Académique Documents
Professionnel Documents
Culture Documents
CO2 Removal From Syngas
Transféré par
Syed Shah Jehan GillaniCopyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
CO2 Removal From Syngas
Transféré par
Syed Shah Jehan GillaniDroits d'auteur :
Formats disponibles
PresentedatNitrogen+Syngas2012,2023February2012,Athens,Greece
*Speaker
CO
2
RemovalfromSyngasUsingPiperazineActivated
MDEAandPotassiumDimethylGlycinate
R.ScottAlvis*,NathanA.Hatcher&RalphH.Weiland
OptimizedGasTreating,Inc.
12337JonesRd.,Suite432
Houston,TX77070
ABSTRACT
Althoughmethyldiethanolamine,MDEA,canbeactivatedbyanumberofamines,piperazineis
themostcommonlyusedpromoterinapplicationsinvolvingCO
2
removalfromsyngas,aswellasfrom
naturalgasinLNGproduction.Becauseofitsverylowregenerationenergy,removingCO
2
usingMDEA
alonewouldbepreferred;however,thereactioninsolutionisextremelyslowandtheabsorption
processiscontrolledentirelybyresistancetomasstransferinthesolventphase.Piperazineishighly
reactivewithCO
2
(abouttentimesfasterkineticsthanmonoethanolamine)whichgreatlyenhancesCO
2
absorptionrates.Yetbecauseonlyrelativelysmallconcentrationsofpiperazineareneeded,solvent
regenerationenergyrequirementsarenotmuchhigherthanforMDEAalone.
Aspartofareviewofasolventchangeoutfromaminepromotedhotpotassiumcarbonateto
piperazineactiveMDEA,thispaperaddressestheeffectsofsuchparametersastotalsolventstrength,
piperazinetoMDEAratioandtreatingtemperatureandpressureonthetreatedgasCO
2
contentand
thenecessaryregenerationenergy.ItalsocomparespiperazineactivatedMDEAwithpiperazine
activatedAlkazidDIK(potassiumsaltoftheaminoaciddimethylglycine,KDiMGly)asapotentialsolvent
forCO
2
removalapplications.AlkazidDIKisasolventusedsincethemid1930sforH
2
Sremovalin
refineryandcoalgasapplicationsbutitfelloutoffavourwiththeadventofMDEA.Itiscompletely
nonvolatileandmaybemoreoxidationresistantthantheconventionalamines.
INTRODUCTION
Intheearlydaysofammoniaproduction,monoethanolamine(MEA)wascommonlyusedfor
CO
2
removalfromthesynthesisgasusingtheGirbotolprocess.Somewhatlater,hotpotassium
carbonate(theBenfield,orHotPotprocess)wasused,ofteninasplitflowconfigurationdescribedasa
twostageBenfieldLoHeatprocessforenergyconservation.Inthelast20years,averysubstantial
fractionoftheseplantshavebeenretrofittedusingBASFsaMDEAprocess.
NMethyldiethanolamine(MDEA)isatertiaryaminewhoseaminogroupisincapableofreacting
withCO
2
.However,itisalkalineandsoisanexcellentsinkforprotonsproducebyCO
2
hydrolysis.
Becauseitisnonreactive,aqueousMDEAbyitselfabsorbsCO
2
fartooslowlytobeaneffectivesolvent
fortreatingammoniasynthesisgas.Butwhenspikedwitharelativelysmallconcentrationofpiperazine,
adiaminethatreactsextraordinarilyfastwithCO
2
,theresultingblendisanexcellentsolventfortreating
syngasandremovingCO
2
intheproductionofLNG.
Inthispaper,wefirstpresenttheresultsofaquantitativestudyofthepiperazinepromotionof
MDEA,specificallytheeffectsofpiperazinetoMDEAratio,totalaminestrength,andthetreating
temperatureonperformanceofatypicalammoniasyngasCO
2
removalsystem.Inarecentpatent,
Wagneretal.(2009)proposedusingthealkalimetalsaltsofanumberoftertiaryaminoacids,
appropriatelypromotedwithreactiveaminessuchasMEA.Someofthepotentiallymoreinteresting
resultsofourstudyincludetheutilityofoperatingathighertemperatureswithlowerratherthanhigher
totalamineconcentrations,andtheexistenceofoperatingboundariesthatcanleadtounstable
operationwhenapproachedtooclosely.
