Académique Documents
Professionnel Documents
Culture Documents
Types of CAM
Transféré par
jojolilimomoDescription originale:
Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Types of CAM
Transféré par
jojolilimomoDroits d'auteur :
Formats disponibles
Ch'in and Tang first described cystic adenomatoid malformation (CAM) as a distinct entity in 1949.
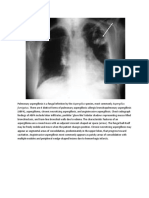
[1] CAM is a developmental hamartomatous abnormality of the lung, with adenomatoid proliferation of cysts resembling bronchioles. CAM represents approximately 25% of all congenital lung lesions. The following 2 radiographs are from the same infant with congenital cystic adenomatoid malformation:
Initial anteroposterior radiograph of the chest in a patient with congenital cystic adenomatoid malformation on the first day of life, with dense lungs and a suggestion that the right lung is
slightly more voluminous than the left lung. On the second day of life (same patient as in previous image), an anteroposterior radiograph shows physiologic fluid resorbed from an area of congenital cystic adenomatoid malformation and replaced with an air-containing cystic area occupying the right upper lung.
Types of CAM
CAM is subdivided into 3 major types[2] :
Type I lesions, the most common, are composed of 1 or more cysts measuring 2-10 cm in diameter. Larger cysts are often accompanied by smaller cysts, and their walls contain muscle, elastic, or fibrous tissue. Cysts are frequently lined by pseudostratified columnar epithelial cells, which occasionally produce mucin. Mucinogenic differentiation is unique to this subtype of CAM.
Type II lesions are characterized by small, relatively uniform cysts resembling bronchioles. These cysts are lined by cuboid-to-columnar epithelium and have a thin fibromuscular wall. The cysts generally measure 0.5-2 cm in diameter. Type III lesions consist of microscopic, adenomatoid cysts and are grossly a solid mass without obvious cyst formation. Microscopic adenomatoid cysts are present.
CAM receives its blood supply from the pulmonary circulation and is not sequestered from the tracheobronchial tree. However, type II and III lesions can occasionally coexist with extralobar sequestration, and in such cases, they may receive systemic arterial supply. CAM may also occur in combination with a polyalveolar lobe. A polyalveolar lobe is a form of congenital emphysema with increased number of alveoli with normal bronchi and pulmonary vasculature. CAM usually occurs early in fetal life, whereas polyalveolar lobe occurs late.[3]
Differential diagnosis
CAM is differentiated from other congenital cystic disease by 5 characteristics[4, 5, 6] :
Absence of bronchial cartilage (unless it is trapped within the lesion) Absence of bronchial tubular glands Presence of tall columnar mucinous epithelium Overproduction of terminal bronchiolar structures without alveolar differentiation, except in the subpleural areas Massive enlargement of the affected lobe that displaces other thoracic structures
Preferred examination
CAM may be initially detected during prenatal ultrasonography. After birth, chest radiography should be performed first.[7] Although lesions remain filled with fluid, postnatal sonography can be used for a more detailed assessment, particularly in type III lesions. Once lesions are air-filled, CT scanning is necessary for determination of the type and extent of the lesions.[8]
Limitations of techniques
Prenatal ultrasonography is accurate in diagnosing CAM. Prenatally diagnosed lesions may be asymptomatic at birth (71%), and they have normal radiographic findings (57%). A concurrent sequestration may not be identified. Usually, radiographic findings are apparent in a symptomatic individual, but they may not be as apparent in an asymptomatic child. Most often, the diagnosis can be made by using plain radiographs. CT scans may be used to diagnose confusing cases. Overlapping CT features exist among cases of CAM, pulmonary sequestration, bronchogenic cyst, and other foregut malformations. CT is more accurate than radiography or ultrasonography in classifying the type of CAM.
