Académique Documents
Professionnel Documents
Culture Documents
Jib 138 2003
Transféré par
Muhammad JavedDescription originale:
Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Jib 138 2003
Transféré par
Muhammad JavedDroits d'auteur :
Formats disponibles
Journal of Inorganic Biochemistry 94 (2003) 138145 www.elsevier.
com / locate / jinorgbio
DNA interactions of new mixed-ligand complexes of cobalt(III) and nickel(II) that incorporate modied phenanthroline ligands
C.V. Sastri, D. Eswaramoorthy , L. Giribabu, Bhaskar G. Maiya*
School of Chemistry, University of Hyderabad, Gachibowli, Hyderabad 500 046, India Received 28 October 2002; accepted 23 November 2002
1
Abstract Four new mixed-ligand complexes, namely [Co(phen) 2 (qdppz)] 31 , [Ni(phen) 2 (qdppz)] 21 , [Co(phen) 2 (dicnq)] 31 and [Ni(phen) 2 (dicnq)] 21 (phen51,10-phenanthroline, qdppz5naptho[2,3-a]dipyrido[3,2-h:29,39-f ]phenazine-5,18-dione and dicnq5 dicyanodipyrido quinoxaline), were synthesized and characterized by FAB-MS, UV/ Vis, IR, 1 H NMR, cyclic voltammetry and magnetic susceptibility methods. Absorption and viscometric titration as well as thermal denaturation studies revealed that each of these octahedral complexes is an avid binder of calf-thymus DNA. The apparent binding constants for the dicnq- and qdppz-bearing complexes are in the order of 10 4 and .10 6 M 21 , respectively. Based on the data obtained, an intercalative mode of DNA binding is suggested for these complexes. While both the investigated cobalt(III) complexes and also [Ni(phen) 2 (qdppz)] 21 affected the photocleavage of DNA (supercoiled pBR 322) upon irradiation by 360 nm light, the corresponding dicnq complex of nickel(II) was found to be ineffective under a similar set of experimental conditions. The physico-chemical properties as well as salient features involved in the DNA interactions of the cobalt(III) and nickel(II) complexes investigated here were compared with each other and also with the corresponding properties of the previously reported ruthenium(II) analogues. 2002 Elsevier Science Inc. All rights reserved.
Keywords: Cobalt(III) and nickel(II) complexes; Modied phenanthroline ligands; Synthesis and spectroscopy; DNA binding and photocleavage
1. Introduction Transition metal complexes of 2,2-bipyridyl (bpy), 1,10phenanthroline (phen) or their modied variants are widely employed in studies of DNA in view of their applications in several research areas, including bioinorganic and biomedicinal chemistries [110]. Recently, efforts have been directed towards the design of complexes containing modied bpy or phen ligands that bind DNA primarily via base-pair intercalation [1120]. Among such metal complexes of intercalatable ligands, those incorporating dipyrido[3,2-a:29,39-c]phenazine (dppz) are known to possess unique characteristics. Various metal complexes of dppz are avid intercalators of DNA due to the extensively p-conjugated and planar structure of this novel ligand
*Corresponding author. Tel.: 191-40-401-500; fax: 191-40-3012460. E-mail address: bgmsc@uohyd.ernet.in (B.G. Maiya). 1 Permanent address: Department of Chemistry, Crescent Engineering College, Vandalur, Chennai, India.
[3,8,10,1826]. An added advantage of this class of DNAbinding species is that the metal ion can also be varied such that a series of complexes having the same set of ligands but with varying properties can be generated to facilitate an individual application. During our initial studies on metal complexDNA interactions [27], we realized that the unique architecture of dppzthe prototype ligand in which 2,29-bipyridyl and phenazine subunits are brought togetherpermits the design of a variety second generation ligands, the complexes of which possess more potent DNA-binding and cleavage proclivities. Two such new modied dppz ligands, viz. naptho[2,3-a]dipyrido[3,2-h:29,39-f ]phenazine5,18-dione (qdppz) and dicyanodipyrido quinoxaline (dicnq), have been synthesized and DNA interactions of their ruthenium(II) complexes have recently been reported [2831]. We sought to compare the effect of substituting ruthenium(II) in these qdppz / dicnq-based systems by cobalt(III) / nickel(II) on the DNA interactions of the ensuing complexes. This article reports the synthesis, spectral characterization and DNA-binding and photo-
0162-0134 / 02 / $ see front matter 2002 Elsevier Science Inc. All rights reserved. doi:10.1016 / S0162-0134(02)00622-0
C.V. Sastri et al. / Journal of Inorganic Biochemistry 94 (2003) 138145
139
Fig. 1. Molecular structures of the complexes investigated in this study.
cleavage properties of the cobalt(III) and nickel(II) complexes, viz. [Co(phen) 2 (qdppz)] 31 , [Ni(phen) 2 (qdppz)] 21 , [Co(phen) 2 (dicnq)] 31 and [Ni(phen) 2 (dicnq)] 21 . The structures of these investigated complexes are given in Fig. 1.
Yield: 0.99 g (74%). FAB-MS (m /z): [M2PF 6 ] 1 , 1122; [M22PF 6 ] 1 , 977; [M23PF 6 2qdppz] 1 , 419. IR, KBr pellets (cm 21 ): 839, 1321, 1431, 1581, 1670, 3105. 1 H NMR, d ppm ((CD 3 ) 2 SO, TMS): 9.92 (d, 2H), 9.18 (m, 4H), 8.92 (d, 2H), 8.60 (d, 6H), 8.29 (d, 2H), 8.00 (m, 8H), 7.70 (d, 4H).