Followingadiscussionofaminoacidsandtheirmodeofoperation,wecriticallyanalyzethe
possibilityofusingthepotassiumsaltofthetertiaryaminoaciddimethylglycine,promotedwith
piperazineasasyngastreatingsolvent.Theresultsshowthatitispossibletotreatsyngasquite
effectivelyusingsuchasolventbutwithmuchlowerconcentrationofthepiperazinepromoter.
Furthermore,resultssuggestthatthecrossexchangercommonlyusedasaheatintegrationtoolin
treatingplantscanbecompletelyeliminated.
USINGTERTIARYAMINESFORCO
2
REMOVAL
Beforelookingatspecificchemicalsolvents,itisbeneficialtodescribethefunctionofanamine
thatiscompletelynonreactivetowardsCO
2
,intheCO
2
absorptionprocess,andtogiveaquantitative
pictureofhoweffectivepiperaziner i s, aminesdissociateinwaterand
producehydroxylion:
eallyis.L ke all amine tertiary
RN + E
2
0 - RNE
+
+ 0E
-
Thehydroxylioniswhatgivesaminestheiralkalinity.However,CO
2
doesnotreactwithatertiaryamino
groupbecausethisgrouplacksthemobilehydrogennecessarytoexchangefortheCO
2
andform
carbamate.Instead,theCO
2
merelyabsorbsintowaterandhydrolyzes:
C0
2
+ E
2
0 - E
+
+ EC0
3
-
Theliberatedprotonisneutralizedbytheaminesalkalinityasrepresentedbythehydroxylgroup.Thus,
unlessonecandirectlycatalyzethehydrolysisreactionitself(ascanbedoneusingcarbonicanhydrase,
forexample),CO
2
absorptionratesarenotenhancedatallbyreactionandwillbenofasterthanthey
wouldbeintoessentiallypurewater.Thefunctionofthetertiaryaminethen,isnottoenhance
absorptionratesthroughchemicalreactionbutrathertoincreasedramaticallythecapacityofthe
solvent.ThetroublewithstraightMDEA,however,isthattheCO
2
absorptionrateisalmostalwaystoo
slowforittobeusedalone,exceptforbulkCO
2
removalathighpressure.Itsimplycannotbeused
effectivelyforthedeepCO
2
removalrequiredforsyngasandLNGproduction.TheCO
2
reactionmustbe
promoted,andpiperazineisanexcellentpromoter.
PiperazineisacyclicdiaminewiththestructureshowninFigure1.ItreactswithCO
2
aboutten
Figure1 Piperazine
timesfasterthanMEA.Itssecondorderrateconstantat25C,forexample,isabout59,000Lmol
1
s
1
versusMEAat6,000Lmol
1
s
1
.ThismakespiperazinethemostreactivepromoterofCO
2
kinetics
availablecommercially.Overthelast30years,theaMDEAprocess,firstpatentedbyBASFin1982,has
capturedthelionsshareoftheammoniasyngaspurificationmarket,anditisstillusedinthemajorityof
theworldsammoniaplants.
Insummarythen,althoughMDEAalonecanbeusedforbulkCO
2
removalathighpressure,its
reactionratewithCO
2
ismuchtooslowforittobeusefullyappliedtodeeperCO
2
removalincolumnsof
reasonableheight.Forthat,anactivatorisrequired,andpiperazinehasevolvedastheactivatorof
choice.Today,mostsolventvendorsofferaformofpiperazinepromotedMDEAunderavarietyof
brandandcodenames.
