancement & Role of DG Technology in Distribution SystemDocument10 pagesAdvancement & Role of DG Technology in Distribution SystemIJRRRPas encore d'évaluation
- Design and Characterizations of Solar Steam EngineDocument7 pagesDesign and Characterizations of Solar Steam EngineIJRRRPas encore d'évaluation
- V.S Naipaul in The Line and Light of Colonial CultureDocument9 pagesV.S Naipaul in The Line and Light of Colonial CultureIJRRRPas encore d'évaluation
- Paper2.pdthe Effect of The Solution Concentration On Structural and Optical Properties CDS Thin Films Prepared by Chemical Bath Deposition TechniqueDocument6 pagesPaper2.pdthe Effect of The Solution Concentration On Structural and Optical Properties CDS Thin Films Prepared by Chemical Bath Deposition TechniqueIJRRRPas encore d'évaluation
- The Theory of Agency Applied To The Relationship BetweenDocument9 pagesThe Theory of Agency Applied To The Relationship BetweenIJRRRPas encore d'évaluation
- Study of Optical Properties of Bilayer ZnTe: Al Film Grown On Glass Substrate by Thermal Evaporation Method2 Al Thin FilmsDocument3 pagesStudy of Optical Properties of Bilayer ZnTe: Al Film Grown On Glass Substrate by Thermal Evaporation Method2 Al Thin FilmsIJRRRPas encore d'évaluation
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5784)
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (890)
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (399)
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (265)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (72)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (344)
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (119)
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)
- KIOTI tractor manual overviewDocument150 pagesKIOTI tractor manual overviewNatsumi KawaiPas encore d'évaluation
- UOP046-85 Wax Content in Petroleum OilsDocument6 pagesUOP046-85 Wax Content in Petroleum OilsZiauddeen Noor100% (1)
- TS1A-13A: Operation and Maintenance ManualDocument204 pagesTS1A-13A: Operation and Maintenance ManualJavier ApontePas encore d'évaluation
- Distance Measurement Methods & CalculationsDocument16 pagesDistance Measurement Methods & CalculationsAlfonso John AnthonyPas encore d'évaluation
- Vena SIF Piping CalculationDocument5 pagesVena SIF Piping CalculationPratap KollapaneniPas encore d'évaluation
- Adobe 2017 Download LinksDocument4 pagesAdobe 2017 Download LinksDaśhīñg ÆžīPas encore d'évaluation
- Reliable Ni-Cd batteries keep railroads running smoothlyDocument8 pagesReliable Ni-Cd batteries keep railroads running smoothlyJesus LandaetaPas encore d'évaluation
- Solid Mechanics: Amit Sir (M-Tech (STRUCTURE), BE, Diploma in Civil), 7020059691/7385990691Document9 pagesSolid Mechanics: Amit Sir (M-Tech (STRUCTURE), BE, Diploma in Civil), 7020059691/7385990691Nikita GonnadePas encore d'évaluation
- Kitchen DetailsDocument12 pagesKitchen DetailsAJ PAJEPas encore d'évaluation
- Instruction Manual - Digital Drybath - ENDocument19 pagesInstruction Manual - Digital Drybath - ENAlain ManceraPas encore d'évaluation
- Icwe14 - Id02441 HfpiDocument36 pagesIcwe14 - Id02441 HfpiSergio StolovasPas encore d'évaluation
- Planning of Electrical NetworksDocument32 pagesPlanning of Electrical NetworksSerge RINAUDOPas encore d'évaluation
- IES Syllabus For Mechanical Engineering IES 2015 Syllabus MEDocument5 pagesIES Syllabus For Mechanical Engineering IES 2015 Syllabus MERohitMishraPas encore d'évaluation
- FintechDocument8 pagesFintechArnab Das50% (4)
- Terminal Blocks: KasugaDocument6 pagesTerminal Blocks: KasugaKs MuraliPas encore d'évaluation
- WSST - FW - Flyer Changi EN PDFDocument5 pagesWSST - FW - Flyer Changi EN PDFAmlan Banerjee0% (1)
- Marine Seawater ValvesDocument8 pagesMarine Seawater ValvesPhornlert WanaPas encore d'évaluation
- GNB Absoltye IIPDocument18 pagesGNB Absoltye IIPFederico Tellez QPas encore d'évaluation
- Mobile Robots & Kinematics: Session 2: Nicol As Ilich Samus February 27, 2014Document5 pagesMobile Robots & Kinematics: Session 2: Nicol As Ilich Samus February 27, 2014Nicolás Ilich SamusPas encore d'évaluation
- Fls Brochure Usa v2 PDFDocument4 pagesFls Brochure Usa v2 PDFXa ViPas encore d'évaluation
- Acoustic Emission-Based Monitoring Approach For Friction Stir Welding of Aluminum Alloy AA6063-T6 With Different Tool Pin ProfilesDocument10 pagesAcoustic Emission-Based Monitoring Approach For Friction Stir Welding of Aluminum Alloy AA6063-T6 With Different Tool Pin ProfileslarryPas encore d'évaluation
- STG SiemensDocument2 pagesSTG SiemensjoncperezPas encore d'évaluation
- I RoboDocument40 pagesI RoboTuấn NguyễnPas encore d'évaluation
- API 5l Grade l245 PipesDocument1 pageAPI 5l Grade l245 PipesMitul MehtaPas encore d'évaluation
- Space Programming 1Document10 pagesSpace Programming 1Mache SebialPas encore d'évaluation
- Gas Stop HTDocument1 pageGas Stop HTbagus918Pas encore d'évaluation
- Operating Systems Concepts and ComponentsDocument42 pagesOperating Systems Concepts and ComponentsgtaclubPas encore d'évaluation
- Resume Hemant ChaurasiaDocument3 pagesResume Hemant Chaurasiachaurasia_hPas encore d'évaluation
- Sap InputDocument211 pagesSap InputdominscribdPas encore d'évaluation
- Preliminary: SPCA702ADocument60 pagesPreliminary: SPCA702Avetchboy0% (1)