Recent studies
In a retrospective study, Scialpi et al proposed that because of similar CT scan patterns between intrapulmonary bronchogenic cyst (IBC) and type I CAM that precluded differentiation, surgical resection of all intrapulmonary cystic lesions in adults is required, as type I CAM is a precursor of mucinous bronchioloalveolar carcinoma.[9] In the study, of 9 patients with a histologic diagnosis of pulmonary cystic disease following surgery, 6 had IBC
and 3 had type I cystic adenomatoid malformation (CAM). (See the CT Scan discussion below.) Komori et al determined the optimal age for surgery in patients with congenital cystic lung disease is younger than 1 year. They used radionuclide imaging to assess long-term pulmonary function following lobectomy for congenital cystic lung disease in 93 infants and children. Patients who were younger than 1 year at surgery had a significantly lower mean transit time (marker for air trapping) at 5 years and 10 years postoperatively than those who were older than 1 year at surgery. Additionally, at 10 years after surgery, perfusion scintigraphy scores were higher and mean transit times were lower in patients without infection compared with patients with infection.[10] In a French study, Zeidan et al showed that prenatal MRI was less accurate than postnatal CT scan, which, according to the authors, remains the most reliable diagnostic modality to specify the location, extent, and type of lesions.[7] The authors evaluated the accuracy of prenatal MRI and postnatal CT imaging in identifying congenital cystic adenomatoid malformation and bronchopulmonary sequestration by comparison with histologic analysis. (See the CT Scan and MRI discussions below.) See the radiographic images of cystic adenomatoid malformation (CAM), below.
Initial anteroposterior radiograph of the chest in a patient with congenital cystic adenomatoid malformation on the first day of life, with dense lungs and a suggestion that the right lung is
slightly more voluminous than the left lung. On the second day of life (same patient as in previous image), an anteroposterior radiograph shows physiologic fluid resorbed from an area of congenital cystic adenomatoid malformation and replaced with an air-containing cystic
area occupying the right upper lung. Anteroposterior radiograph of a patient with congenital cystic adenomatoid malformation with bilateral basilar cystic disease at several weeks of age. The patient's mediastinum is positioned toward the right, secondary to congenital scoliosis and larger left-sided cysts.
Usually, the radiographic pattern appears as an expansile soft-tissue mass containing multiple air-filled cystic masses of varying size and shifting of the mediastinum. Initially and early in life, a homogeneous fluid-opacity pulmonary mass may present and evolve to demonstrate an air-filled cystic radiographic appearance. The initial dense appearance is a result of delayed emptying of alveolar fluid via either the bronchi or lymphatic and circulatory systems. In patients with CAM, the pattern in the lung demonstrates multiple radiolucent areas that vary greatly in size and shape. Cysts are separated from each other by strands of opaque pulmonary tissue. The involved lung may appear honeycombed or spongy, but occasionally, 1 large cyst may overshadow the others. Air-trapping within cystic spaces can cause rapid enlargement of the CAM and subsequent respiratory embarrassment. Findings are usually apparent in a symptomatic individual, but they may not be as apparent in an asymptomatic child.
Degree of confidence
Most often, the diagnosis of CAM can be made by using plain radiographs. Most commonly, CAM appears as a mass composed of numerous air-containing cysts scattered irregularly throughout a segment of the lung. The mass is space occupying, expanding the ipsilateral hemithorax and shifting the mediastinum to the contralateral side. Usually, CT is not necessary for the diagnosis of CAM in the neonatal period. Identifying the lobe involved or determining the extent of mass effect on the uninvolved lung may be possible. CT can be used to diagnose confusing cases encountered in infancy, childhood, or adult life or for planning surgery.
False positives and negatives
Pneumatoceles that form subsequent to bacterial pneumonia (eg, streptococcal, staphylococcal) can be mistaken for CAM, particularly in the older child. Congenital lobar emphysema refers to overexpansion of 1 lobe, typically an upper lobe or right middle lobe, that leads to mass effect and respiratory distress. Although this entity could
potentially be confused with CAM, typical features of overexpanded but normal parenchyma can be observed and confirmed with CT if necessary. Pulmonary interstitial emphysema may resemble CAM when it is complicated by large air collections. However, these are also typically associated with linear collections and preceded by high-pressure ventilation and barotrauma. The air collections are located in the interstitial lymphatics. On plain radiographs, intrapulmonary sequestration with infection and abscess formation can be difficult to differentiate from CAM. Bronchogenic cysts are usually fluid filled and well circumscribed. Neuroenteric cysts are posterior mediastinal soft-tissue masses that are usually associated with vertebral anomalies. See the CT images of cystic adenomatoid malformation (CAM) below.