DEEPCO
2
REMOVALUSINGANMDEABASEDSOLVENT
Usinganamineprocesssimulatorthatisfoundeduponreal,massandheattransferratesallows
avirtualplanttobeconstructedonacomputer.Inthepresentwork,theProTreatsimulatorwas
used.ProTreatistheworldsonlytruemasstransferratebasedcommercialsimulatordedicatedtogas
treating.AvirtualplantbuiltusingProTreatallowsonetoexperimentwithawidevarietyofdifferent
processconfigurationsandwhatifscenarios.Itisrelativelyeasytolearnagreatdealaboutaparticular
plantsperformancecharacteristicsandquantitativelyunderstandhownumerousplantoperating
parametersaffectthatperformance.Inwhatfollows,wehaveusedProTreattoanswersuchquestions
as,Whatisthebestsolventconcentrationandformulationtouse?andHowdoesthetreating
temperatureaffectperformance?.Althoughnopublicdomainplantdataexist,orareavailabletous
ontheuseofDiMGlyasasolventforsyngaspurification,aratherlimitedamountoffundamentaldata
onvaporliquidequilibriumandphysicalpropertiesissufficienttodetermineagreatdealabouthowthis
chemicalwillperforminasyngastreatingsituation.Butthatisforthenextsectionofthepaper.
AProTreatPFDoftheaminesectionofatypicalammoniasyngasplantisshowninFigure2.It
hasasingleabsorberbutthereareidenticaltwinregeneratorsinparallel.Thereisalsoarather
complexschemeforreturningpartofthecondensatefromtheregeneratoroverheadcondenserstoa
shortwashsectionintheabsorberforrecoveringaminevapors.Theflowsheetisquiteabitmore
complexthanneededtoillustratethemainpointsofthepapersoithasbeengreatlysimplifiedtothe
PFDshowninFigure3.Thesimplifiedflowsheethasasingleregenerator,andwehaveeliminatedany
considerationofanaminerecoverysectionatoptheabsorber.Theplantwasdesignedtotreat208000
NCMHrawgasat26.4baraand37Ccontaining60.2mol%H
2
,20.9mol%N
2
,and17.9mol%CO
2
with
thebalancebeingtraceamountsofargon,methane,carbonmonoxide,andwatervapor.Forthisstudy,
theabsorberwaspackedwith15.25m(50ft)of#50IMTPtowerpacking.Theregeneratorwasalso
packed,andcontains10.4m(34ft)of#3CascadeMinirings.Althoughthisabsorberhadalittledeeper
packedbedthanfoundinmanysyngasCO
2
plants,theconclusionsofthepaperareinnowayaffected.
Figure2 OriginalAmineTreatingUnitforAmmoniaSyngas
Figure3 SimplifiedSyngasTreatingPFD
MDEAPiperazineComposition
ThemostcommonlyusedtotalaminestrengthforgenericMDEAandMDEAbasedblendsis50
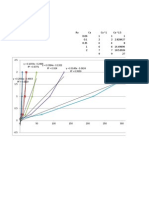
wt%although,asweshallsee,thisissometimesnotthebestconcentrationtouse.Figure4showshow
therelativeconcentrationsofpiperazineandMDEAina50wt%totalamineblendaffecttheCO
2
leftin
thetreatedgas.Itisapparentfromthefigurethatabout4wt%piperazineisneededtoachieve<1000
ppmvCO
2
andthat5or6wt%piperazineallowsonetoachieveinthevicinityof100ppmvCO
2
.The
treatingperformanceisverysensitivetotheconcentrationthepiperazineadditiveandthisiswhyitisso
importanttomonitorsolutionstrengthforpiperazinecontent.Unfortunately,piperazineissomewhat
volatilesoitsconcentrationtendstofallwithtimemorerapidlythandoesMDEA.Plantperformance
dependscriticallyonmaintainingtherightconcentrationsofthesolventingredients.
Figure4 EffectofPiperazineconcentrationinMDEA(Total
50wt%amine)onCO
2
LevelinTreatedSyngas
SolventTotalAmineStrength
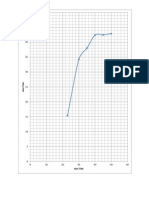
Havingestablishedthatthisplantwilloperatequitecomfortablywith5wt%piperazineand45wt%
MDEA,wethenaskedthequestion,whateffectdoestotalaminestrengthhaveontheCO
2
contentof
thetreatedgas?Figure5summarizesthefindings.ThenumbersnexttopointsontheppmvCO
2
curve
arethecorrespondingCO
2
moleloadings(permoletotalamine)oftherichsolventfromtheabsorber.