2. Experimental
2.1. Materials
CoCl 2 ?6H 2 O, NiCl 2 ?6H 2 O and 1,10-phenanthroline? monohydrate were purchased from Merck (India). Calfthymus (CT) DNA, tetrabutylammonium chloride (TBACl) and tetrabutylammonium hexauorophosphate (TBAPF 6 ) were obtained from Sigma (St. Louis, MO, USA). The supercoiled (CsCl puried) pBR 322 DNA (Bangalore Genie, India) was used as received. All other common chemicals and solvents were procured from locally available sources. All the solvents were puried before use as per standard procedures [32]. Deionised, triply distilled water was used for preparing various buffers. The ligands qdppz and dicnq were prepared by the reaction of 1,10-phenanthroline-5,6-dione (phen-dione) [33] with 1,2-diaminoanthraquinone or diaminomaleonitrile (Aldrich, USA), as detailed earlier [2831]. [Co(phen) 2 Cl 2 ]Cl?3H 2 O and [Ni(phen) 2 Cl 2 ] were prepared as reported previously [34,35].
2.1.2. [ Ni( phen)2 ( qdppz)]( PF6 )2 ?2 H2 O To a 10 ml methanolic solution of [Ni(phen) 2 Cl 2 ] (0.49 g, 1 mmol) was added qdppz (0.41 g, 1 mmol) dissolved in ethylene glycol (25 ml). The resulting solution was reuxed for 4 h, allowed to cool and then ltered. The crude complex was precipitated upon addition of a saturated solution of NH 4 PF 6 . The complex was ltered and further dried under vacuum before being recrystallized from acetonediethyl ether. Yield: 0.95 g (82%). FAB-MS (m /z): [M2PF 6 ] 1 , 976; [M2PF 6 2qdppz] 1 , 563; [M2 2PF 6 2qdppz] 1 , 418. IR, KBr pellets (cm 21 ): 839, 1333, 1426, 1587, 1672. meff 5 2.7660.02 B.M. 2.1.3. [ Co( phen)2 (dicnq)]( PF6 )3 ? H2 O A solution containing [Co(phen) 2 Cl 2 ]Cl?3H 2 O (0. 58 g, 1 mmol) and dicnq (0. 28 g, 1 mmol) in methanol was reuxed for 1 h. Work up of the reaction mixture, as described above for the corresponding qdppz complex, afforded the desired complex. Yield: 0. 92 g (80%). FABMS (m /z): [M2PF 6 ] 1 , 991; [M22PF 6 ] 1 , 846; [M2 3PF 6 ] 1 , 419. IR, KBr pellets (cm 21 ): 716, 837, 1375, 1 1522, 3106, 3654. H NMR, d ppm ((CD 3 ) 2 SO, TMS): 9.82 (m, 2H), 9.13 (d, 4H), 8.60 (d, 4H), 8.18 (m, 2H), 8.00 (m, 4H), 7.80 (t, 2H), 7.65 (d, 4H). 2.1.4. [ Ni( phen)2 (dicnq)]( PF6 )2 ? H2 O A solution containing [Ni(phen) 2 Cl 2 ] (0.49 g, 1 mmol) and dicnq (0. 28 g, 1 mmol) in methanol was reuxed for 1 h. Work up of the reaction mixture, as described above for the corresponding qdppz complex, afforded [Ni(phen) 2 (dicnq)](PF 6 ) 2 ?2H 2 O. Yield: 0.79 g (78%). FAB-MS (m /z): [M2PF 6 ] 1 , 851; [M2PF 6 2dicnq] 1 ,
2.1.1. [ Co( phen)2 ( qdppz)]( PF6 )3?4 H2 O [Co(phen) 2 Cl 2 ]Cl?3H 2 O (0. 58 g, 1 mmol) and qdppz (0.41 g, 1 mmol) were taken up in a solvent mixture containing 20 ml ethylene glycol and 5 ml methanol. The resulting mixture was reuxed for 4 h, allowed to cool and then ltered. The desired complex was precipitated upon addition of a methanolic solution of NH 4 PF 6 to the ltrate. The complex was ltered, further dried under vacuum and recrystallized from an acetonediethyl ether mixture.
140
C.V. Sastri et al. / Journal of Inorganic Biochemistry 94 (2003) 138145
564; [M22PF 6 2dicnq] 1 , 419. IR, KBr pellets (cm -1 ): 725, 839, 1377, 1554, 3086, 3650. meff 5 2.7860.02 B.M. Each of the above PF 6 salts was dissolved in the minimum amount of acetone, and a saturated solution of TBACl in acetone was added drop-wise until precipitation was complete. The chloride salts thus obtained were ltered, washed thoroughly with acetone and vacuum dried (yield |95% in each case).
The data were then tted to Eq. (1) to obtain the intrinsic binding constant, Kb [38]: [DNA] /(a 2 f ) 5 [DNA] /(b 2 f ) 1 1 /Kb (b 2 f ) (1)
2.2. Instrumentation
Elemental analysis was carried out with a Perkin-Elmer Model 240-C CHN analyzer. IR spectra were recorded with a JASCO Model 5300 FT-IR spectrophotometer by dispersing the solid sample in KBr pellets. FAB-MS spectra were recorded with a JEOL SX 102 / DA-6000 mass spectrometer / data system. 1 H NMR spectra were recorded on a Brucker NR-FT 200 MHz spectrometer using (CD 3 ) 2 SO as the solvent and tetramethylsilane (TMS) as an internal standard. Room temperature magnetic susceptibility measurements were carried out using a Cahn (Model 6612) magnetic susceptibility system. The magnetic moment ( meff in units of Bohr Magnetrons, B.M.) values were calculated from these data after incorporating the diamagnetic corrections as described [36]. HgCo(NCS) 4 was used as a calibration standard. Absorption spectra were recorded using a JASCO Model UV 7800 spectrophotometer (cuvette path length 10 mm). Cyclic voltammetric experiments (CH 3 CN, 0.1 M TBAP, scan rate 50500 mV s 21 ) were performed on a CH Instruments Model CHI 620A electrochemical analyzer as described previously [2731]. A glassy carbon working electrode, a Pt-wire counter electrode and a saturated calomel reference electrode (SCE) were employed. The Fc 1 / Fc couple (Fc5ferrocene) was used to calibrate the redox potential values.