CT scan of the chest demonstrating a multiseptated cystic lesion in the right upper lobe consistent with localized congenital cystic adenomatoid
malformation. CT image of the bases of the lungs showing an unusual bilateral congenital cystic adenomatoid malformation. CT findings are correlated with the pathologic findings.[11, 12, 13]
Areas of small cysts (< 2 cm in diameter) appearing with other abnormalities (a larger cystic area, consolidation, or low attenuation) are the most frequent findings. Multiple large cystic lesions (>2 cm in diameter) are seen alone or with other abnormalities (areas of small cysts, consolidation, or low attenuation). Low-attenuation areas are clusters of microcysts.
Air-fluid levels can be seen in some cysts. These lesions may be predominantly type I, type II, or a combination of both. CAM may completely resolve, as indicated by sonographic and plain radiographic criteria, but persistent abnormalities are well demonstrated on CT examination.
Approximately 25% of lesions diagnosed as CAM may be either pulmonary sequestration or bronchogenic cysts. Overlapping CT features can also exist among other foregut malformations. In a retrospective study, Scialpi et al proposed that because of similar CT scan patterns between intrapulmonary bronchogenic cyst (IBC) and type I CAM that precluded differentiation, surgical resection of all intrapulmonary cystic lesions in adults is required, as type I CAM is a precursor of mucinous bronchioloalveolar carcinoma.[9] In the study, of 9 patients with a histologic diagnosis of pulmonary cystic disease following surgery, 6 had IBC and 3 had type I CAM. In a French study, Zeidan et al showed that prenatal MRI was less accurate than postnatal CT scan, which, according to the authors, remains the most reliable diagnostic modality to specify the location, extent, and type of lesions.[7] The authors evaluated the accuracy of prenatal MRI and postnatal CT imaging in identifying congenital cystic adenomatoid malformation and bronchopulmonary sequestration by comparison with histologic analysis Prenatal sonography enables the identification of cystic adenomatoid malformation (CAM) in a population of infants who are asymptomatic at birth. Regression of CAM on prenatal sonograms is common, but this process usually does not continue postnatally.
Partially cystic, partially echogenic masses are characteristic of type I or type II lesions; the size or dimension of the cysts distinguishes the 2 types Type III lesions may be large and entirely echogenic Usually, the newborn is symptomatic at birth, with the finding of a lesion exceeding 50% of the hemithorax
The accuracy of prenatal ultrasonography in classifying the lesions of CAM is approximately 77%. Prenatally diagnosed lesions may be asymptomatic at birth (71%), and there may be normal findings on radiographic examinations (57%). If radiographic results are normal, CT should be performed because it is more sensitive for detection of smaller lesions. False-positive findings include bronchogenic cysts and pulmonary sequestration Komori et al used radionuclide imaging to assess long-term pulmonary function following lobectomy for congenital cystic lung disease in 93 infants and children, and they determined the optimal age for surgery in patients with congenital cystic lung disease is younger than 1 year. Patients who were younger than 1 year at surgery had a significantly lower mean transit time (marker for air trapping) at 5 years and 10 years postoperatively than those who were older than 1 year at surgery. Additionally, at 10 years after surgery, perfusion scintigraphy scores were higher and mean transit times were lower in patients without infection compared with patients with infection.[10]