Figure5 EffectofTotalAmineStrengthonCO
2
Levelin
TreatedSyngasandonSolventViscosity
Itmightbesurprisingthatthehighertheaminestrengththeworsethetreating.Using50wt%
totalamine(BaseCase),thesimulatedtreatedgashas208ppmvCO
2
andaverysatisfactoryrich
solutionloadingof0.423molesCO
2
permoleoftotalamine.However,45wt%amineallowsa75ppmv
CO
2
gastobeproducedand40wt%aminereducestheCO
2
toabout30ppmv.With30wt%aminethe
residualCO
2
isevenlower.However,onthenegativeside,therichsolutionloadingincreasesto
unacceptablyhighlevelsfromacarbonsteelmetallurgycorrosionstandpoint.Ofcourse,stainless
claddingintherightplaces,andtheuseofstainlessexchangerbundles,wouldovercomethisobjection.
Thereasonforimprovedtreatingwithlowersolventstrengthsisthelowersolventviscositythat
accompaniesreducedamineconcentration.CO
2
absorptionisaprocesswhoserateiscontrolledby
masstransferresistanceintheliquid,orsolvent,phase,andthemoreviscousthatphaseis,thegreater
theresistance.AsFigure5shows,droppingamineconcentrationfrom50wt%to30wt%reduces
solventviscositybyafactorofnearlythree.ThisallowsCO
2
tobeabsorbedfasterandallowsthe15
+
metresofpackingtoextractenoughextraCO
2
toreducethetreatedgasconcentrationfrom208ppmv
tojustalittleover7ppmv.Solventviscosityisanimportantfactorinabsorberperformance,andany
successfulefforttoloweritwillpaydividends.Anotherwaytolowerviscosityistouseahottersolvent.
SolventTemperature
AsFigure6shows,settingthesolventtemperatureat50C,say,ratherthan26Clowerstheviscosityby
afactorofnearlythreeaswell,andtreatingimprovesdramaticallyovertheBaseCase.Thesimulated
resultsshowninthefigurearefora45wt%plus5wt%piperazinesolution.TreatedgasCO
2
content
dropsrapidlywithtemperaturebuteventuallyitlevelsout,thenexperiencesaprecipitousrise.Inthis
case,atemperatureofjustover50Cpresentsanoperationalcliff.Astemperaturerises,ofcourse,the
vaporpressureofCO
2
overthesolventgoesupaswell,andnear50Cthepointisreachedwhere
reducedsolubilitypreventsabsorptionofenoughCO
2
eventocomeclosetomeetinga<1000ppmv
CO
2
specification.TheppmCO
2
levelsuddenlyrisesbecausethesolventscapacityhasbeenreducedto
alevelinsufficienttoabsorbenoughCO
2
.TheexcessCO
2
intheinletsimplypassesrightthroughthe
columnandCO
2
breakthroughisexperienced.Operationally,itwouldbeverydangeroustooperatetoo
closetothistemperaturebecauseitwouldbecomeimpossibletomaintaincontrolofoutletCO
2
levels.
Figure6 EffectofSolventTemperatureonCO
2
Levelin
TreatedSyngasandonSolventViscosity
AMINOACIDSALTBASEDSOLVENTPOTASSIUMDIMETHYLGYCINATE(KDiMGly)
Potassiumdimethylglycinateisthepotassiumsaltofdimethylglycine,andwasknownunderthe
BASFtradenameAlkazidDIK.Thisisatertiaryaminoacidsaltthatsince1935hadbeenusedquite
successfullyandextensively,mainlyinEurope,forselectivelyremovingH
2
Sfromsuchproblematic
streamsasrefineryandcokeovengases.Thesegasestendtobeheavilycontaminatedwithvarious
componentsknowntobehardonconventionalamines.WiththeadventofMDEA,itsusediminished,
andeventuallyitstoppedbeingofferedcommercially.However,KDiMGlyhascertainpropertiessuchas
zerovaporpressureandpossiblyoxidationresistancethataremakingitmoreattractiveinpost
combustionCO
2
capture,andwhichmaymakeitausefulalternativetoMDEAforammoniasyngas.