where a , f , and b are the apparent, free and bound metal complex extinction coefcients, respectively. A plot of [DNA] /(a 2 f ) vs. [DNA] gave a slope of 1 /(b 2 f ) and a Y intercept equal to 1 /Kb (b 2 f ); Kb is the ratio of the slope to the Y intercept. Thermal denaturation experiments were carried out with a Shimadzu Model UV-160A spectrophotometer coupled to a temperature controller (Model TCC-240A) by monitoring the absorption at 260 nm of CT DNA at various temperatures, both in the presence and absence of each complex. The melting temperature (T m , the temperature at which 50% of doublestranded DNA becomes single-stranded) and the curve width (sT , the temperature range between which 10 to 90% of the absorption increase occurred) were calculated as reported [39,40]. Viscosity measurements were made using a Cannon-Ubbelohde capillary viscometer at 2661 8C. The DNA concentration was xed at 600 mM and nitrogen gas was bubbled through the solution to ensure complete mixing after each addition of the complex. Flow time measurements were repeated at least four times and accepted if successive values agreed within 0.2 s. For the gel electrophoresis experiments, supercoiled pBR 322 DNA (100 mM in nucleotides) in TrisHCl buffer (pH 8.0) was treated with the metal complex (10 mM) and the mixture was incubated for 1 h in the dark. The samples were analyzed by 0.8% agarose gel electrophoresis (Trisacetic acidEDTA buffer, pH 8.0) and the data documented using the UVP Gel Documentation System as described previously [2931,41,42]. Irradiation experiments were carried out by keeping the pre-incubated (dark, 1 h) samples inside the sample chamber of a JASCO Model FP-777 spectrouorimeter for 30 min ( lexc 5 36065 or 39065 nm; slit width 5 nm).
2.3. DNA-binding and cleavage experiments
3. Results and discussion The concentration of CT DNA (in nucleotide phosphate, NP) was measured by using its known extinction coefcient at 260 nm (6600 M 21 cm 21 ) [37]. DNA concentrations are reported in units of NP throughout this paper. Buffer A (5 mM Tris, pH 7.1, 50 mM NaCl) was used for absorption titration experiments, buffer B (1 mM phosphate, pH 7.0, 2 mM NaCl) was used for thermal denaturation experiments and buffer C (1.5 mM Na 2 HPO 4 , 0.5 mM NaH 2 PO 4 , 0.25 mM Na 2 EDTA, pH 7.0) was used for the viscometric titrations. Absorption titration experiments were carried out by varying the DNA concentration (0600 mM) and maintaining the complex concentration constant (230 mM). Absorbance values were recorded after each successive addition of DNA solution and equilibration (ca. 10 min).
3.1. Synthesis and characterization
The FAB-MS, IR, 1 H NMR and magnetic moment data of the new complexes are summarized in the Experimental section. UV/ Vis and redox potential data are provided in Table 1. The IR spectrum of the PF 6 salt of each complex showed a strong band in the 837839 cm 21 region ascribable to the counter anion and this band was absent for the corresponding chloride salts [43]. In the 1 H NMR spectra of the two cobalt(III) complexes, the peaks due to various protons of phen, qdppz and dicnq are seen to be shifted in comparison with the corresponding free ligands, suggesting complexation. Unlike the cobalt(III) complexes, which are diamagnetic, both [Ni(phen) 2 (qdppz)] 21 and
C.V. Sastri et al. / Journal of Inorganic Biochemistry 94 (2003) 138145 Table 1 UV/ Vis (CH 3 CN) and redox potential (CH 3 CN, 0.1 M TBAP) data Complex
141
lmax , nm (log )a
31
Potential, V. vs. SCE b E1 / 2 (red.) E1 / 2 (ox.) 0.40 0.39
[Co(phen) 2 (qdppz)] [Ni(phen) 2 (qdppz)] 21 [Co(phen) 2 (dicnq)] 31 [Ni(phen) 2 (dicnq)] 21
a b
227 (5.15), 272 (5.17), 381 (4.36), 398 (4.36) 230 (4.98), 272 (5.06), 382 (4.23), 399 (4.23) 222 (5.00), 271 (4.93), 300 (4.58), 338 (4.06), 355 (3.97) 227 (5.05), 269 (5.11), 294 (4.70), 355 (4.17), 369 (4.20)
20.42, 20.70, 21.03 20.43, 20.82, 21.28 20.60, 20.97, 21.17 20.58, 20.91, 21.30
Error limits: lmax , 61 nm; , 67%. Error limits: E1 / 2 , 60.03 V.
[Ni(phen) 2 (dicnq)] 21 were found to be paramagnetic with a meff value of 2.7760.02 B.M., as expected for typical d 8 systems. The UV/ Vis spectra of qdppz and dicnq are characterized by prominent bands ascribable to the pp* transitions of their phenanthroline (ca. ,290 nm) and phenazine / quinoxaline (.290 nm) parts [30,31]. The spectra of the complexes show bands arising from these same intra-ligand transitions, the wavelength maxima ( lmax ) and molar extinction coefcients () of which do not change appreciably in comparison with those of the respective free ligands (Table 1). No charge-transfer transitions are discernable from the spectra of these complexes unlike in the case of the corresponding ruthenium(II) complexes, which show MLCT bands in the 440450 nm region in CH 3 CN [30,31]. Consequently, MLCT emission was also absent in the presently investi-
gated cobalt(III) and nickel(II) complexes; in fact, these complexes are totally non-luminescent. Representative cyclic voltammograms of [Co(phen) 2 (dicnq)] 31 and [Ni(phen) 2 (dicnq)] 21 are illustrated in Fig. 2 and data for all four complexes are summarized in Table 1. Wave analysis suggested that while the rst two reduction peaks of each complex and also the oxidation peaks of the two cobalt(III) complexes are reversible reactions, the subsequent reduction steps are, in general, either quasi-reversible or totally irreversible [44]. It is observed that the reduction potentials of the ligands [30,31] are anodically shifted upon chelation, consistent with the fact that the electron density on these ligands decreases upon electron donation to the metal, thus making the electron addition easier [33]. In the anodic scan, the peak due to the Co 31 / Co 21 couple was observed at about
Fig. 2. Cyclic voltammograms of (a) [Co(phen) 2 (dicnq)] 31 and (b) [Ni(phen) 2 (dicnq)] 21 in CH 3 CN, 0.1 M TBAP. Scan rate 100 mV s 21 . Fc 1 / Fc represents the currentvoltage response of ferrocene under the same set of experimental conditions.