Vous aimerez peut-être aussi
- Radiological Signs (Shënja Radiologjike) - 1Document122 pagesRadiological Signs (Shënja Radiologjike) - 1Sllavko K. KallfaPas encore d'évaluation
- Allure USA 2013-09Document256 pagesAllure USA 2013-09jojolilimomo100% (2)
- Allure USA 2013-09Document256 pagesAllure USA 2013-09jojolilimomo100% (2)
- InfertilityDocument101 pagesInfertilityjojolilimomo100% (1)
- SGOP 2008 (Guidelines On Management)Document87 pagesSGOP 2008 (Guidelines On Management)Via Alip100% (1)
- ABC of Pleural DiseasesD'EverandABC of Pleural DiseasesNajib M. RahmanPas encore d'évaluation
- Gene TherapyDocument28 pagesGene TherapyKaycee Gretz LorescaPas encore d'évaluation
- Triple Warmer Meridian Acupoint Chart PDFDocument1 pageTriple Warmer Meridian Acupoint Chart PDFVicaas VSPas encore d'évaluation
- Vojta TherapyDocument4 pagesVojta TherapyPrashu Jain100% (1)
- Anemia in Pregnancy - Anahita ChauhanDocument45 pagesAnemia in Pregnancy - Anahita ChauhanjojolilimomoPas encore d'évaluation
- 06 ChestDocument16 pages06 ChestANAS ALIPas encore d'évaluation
- Schilling Test: DR - CSBR.Prasad, M.D.Document26 pagesSchilling Test: DR - CSBR.Prasad, M.D.muhammad100% (1)
- Cystic Adenomatoid Malformation (CAM)Document38 pagesCystic Adenomatoid Malformation (CAM)Aliyah Tofani PawelloiPas encore d'évaluation
- AtpdDocument48 pagesAtpddrkadiyala2100% (2)
- Genital Tract InjuryDocument24 pagesGenital Tract InjuryAbdulJabar Riadh100% (1)
- Congenital Pulmonary Airway MalformationDocument5 pagesCongenital Pulmonary Airway MalformationKiagusRoyPas encore d'évaluation
- Approach To Interstitial Lung DiseasesDocument38 pagesApproach To Interstitial Lung DiseasesmeawchiPas encore d'évaluation
- Thyroid StormDocument11 pagesThyroid StormAndrew UtamaPas encore d'évaluation
- Section II - Chest Radiology: Figure 1ADocument35 pagesSection II - Chest Radiology: Figure 1AHaluk Alibazoglu100% (1)
- Direpub 2063 OnlineDocument9 pagesDirepub 2063 OnlinephobicmdPas encore d'évaluation
- SGL5 - HaemoptysisDocument73 pagesSGL5 - HaemoptysisDarawan MirzaPas encore d'évaluation
- Pneumothoraks Jurnal RadiologiDocument9 pagesPneumothoraks Jurnal RadiologiRachmi MerrinaPas encore d'évaluation
- International Journal of Cancer and Clinical ResearchDocument5 pagesInternational Journal of Cancer and Clinical ResearchDenny SutantoPas encore d'évaluation
- Tumores Solitarios Pulmonares InusualesDocument8 pagesTumores Solitarios Pulmonares InusualesSasha de la CruzPas encore d'évaluation
- Imaging of Solitary and Multiple Pulmonary NodulesDocument75 pagesImaging of Solitary and Multiple Pulmonary NodulesAnshulVarshneyPas encore d'évaluation
- Radiological Signs (Shënja Radiologjike) - 1Document122 pagesRadiological Signs (Shënja Radiologjike) - 1Sllavko K. KallfaPas encore d'évaluation
- Congenital Lung.Document159 pagesCongenital Lung.novitafitri123Pas encore d'évaluation
- Congenital Pulmonary Airway Malformation Radiology Reference ArticleDocument1 pageCongenital Pulmonary Airway Malformation Radiology Reference ArticleWirda Elya SariPas encore d'évaluation
- Antenatally DiagnosedDocument9 pagesAntenatally DiagnosedAsty LebaoPas encore d'évaluation