142
C.V. Sastri et al. / Journal of Inorganic Biochemistry 94 (2003) 138145
10.4 V for both [Co(phen) 2 (qdppz)] 31 and 31 [Co(phen) 2 (dicnq)] , the range in which the nickel(II) complexes were found to be electro-inactive (see Fig. 2). Overall, analysis of the spectroscopic and electrochemical properties of the four new complexes investigated in this study reveals that, in general, these properties are rather sensitive to the type of ligand, i.e. dicnq or qddpz, rather than to the central metal ion, i.e. cobalt(III) or nickel(II). Thus, the overall charge on the complex does not seem to inuence the properties of these polypyridyl complexes very much [27].
3.2. DNA binding
It should be noted here that all four new complexes were isolated in their racemic forms and that the DNA-binding behaviours described below are a composite of those of two enantiomers. Initially, the interaction of these new complexes with DNA was monitored by the absorption titration method using their chloride salts, the absorption spectra of which in aqueous buffered solutions were found to be nearly identical to the corresponding spectra of the PF 6 salts in CH 3 CN. In the presence of increasing amounts of CT DNA, both [Co(phen) 2 (dicnq)] 31 and [Ni(phen) 2 (dicnq)] 21 (Fig. 3) showed a strong decrease in intensity (hypochromicity: 35% for nickel(II) and 28% for cobalt(III) complexes) and bathochromic shifts (maximum: 561 nm for nickel(II) and 361 nm for cobalt(III) complexes) for their most red-shifted absorption peak maxima. Isosbestic points appeared at 335 and 395 nm for
[Ni(phen) 2 (dicnq)] 21 and at 375 nm for 31 [Co(phen) 2 (dicnq)] during these titrations. The change in the absorbance values (at 409 nm for [Co(phen) 2 (dicnq)] 31 and at 369 nm for [Ni(phen) 2 (dicnq)] 21 ) with increasing amounts of CT DNA were used to evaluate the intrinsic binding constants (Kb ) for the complexes. The values of Kb evaluated for [Co(phen) 2 (dicnq)] 31 and [Ni(phen) 2 (dicnq)] 21 , using Eq. 3 4 21 (1), are 6.0310 and 2.1310 M , respectively. In the presence of increasing amounts of CT DNA, UV/ Vis absorption spectra of [Co(phen) 2 (qdppz)] 31 and [Ni(phen) 2 (qdppz)] 21 also showed bathochromic shifts (maximum: 461 nm for both complexes) and hypochromism (hypochromicity: 51 and 47% for nickel(II) and cobalt(III) complexes, respectively) as is the case with the corresponding dicnq complexes described above. It was not possible to estimate the accurate binding constants for DNA interactions of these complexes because they were found to bind too strongly even at micromolar concentrations of DNA as is the case with [Ru(phen) 2 (qdppz)] 21 and a host of other dppz-based complexes [2126,30,45 47]. Thus, it is reasonable to expect that the Kb values for the presently investigated qdppz complexes are .10 6 M 21 as is the case for [Ru(phen) 2 (qdppz)] 21 [30]. Thermal melting studies were carried out at [(DNA)] / [complex]525 and T m and sT were determined by monitoring the absorbance of DNA at 260 nm as a function of temperature. The T m of DNA was found to be 6061 8C under our experimental conditions. Under the same set of conditions, addition of [Ni(phen) 2 (qdppz)] 21 ,
Fig. 3. UV/ Vis spectra of (a) [Ni(phen) 2 (dicnq)] 21 and (b) [Co(phen) 2 (dicnq)] 31 in the absence (top curve, in each case) and presence (subsequent curves) of increasing concentrations of CT DNA (0300 mM) in buffer A. The data were t to Eq. (1) to obtain the binding constants (see text for details).
C.V. Sastri et al. / Journal of Inorganic Biochemistry 94 (2003) 138145
143
[Co(phen) 2 (dicnq)] 31 and [Ni(phen) 2 (dicnq)] 21 increased T m (61 8) by 7, 6 and 4 8C, respectively. While the sT value (61 8C) for DNA alone is 21 8C, the corresponding values in the presence of [Ni(phen) 2 (qdppz)] 21 , 31 21 [Co(phen) 2 (dicnq)] and [Ni(phen) 2 (dicnq)] were found to be 24, 23 and 25 8C, respectively. Thermal denaturation experiments could not be satisfactorily carried out with [Co(phen) 2 (qdppz)] 31 due to the appearance of turbidity for DNA solutions containing this complex at higher temperatures. The relative viscosity data were calculated for all the complexes studied at a complex-to-DNA ratio of between 0.0 and 0.15 adopting the method described by Cohen and Eisenberg [48]. Representative plots of h /h0 vs. [drug] / DNA are shown in Fig. 4. As can be seen, while [Ru(phen) 3 ] 21 does not affect the DNA viscosity, as reported previously [30], there is a positive change of viscosity with increasing addition of the complex for both [Co(phen) 2 (qdppz)] 31 and [Co(phen) 2 (dicnq)] 31 . Positive changes in viscosity were also observed for the corresponding nickel(II) complexes. The reduction in peak intensities and the bathochromic shifts in the absorption titration experiments, the increase in the T m and sT values in the thermal denaturation experiments and the positive changes of the viscosity observed in the presence of DNA for the new complexes of qdppz and dicnq discussed above are reminiscent of the similar observations made earlier for various metallointercalator species, including dppz-based complexes [21 26,4547,49], and for [Ru(phen) 2 (qdppz)] 21 and 21 [Ru(phen) 2 (dicnq)] [30,31]. Based on these similarities, we propose that the bound qdppz or dicnq ligand is involved in the intercalation with DNA. qdppz, being a more extended p-system, is obviously a better intercalator than dicnq.