- 4.pneumothorax Imaging - Practice Essentials, Radiography, Computed TomographyDocument11 pages4.pneumothorax Imaging - Practice Essentials, Radiography, Computed TomographyAchmadRizaPas encore d'évaluation
- Bronchogenic Malignancy and Metastatic Disease: Hilar MassesDocument4 pagesBronchogenic Malignancy and Metastatic Disease: Hilar MassesTira SariPas encore d'évaluation
- Chest Radiography Diagnostic Value and Interpretation: Imaging ModalitiesDocument12 pagesChest Radiography Diagnostic Value and Interpretation: Imaging Modalitiesdoctor evanPas encore d'évaluation
- 2021-Congenital Pulmonary Airway MalformationDocument8 pages2021-Congenital Pulmonary Airway MalformationAzucena La QuirozPas encore d'évaluation
- Solitary Pulmonary Nodule: Figure 19-14Document2 pagesSolitary Pulmonary Nodule: Figure 19-14LORENZO GABRIEL BANAYOPas encore d'évaluation
- The Radiologic Appearance of Lung Cancer: Review Article Peter B. O'donovan, MDDocument12 pagesThe Radiologic Appearance of Lung Cancer: Review Article Peter B. O'donovan, MDAdrianus KevinPas encore d'évaluation
- Radiologicalfeaturesofbronchogeniccarcinoma 160608214533Document135 pagesRadiologicalfeaturesofbronchogeniccarcinoma 160608214533Abdi KumalaPas encore d'évaluation
- Lung Cancer in ChildDocument4 pagesLung Cancer in Childtonirian99Pas encore d'évaluation
- Keywords: Benign Lung Nodules, CT, Lung Cancer, PET/CT, Pulmonary NodulesDocument15 pagesKeywords: Benign Lung Nodules, CT, Lung Cancer, PET/CT, Pulmonary NodulesFadhil NugrahaPas encore d'évaluation
- Malignant Mesothelioma ImagingDocument22 pagesMalignant Mesothelioma ImagingFatya WelinsaPas encore d'évaluation
- Lung CancerDocument2 pagesLung CancerShalini ShanmugalingamPas encore d'évaluation
- Cyst and Cystlike Lung LesionsDocument57 pagesCyst and Cystlike Lung LesionsAna StankovicPas encore d'évaluation
- An Unusual Presentation of Multiple Cavitated Lung Metastases From Colon CarcinomaDocument4 pagesAn Unusual Presentation of Multiple Cavitated Lung Metastases From Colon CarcinomaGita AmeliaPas encore d'évaluation
- Congenital Pulmonary Airway (Cystic Adenomatoid) Malformation - UpToDateDocument28 pagesCongenital Pulmonary Airway (Cystic Adenomatoid) Malformation - UpToDateTung PhamPas encore d'évaluation
- Journal Radiologi 3Document14 pagesJournal Radiologi 3WinayNayPas encore d'évaluation
- Articulo 20 de OctubreDocument6 pagesArticulo 20 de OctubreAlba PalaciosPas encore d'évaluation
- 521 FullDocument11 pages521 FulljhordyafsPas encore d'évaluation
- Pitfalls of EmphysemaDocument7 pagesPitfalls of EmphysemaWahyu Puspita IrjayantiPas encore d'évaluation
- Answer IsDocument2 pagesAnswer Iscatherine robertPas encore d'évaluation
- 2 Complications of Pulmonary TB PDFDocument18 pages2 Complications of Pulmonary TB PDFGuling SetiawanPas encore d'évaluation
- High Resolution CT of The Lungs: Technique, Indications and FindingsDocument9 pagesHigh Resolution CT of The Lungs: Technique, Indications and FindingsHari Baskar SPas encore d'évaluation
- The Solitary Pulmonary NoduleDocument8 pagesThe Solitary Pulmonary NoduleRafa IsaacPas encore d'évaluation
- EGFR Mutation of Adenocarcinoma in Congenital Cystic Adenomatoid Malformation/Congenital Pulmonary Airway Malformation: A Case ReportDocument6 pagesEGFR Mutation of Adenocarcinoma in Congenital Cystic Adenomatoid Malformation/Congenital Pulmonary Airway Malformation: A Case ReportSarah QonitahPas encore d'évaluation
- PDF To WordDocument17 pagesPDF To Wordanisa ghinaPas encore d'évaluation
- Back To Basics - Must Know' Classical Signs in Thoracic RadiologyDocument30 pagesBack To Basics - Must Know' Classical Signs in Thoracic Radiologyvalencia suwardiPas encore d'évaluation
- Thorax 2003 Lordan 814 9Document7 pagesThorax 2003 Lordan 814 9Ari KuncoroPas encore d'évaluation
- MUCORMYCOSISDocument37 pagesMUCORMYCOSISSK MOMTAZUL KARIMPas encore d'évaluation
- Congenital Lobar Overinflation Congenital Lobar Overinflation, Also KnownDocument3 pagesCongenital Lobar Overinflation Congenital Lobar Overinflation, Also KnownIqbal Muhammad Yaa-BegitulahPas encore d'évaluation
- 1229 6490 1 PB PDFDocument10 pages1229 6490 1 PB PDFSitti_HazrinaPas encore d'évaluation
- Jurnal Radiologi TBDocument7 pagesJurnal Radiologi TBrian00019Pas encore d'évaluation
- Rad PathologyDocument178 pagesRad PathologyJames MoralesPas encore d'évaluation
- Maq 2Document5 pagesMaq 2eduardoPas encore d'évaluation
- Nader Kamangar, MD, FACP, FCCP FCCM, FAASM, Associate Professor of Clinical Medicine, UniversityDocument7 pagesNader Kamangar, MD, FACP, FCCP FCCM, FAASM, Associate Professor of Clinical Medicine, UniversitynurulfitriantisahPas encore d'évaluation
- 2.2 Pulmonary Radiology 2Document41 pages2.2 Pulmonary Radiology 2Casey Rae YanoPas encore d'évaluation
- 2009 Tumores DiafragmaDocument9 pages2009 Tumores DiafragmaJavier VegaPas encore d'évaluation
- 07.10.22. (A) Bronchogenic Cyst. StatPearls 2022Document5 pages07.10.22. (A) Bronchogenic Cyst. StatPearls 2022Juan Davio Quispe FernándezPas encore d'évaluation
- Lesiones Cavitarias - Chest - Sper BuenoDocument11 pagesLesiones Cavitarias - Chest - Sper BuenoMary CogolloPas encore d'évaluation
- Complications of Pulmonary TuberculosisDocument18 pagesComplications of Pulmonary TuberculosisedhobiondiPas encore d'évaluation
- Bronquiolite DiagnosticoDocument14 pagesBronquiolite DiagnosticoFrederico PóvoaPas encore d'évaluation
- Inadvertent Chest Tube Insertion in Congential Cystic Adenomatoid Malformation and Congential Lobar Emphysema Highlighting An Important ProblemDocument0 pageInadvertent Chest Tube Insertion in Congential Cystic Adenomatoid Malformation and Congential Lobar Emphysema Highlighting An Important ProblemWenny EudensiaPas encore d'évaluation
- Practical No 28 - Respiratory SystemDocument8 pagesPractical No 28 - Respiratory Systemkartik.patil151106Pas encore d'évaluation
- Chest Radio GraphsDocument13 pagesChest Radio Graphsdranees12Pas encore d'évaluation
- Difficult Acute Cholecystitis: Treatment and Technical IssuesD'EverandDifficult Acute Cholecystitis: Treatment and Technical IssuesPas encore d'évaluation
- How Are Questionnaires Prepared?: List The Variables To Be MeasuredDocument8 pagesHow Are Questionnaires Prepared?: List The Variables To Be MeasuredjojolilimomoPas encore d'évaluation
- Quantitative ResearchDocument57 pagesQuantitative ResearchjojolilimomoPas encore d'évaluation
- PCHRD'S Guideline of ProposalsDocument17 pagesPCHRD'S Guideline of ProposalsjojolilimomoPas encore d'évaluation
- POGS ISIS Excel INSTRUCTIONSDocument24 pagesPOGS ISIS Excel INSTRUCTIONSjojolilimomoPas encore d'évaluation
- Isis User ManualDocument38 pagesIsis User Manualjojolilimomo0% (1)
- Dermoid Cyst-Grand RoundsDocument13 pagesDermoid Cyst-Grand RoundsjojolilimomoPas encore d'évaluation
- Two Up Cont CPG Intrapartum PDFDocument98 pagesTwo Up Cont CPG Intrapartum PDFjojolilimomoPas encore d'évaluation
- Philippine Civil Service Examination ReviewerDocument33 pagesPhilippine Civil Service Examination Reviewermaverick_ariel_18100% (1)