Fig. 5. Light-induced nuclease activity of [Ru(phen) 2 (qdppz)] 21 , [Co(phen) 2 (qdppz)] 31 and [Ni(phen) 2 (qdppz)] 21 . Dark and light experiments. Lanes 1 and 5: untreated pBR 322 (100 mM) in the dark and upon irradiation ( lirrd. 5 360 nm, 30 min). Lanes 2, 3 and 4: pBR 3221 [Ru(phen) 2 (qdppz)] 21 , [Co(phen) 2 (qdppz)] 31 and [Ni(phen) 2 (qdppz)] 21 , respectively (10 mM). Lanes 6, 7 and 8: pBR 3221 [Ru(phen) 2 (qdppz)] 21 , [Co(phen) 2 (qdppz)] 31 and [Ni(phen) 2 (qdppz)] 21 , respectively, upon irradiation ( lirrd. 5 360 nm, 30 min).
3.3. DNA photocleavage
Fig. 5 summarizes the results of DNA photocleavage experiments carried out with the qddpz complexes of cobalt(III), nickel(II) and ruthenium(II) (for comparison) as monitored by the agarose gel electrophoresis method. Control experiments suggested that untreated DNA does not show any cleavage in the dark and even upon irradiation by 360 nm light (compare lanes 1 and 5). Similarly, DNA nicking was not observed for pBR 322 treated with [Ni(phen) 2 (qdppz)] 21 , [Co(phen) 2 (qdppz)] 31 and [Ru(phen) 2 (qdppz)] 21 in the dark experiments (lanes 24). Additional control experiments suggested that phen, qdppz and dicnq (free ligands, dissolved in 10% DMF) are not detectably active under our irradiation conditions. Moreover, no perceptible DNA cleavage was observed when samples of pBR 322 containing the tris-phen or tris-phen dione complexes of cobalt(III) and nickel(II) were irradiated at 360 nm, as these complexes do not appreciably absorb light at this wavelength. On the other hand, irradiation of DNA in the presence of [Ru(phen) 2 (qdppz)] 21 at 360 nm was seen to completely photocleave the DNA (see lane 6). Irradiation of [Co(phen) 2 (qdppz)] 21 (lane 7) and [Ni(phen) 2 (qddpz)] 21 (lane 8) results in the conversion of nearly 50% of pBR 322 from form I to form II under the present set of experimental conditions. Increasing the irradiation time resulted in further relaxation of the DNA in both cases. As far as the photocleavage abilities of the dicnq complexes are concerned, [Co(phen) 2 (dicnq)] 31 was found to be about half as effective as the corresponding qdppz complex. The nickel(II) analogue, on the other hand, was completely ineffective under the present set of experimental conditions (data not shown). It should be noted that switching the irradiation wavelength from 360 to 390 nm in these experiments did not change the photocleavage abilities of any of the complexes. Although the exact mechanism of the photocleavage
Fig. 4. Results of viscometric titrations carried out for CT DNA (600 mM, buffer C) in the presence of (s) [Co(phen) 2 (qdppz)] 31 (m) [Co(phen) 2 (dicnq)] 31 and (,) [Ru(phen) 3 ] 21 in buffer C.
144
C.V. Sastri et al. / Journal of Inorganic Biochemistry 94 (2003) 138145
reactions of the individual complexes was not investigated in the present study, the following observations can be made. We have reported earlier that [Co(phen) 2 (dppz)] 31 is able to photocleave DNA and that, in comparison, [Ni(phen) 2 (dppz)] 21 is an ineffective photocleaving agent [27]. Thus, the observed abilities of both [Co(phen) 2 (qddpz)] 31 and [Ni(phen) 2 (qddpz)] 21 to photocleave DNA underscores the importance of the quinone functionality in the qdppz structure in the photochemical cleavage process. Excited states of quinones are known to photocleave DNA both by electron transfer and H-abstraction mechanisms [50]. In addition, by exploiting their metal-centred redox activity, cobalt(III) complexes are capable of photogenerating various radicals, including reactive oxygen species, that can cleave the duplex by various mechanisms [51,52]. The DNA photocleavage activity of [Co(phen) 2 (dicnq)] 31 observed here is thus consistent with the facile metal-centred redox activity of this complex (vide infra) and is similar to the photocleaving abilities reported for other cobalt(III) polypyridyl complexes [27,51,52]. Finally, the photoinactivity of 21 [Ni(phen) 2 (dicnq)] can be rationalized in terms of the paramagnetic nature of the complex, which renders the excited state of the complex ineffective [27].
and photocleavage abilities of the metal complexes of dppz-based systems.
5. Abbreviations bp bpy CT DNA dicnq dppz FAB-MS MLCT NP phen phen-dione qdppz SCE TBACl TBAPF 6 base pairs 2,2-bipyridyl calf-thymus DNA dicyanodipyrido quinoxaline dipyrido[3,2-a:29,39-c]phenazine Fast atom bombardment mass spectroscopy metal-to-ligand charge transfer nucleotide phosphate 1,10-phenanthroline 1,10-phenanthroline-5,6-dione naptho[2,3-a]dipyrido[3,2-h:29,39f ]phenazine-5,18-dione saturated calomel reference electrode tetrabutylammonium chloride tetrabutylammonium hexauorophosphate
Acknowledgements We are grateful to the DST (New Delhi) and BRNS (Mumbai, India) for nancial support of this work. In addition, we thank the Central Drug Research Institute, Lucknow, for recording the mass spectra. C.V.S. and L.G. acknowledge research fellowships received from the CSIR (New Delhi) and D.E. thanks the TNCST (Chennai, India) for the award of a young scientists fellowship.