- Gestational DiabetesDocument52 pagesGestational Diabetestammycristobalmd100% (8)
- Antepartum Haemorrhage (APH) : Prepared by Helen CookeDocument22 pagesAntepartum Haemorrhage (APH) : Prepared by Helen CookejojolilimomoPas encore d'évaluation
- MG 17Document22 pagesMG 17jojolilimomoPas encore d'évaluation
- ICD-9-CM Coding Proposals: Phlebitis and Thrombophlebitis Venous Complications in PregnancyDocument16 pagesICD-9-CM Coding Proposals: Phlebitis and Thrombophlebitis Venous Complications in PregnancyjojolilimomoPas encore d'évaluation
- Consequences of Adverse Childhood ExperiencesDocument4 pagesConsequences of Adverse Childhood ExperiencesChris PenPas encore d'évaluation
- A Preliminary Study On The Safety and Efficacy of HD-03/ES Therapy in Patients With Chronic Hepatitis BDocument5 pagesA Preliminary Study On The Safety and Efficacy of HD-03/ES Therapy in Patients With Chronic Hepatitis BalkaPas encore d'évaluation
- 7 Implant Post Op InstructionsDocument2 pages7 Implant Post Op InstructionsmrcalvintinePas encore d'évaluation
- Case Report PresentationDocument19 pagesCase Report PresentationCheyenne PetersPas encore d'évaluation
- Thromboangiitis ObliteransDocument5 pagesThromboangiitis Obliteransklinik mandiriPas encore d'évaluation
- HIV:AIDS Determinants and Control of The EpidemicDocument4 pagesHIV:AIDS Determinants and Control of The EpidemicahiPas encore d'évaluation
- HSB Pneumonia Antibiotic AlgorithmDocument4 pagesHSB Pneumonia Antibiotic AlgorithmDr.Senthil KumarPas encore d'évaluation
- PARASITOLOGYDocument15 pagesPARASITOLOGYJoshua EnzonPas encore d'évaluation
- CH 13 Microbe-Human Interactions (Student) .PPT (Compatibility Mode)Document12 pagesCH 13 Microbe-Human Interactions (Student) .PPT (Compatibility Mode)shanika100% (1)
- Matusiewicz Reynolds Lejuez 2010Document87 pagesMatusiewicz Reynolds Lejuez 2010NISHANTHA KARUNARATHNAPas encore d'évaluation
- Cystic Fibrosis PresentationDocument27 pagesCystic Fibrosis PresentationVy LêPas encore d'évaluation
- Groin Pain in Football Players: A Systematic Diagnostic ApproachDocument5 pagesGroin Pain in Football Players: A Systematic Diagnostic ApproachCherbiti Mohammed Amine100% (1)
- Complication of Blood TransfusionDocument61 pagesComplication of Blood Transfusionতৌহিদ তপুPas encore d'évaluation
- Impression HerniaDocument3 pagesImpression Herniakjeseo8Pas encore d'évaluation
- (Cô Vũ Mai Phương) Tài liệu LIVESTREAM - Tổng ôn 2 tuần cuối - Chủ đề nóng Covid 19Document3 pages(Cô Vũ Mai Phương) Tài liệu LIVESTREAM - Tổng ôn 2 tuần cuối - Chủ đề nóng Covid 19Mon MonPas encore d'évaluation
- ENEMADocument53 pagesENEMAJocel Mae OrtegaPas encore d'évaluation
- Vanessa LeprosyDocument18 pagesVanessa LeprosyMichael Angelo SeñaPas encore d'évaluation
- Presentasi Kasus Acne VulgarisDocument27 pagesPresentasi Kasus Acne VulgarisAndi Rizki TenryayuPas encore d'évaluation
- Blood Typing Lab HandoutDocument2 pagesBlood Typing Lab HandoutAlex BennettPas encore d'évaluation
- Reviewer in Ab PsychDocument8 pagesReviewer in Ab PsychMica MoradaPas encore d'évaluation
- Kista BartoliniDocument24 pagesKista BartoliniTri HariadyPas encore d'évaluation
- Questions and Answers On Parkinsons DiseaseDocument2 pagesQuestions and Answers On Parkinsons DiseaseslapunkPas encore d'évaluation
- Drug Study On Mesalamine MercaptopurineDocument15 pagesDrug Study On Mesalamine Mercaptopurineسوما الشمريPas encore d'évaluation