4. Summary While reports on the DNA interactions of ruthenium(II) complexes of polypyridyl ligands are numerous in the literature, those on interactions of the corresponding cobalt(III) and, more so, of nickel(II) complexes are relatively scarce [13,27,5154]. Various nickel(II) complexes of non-polypyridyl ligands are, however, known to recognize and also to cleave DNA [55,56]. In this study, we have attempted to unravel the DNA interactions of four new mixed-ligand polypyridyl complexes containing cobalt(III) or nickel(II) ions. Based on absorption and viscometric titration as well as thermal denaturation data, an intercalative mode of DNA binding involving qdppz / dicnq is suggested for these complexes. In general, the qdppz complexes are found to be better DNA-binding and photocleaving agents than the corresponding dicnq complexes, suggesting the importance of the quinone functionality in the DNA interactions of the complexes containing this ligand. Except for the luminescence features, the remainder of the physico-chemical properties and also the DNA-binding constants of the cobalt(III) and nickel(II) complexes of qdppz / dicnq are found to be somewhat similar to the corresponding properties of the ruthenium(II) analogues. On the other hand, the DNA photocleaving abilities of these complexes follow the order ruthenium(II).cobalt(III).nickel(II). Overall, the results described in this study underscore the importance of variation of the ligand and metal ion on the DNA-binding
References
[1] L.-N. Ji, X.-H. Zou, J.-G. Liu, Coord. Chem. Rev. 216 / 217 (2001) 513536. [2] N.A.P. Kane-Maguire, J.F. Wheeler, Coord. Chem. Rev. 211 (2001) 145162. [3] K.E. Erkkila, D.T. Odom, J.K. Barton, Chem. Rev. 99 (1999) 27772796. [4] Y. Xiong, L.-N. Ji, Coord. Chem. Rev. 185 / 186 (1999) 711733. [5] S.O. Kelly, J.K. Barton, in: A. Sigel, H. Sigel (Eds.), Metal Ions in Biological Systems, Vol. 39, Marcel Dekker, New York, 1998, pp. 211249. [6] B. Norden, P. Lincoln, B. Akerman, E. Tuite, in: A. Sigel, H. Sigel (Eds.), Metal Ions in Biological Systems, Vol. 33, Marcel Dekker, New York, 1996, pp. 177252. [7] A.K. Mesmaeker, J.P. Lecomte, J.M. Kelly, in: J. Mattay (Ed.), Topics in Current Chemistry, Vol. 177, 1996, pp. 2576. [8] H.H. Thorp, Adv. Inorg. Chem. 43 (1995) 127177. [9] D.S. Sigman, A. Mazumder, D.M. Perrin, Chem. Rev. 93 (1993) 22952316. [10] C.J. Murphy, J.K. Barton, Methods Enzymol. 226 (1993) 576594. [11] K.R. Patel, E.A. Plummer, M. Darwish, A. Rodger, M.J. Hannon, J. Inorg. Biochem. 91 (2002) 220229. [12] C. Stinner, M.D. Wightman, S.O. Kelley, M.G. Hill, J.K. Barton, Inorg. Chem. 40 (2001) 52455250.
C.V. Sastri et al. / Journal of Inorganic Biochemistry 94 (2003) 138145 [13] Q.-L. Zhang, J.-G. Liu, H. Chao, G.-Q. Xue, L.-N. Ji, J. Inorg. Biochem. 83 (2001) 4955. [14] B. Onfelt, P. Lincoln, B. Norden, J. Am. Chem. Soc. 123 (2001) 36303637. [15] Q.-X. Zhen, Q.-L. Zhang, J.-G. Liu, B.-H. Ye, L.-N. Ji, L. Wang, J. Inorg. Biochem. 78 (2000) 293298. [16] G. Albano, P. Belser, L. De Cola, M.T. Gandol, J. Chem. Soc., Chem. Commun. (1999) 11711172. [17] J.G. Collins, J.R. Aldrich-Wright, I.D. Greguric, P.A. Pellegrini, Inorg. Chem. 38 (1999) 55025509. [18] V.W.-W. Yam, K.K.-W. Lo, K.-K. Cheung, R.Y.-C. Kong, J. Chem. Soc., Dalton Trans. (1997) 20672072. [19] C. Moucheron, A.K. Mesmaeker, S. Choua, Inorg. Chem. 36 (1997) 584592. [20] H.D. Stoefer, N.B. Thornton, S.L. Temkin, K.S. Schanze, J. Am. Chem. Soc. 117 (1995) 71197128. [21] R.M. Hartshorn, J.K. Barton, J. Am. Chem. Soc. 114 (1992) 59195925. [22] Y. Jenkins, A.E. Friedman, N.J. Turro, J.K. Barton, Biochemistry 31 (1992) 1080910816. [23] N. Gupta, N. Grover, G.A. Neyhart, W. Liang, P. Singh, H.H. Thorp, Angew. Chem. Int. Ed. Engl. 31 (1992) 10481050. [24] A.E. Friedman, J.C. Chambron, J.P. Sauvage, N.J. Turro, J.K. Barton, J. Am. Chem. Soc. 112 (1990) 49604962. [25] E.J.C. Olson, D. Hu, A. Hormann, A.M. Jonkman, M.R. Arkin, E.D.A. Stemp, J.K. Barton, P.F. Barbara, J. Am. Chem. Soc. 119 (1997) 1145811467. [26] R.E. Holmlin, J.A. Yao, J.K. Barton, Inorg. Chem. 38 (1999) 174189. [27] S. Arounaguiri, B.G. Maiya, Inorg. Chem. 35 (1996) 42674270. [28] S. Arounaguiri, B.G. Maiya, Inorg. Chem. 38 (1999) 842843. [29] S. Arounaguiri, D. Eswaramoorthy, A.A. Kumar, A. Dattagupta, B.G. Maiya, Proc. Indian Acad. Sci. (Chem. Sci.) 112 (2000) 117. [30] A. Ambaroise, B.G. Maiya, Inorg. Chem. 39 (2000) 42564263. [31] A. Ambaroise, B.G. Maiya, Inorg. Chem. 39 (2000) 42644272. [32] D.D. Perrin, W.L.F. Armarego, D.R. Perrin, Purication of Laboratory Chemicals, 2nd Edition, Pergamon Press, New York, 1980. [33] E. Amouyal, A. Homsi, J.-C. Chambron, J.-P. Sauvage, J. Chem. Soc., Dalton Trans. (1990) 18411845. [34] A.A. Vlcek, Inorg. Chem. 6 (1967) 14251427.
145
[35] C.M. Harris, E.D. Mckenzie, J. Inorg. Nucl. Chem. 29 (1967) 10471068. [36] A. Earnshaw, Introduction to Magnetochemistry, Academic Press, New York, 1968. [37] M.E. Reichmann, S.A. Rice, C.A. Thomas, P. Doty, J. Am. Chem. Soc. 76 (1954) 30473053. [38] A. Wolfe, G.H. Shimer, T. Meehan, Biochemistry 26 (1987) 6392 6396. [39] J.M. Kelly, A.B. Tossi, D.J. McConnell, C. OHuigin, Nucleic Acids Res. 13 (1985) 60176034. [40] J. Marmur, P. Doty, J. Mol. Biol. 5 (1962) 109118. [41] G. Mehta, T. Sambaiah, B.G. Maiya, M. Sirish, A. Dattagupta, J. Chem. Soc., Perkin Trans. 1 (1995) 295297. [42] G. Mehta, S. Muthusamy, B.G. Maiya, M. Sirish, J. Chem. Soc., Perkin Trans. 1 (1996) 24212423. [43] K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, WileyInterscience, New York, 1977. [44] R.S. Nicholson, I. Shain, Anal. Chem. 36 (1964) 706723. [45] C.H. Hiort, P. Lincoln, B. Norden, J. Am. Chem. Soc. 115 (1993) 34483454. [46] J.-C. Chambron, J.-P. Sauvage, Chem. Phys. Lett. 182 (1991) 603607. [47] A.E. Frieman, C.V. Kumar, N.J. Turro, J.K. Barton, Nucleic Acids Res. 19 (1991) 25952602. [48] G. Cohen, H. Eisenberg, Bioplymers 8 (1969) 4555. [49] E.C. Long, J.K. Barton, Acc. Chem. Res. 23 (1990) 271273. [50] D.T. Breslin, J.E. Coury, J.R. Anderson, L. Mcfail-Isom, Y. Kan, L.D. Williams, L.A. Bottomley, G.B. Schuster, J. Am. Chem. Soc. 119 (1997) 50435044. [51] Q.-L. Zhang, J.-G. Liu, J. Liu, G.-Q. Xue, H. Li, J.-Z. Liu, H. Zhou, L.-H. Qu, L.-N. Ji, J. Inorg. Biochem. 85 (2001) 291296. [52] Q.-L. Zhang, J.-G. Liu, H. Xu, H. Li, J.-Z. Liu, H. Zhou, L.-H. Qu, L.-N. Ji, Polyhedron 20 (2001) 30493055. [53] X.-F. He, L. Wang, H. Chen, L. Xu, L.-N. Ji, Polyhedron 17 (1998) 31613166. [54] Y.F. Song, P. Yang, Polyhedron 20 (2001) 501506. [55] J.G. Muller, L.A. Kayser, S.J. Paikoff, V. Duarte, N. Tang, R.J. Perez, S.E. Rokita, C.J. Burrows, Coord. Chem. Rev. 185 / 186 (1999) 761774. [56] C.J. Burrows, J.G. Muller, Chem. Rev. 98 (1998) 11091151.
Vous aimerez peut-être aussi
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (587)
- ExercisesDocument3 pagesExercisesMuhammad JavedPas encore d'évaluation
- Low-Temperature NMR Spectra of Fluoride - Acetic Acid Hydrogen-Bonded Complexes in Aprotic Polar EnvironmentDocument10 pagesLow-Temperature NMR Spectra of Fluoride - Acetic Acid Hydrogen-Bonded Complexes in Aprotic Polar EnvironmentMuhammad JavedPas encore d'évaluation
- ImidazoleDocument1 pageImidazoleMuhammad JavedPas encore d'évaluation
- M.sc. Inorganic Chemisty Book ListDocument2 pagesM.sc. Inorganic Chemisty Book ListMuhammad JavedPas encore d'évaluation
- Gratz ElDocument12 pagesGratz ElMuhammad JavedPas encore d'évaluation
- Lists of Things Needed For ConferenceDocument1 pageLists of Things Needed For ConferenceMuhammad JavedPas encore d'évaluation
- Nobel Prize List ChemistryDocument24 pagesNobel Prize List ChemistryMuhammad JavedPas encore d'évaluation
- GATE Question Parer Chemistry 2007Document21 pagesGATE Question Parer Chemistry 2007Muhammad JavedPas encore d'évaluation
- Lists of Things Needed For ConferenceDocument1 pageLists of Things Needed For ConferenceMuhammad JavedPas encore d'évaluation
- Low-Temperature NMR Spectra of Fluoride - Acetic Acid Hydrogen-Bonded Complexes in Aprotic Polar EnvironmentDocument10 pagesLow-Temperature NMR Spectra of Fluoride - Acetic Acid Hydrogen-Bonded Complexes in Aprotic Polar EnvironmentMuhammad JavedPas encore d'évaluation
- Information Brochure OCES/ DGFS - 2013Document13 pagesInformation Brochure OCES/ DGFS - 2013Muhammad JavedPas encore d'évaluation
- GATE 2013 BrochureDocument93 pagesGATE 2013 BrochureSurbhi KumariPas encore d'évaluation
- Heterocycles Essentials1-2009Document2 pagesHeterocycles Essentials1-2009Aravindan NatarajanPas encore d'évaluation
- AcSIR Advt Jan-2013Document1 pageAcSIR Advt Jan-2013Muhammad JavedPas encore d'évaluation
- AcSIR Advt Jan-2013Document1 pageAcSIR Advt Jan-2013Muhammad JavedPas encore d'évaluation
- Radziszewskis Imidazole SynthesisDocument5 pagesRadziszewskis Imidazole SynthesisZahide Öztaş100% (1)
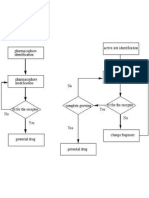
- Flow Charts of Two Strategies of Structure Based Drug DesignDocument1 pageFlow Charts of Two Strategies of Structure Based Drug DesignMuhammad JavedPas encore d'évaluation
- Cy 10Document24 pagesCy 10Joy KunduPas encore d'évaluation
- API DrugsDocument1 pageAPI DrugsMuhammad JavedPas encore d'évaluation
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (890)
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (399)
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (73)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5794)
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2219)
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (344)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (265)
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (119)
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)
- CALIS ScoringDocument2 pagesCALIS ScoringIqbal BaryarPas encore d'évaluation
- Prosecution and elements of crimes under Philippine lawsDocument14 pagesProsecution and elements of crimes under Philippine lawsNoel Cagigas FelongcoPas encore d'évaluation
- Carte Tehnica Partea IDocument22 pagesCarte Tehnica Partea IadrianPas encore d'évaluation
- START-HERE Ch11 LectureDocument84 pagesSTART-HERE Ch11 LecturePraveen VootlaPas encore d'évaluation
- Pos 324 Module 2 Unit 1 ElaborateDocument2 pagesPos 324 Module 2 Unit 1 ElaborateMaricar PaduaPas encore d'évaluation
- Prof Educ 2: Foundation of Special and Inclusive EducationDocument12 pagesProf Educ 2: Foundation of Special and Inclusive EducationNerissa Custosa BastoPas encore d'évaluation
- Chapter 1: Abnormal Behavior in Historical ContextDocument22 pagesChapter 1: Abnormal Behavior in Historical ContextEsraRamos100% (2)
- Assignment On Types of Retail Marketing: Submitted By: MR - Swapnil S. Ghag. Roll No.10 (A)Document9 pagesAssignment On Types of Retail Marketing: Submitted By: MR - Swapnil S. Ghag. Roll No.10 (A)Swapnil Ghag100% (1)
- Diamond-Blackfan AnemiaDocument5 pagesDiamond-Blackfan AnemiaTalal 197Pas encore d'évaluation
- JDP Rainbox Attenuation CratesDocument6 pagesJDP Rainbox Attenuation CratesBerat DalyabrakPas encore d'évaluation
- Environmental Pollution and DegradationDocument2 pagesEnvironmental Pollution and DegradationCharLene MaRiePas encore d'évaluation
- Insulation MBMA-NAIMA Acousticical Performance Guide Noise SoundDocument26 pagesInsulation MBMA-NAIMA Acousticical Performance Guide Noise SoundDianna LambertPas encore d'évaluation
- McDonlads Vs Burger KingDocument6 pagesMcDonlads Vs Burger KingSamuel Tyre Jr.Pas encore d'évaluation
- Makalah ThoughtDocument5 pagesMakalah Thoughtifa safiraPas encore d'évaluation
- Chemistry Code No. 1/2 Set: 3 Time Allowed: 3 Hours Maximum Marks: 100 General InstructionsDocument5 pagesChemistry Code No. 1/2 Set: 3 Time Allowed: 3 Hours Maximum Marks: 100 General InstructionsShalini KumariPas encore d'évaluation
- MicrosystemDocument5 pagesMicrosystembabalalaPas encore d'évaluation
- Cabuyao Integrated National High School: The Problem and Its BackgroundDocument4 pagesCabuyao Integrated National High School: The Problem and Its BackgroundJohn Carlo MolinaPas encore d'évaluation
- CD - 15. Republic of The Philippines Vs Sunlife Assurance of CanadaDocument2 pagesCD - 15. Republic of The Philippines Vs Sunlife Assurance of CanadaAlyssa Alee Angeles JacintoPas encore d'évaluation
- Calculate Size of Transformer / Fuse / Circuit Breaker: Connected Equipment To TransformerDocument16 pagesCalculate Size of Transformer / Fuse / Circuit Breaker: Connected Equipment To TransformerHari OM MishraPas encore d'évaluation
- MSDS - ENTEL BatteryDocument3 pagesMSDS - ENTEL BatteryChengPas encore d'évaluation
- Electric Vehicle BatteryDocument15 pagesElectric Vehicle BatteryTotal Acess100% (1)
- Home & Garden 2015Document32 pagesHome & Garden 2015The Standard NewspaperPas encore d'évaluation
- Spoiled and Improper PackingDocument4 pagesSpoiled and Improper PackingshirvenaPas encore d'évaluation
- Lesson 1:: Introduction To Science, Technology and SocietyDocument17 pagesLesson 1:: Introduction To Science, Technology and SocietyAlexis A. AguilarPas encore d'évaluation
- Yoga Nidra MethodDocument13 pagesYoga Nidra MethodPrahlad Basnet100% (2)
- Dimensions-Mm (Inch) : Valve Regulated Lead Acid Battery (VRLA)Document2 pagesDimensions-Mm (Inch) : Valve Regulated Lead Acid Battery (VRLA)orunmila123Pas encore d'évaluation
- Global Chlor - Alkali Market Statistics Update June 16 2014Document31 pagesGlobal Chlor - Alkali Market Statistics Update June 16 2014diego_cáceres_30Pas encore d'évaluation
- 2022 Intro To Process and Plant Safety NewDocument163 pages2022 Intro To Process and Plant Safety Newdavid gabriel100% (2)
- Smartphone Technician Cum App Tester: Trade PracticalDocument218 pagesSmartphone Technician Cum App Tester: Trade PracticalF ZaidiPas encore d'évaluation
- LLL'DSWD: Administrative Order No. Series 2017Document18 pagesLLL'DSWD: Administrative Order No. Series 2017SGTPas encore d'évaluation