Asthenameimplies,aminoaci n atoneendofthemoleculeanda
carboxylicacidgroupattheother.In lycineexistsasasocalledzwitterion:
dscontaina aminogroup
aqueous solution,dimethylg
C00 - CE
2
- N(CE
3
)
2
E
+
-
Because the amino group is protonated, it is completely nonreactive towards CO
2
and it is pH neutral
which, from the standpoint of life on earth, is probably a good thing. But as a solvent for CO
2
it is
virtually useless, having neither reactivity nor absorption capacity. However, when the acid group is
titrated(neut d with amino ralize ) KOHthe groupdeprotonates,
K0E + C00 - C
-
E
2
-N(CE
3
)
2
E
+
- K
+
0
-
0C -CE
2
- N(CE
3
)
2
+ E
2
0
andproduceswhatisnowanalkaline,tertiaryamine.AnimportantpropertyisthatKDiMGlyandall
otheraminoacidsaltsexistinwaterasfullydissociatedsalts,makingthemcompletelynonvolatile.
Furthermore,aminoacidsarealreadypartiallyoxidizedwhichmaygivethembetterresistanttofurther
oxidation.
InthecaseofKDiMGly,thetwoadditionalmethylgroupsareattachedtonitrogenattheamino
endofthemolecule,deprivingtheaminogroupoftheprotonnecessaryforreactionwithCO
2
.The
aminogroupistertiary;however,is thesamewasasMDEAand,in
solution,dissociatesaccordingto
itnonethelesshighlyalkalinein
RN + E
2
0 - RNE
+
+ 0E
-
JustasforMDEA,tertiaryaminesdonotreactwithCO
2
(nocarbamateorotherreactionproductswith
theamineareformed)sotheCO
2
absorptionratewillbenearlyidenticalwithwhatitwouldbeinwater,
i.e.,extremelyslow.ButthealkalinitygivesthesolventhighCO
2
holdingcapacity,ifonlytheCO
2
could
getintosolutioninthefirstplace.Thisisdonebypromotingthesolventwithveryfastreacting
piperazine.
ModelStudiesoftheKDiMGlyPiperazineSolvent
Becausechemicalsareboughtandsoldbyweight,weareusedtothinkingaboutsolvent
strengthintermsoftheweightpercentoftheactiveingredients.However,chemistryworksonthe
basisofmoles.TodoasnearlyaonetoonecomparisonwithpiperazinepromotedMDEAaspossibleit
isnecessarytouseequalmolarconcentrationsofthemainconstituent,anditturnsoutthat54.25wt%
KDiMGlyisequivalentto50wt%MDEA.Therefore,thiswasthebaseconcentrationusedintheamino
acidstudy.
KDiMGlyPiperazineComposition
Figure7showsthatevenwithoutthepiperazineadditive,KDiMGlyremovesmoreCO
2
(6.96
mol%CO
2
inthetreatedgas)thantheMDEAmolarequivalent(9.068mol%CO
2
).Thissuggestshigher
drivingforceforabsorption(lowvaporpressureofCO
2
)whenKDiMGlyisusedinaplantoperating
underotherwiseidenticalconditionsofsolventrate,reboilerduty,equipmentdetails,andsoon.
Considerablylesspiperazine(2wt%versus4wt%)isneededtoachieve1000ppmvtreatedgas,andthe
responsetopiperazineadditionishigher.Lowerpiperazineconcentrationsmeanreducedpotential
vaporizationlossesand,ofcourse,thecompletelynonvolatilenatureofKDiMGlyeliminatesthis
inventorylossmechanism.
EffectofTotalAmineStrength
LowertotalamineconcentrationissimulatedtohaveaneffectsimilartoMDEA,althoughtheviscosity
reductionisnotasgreat(afactoroftwoversusafactorofthreeforMDEA)andthereforetheimpactof
lowertotalamineconcentrationisnotasgreat.Figure8showsthesimulationresults.Thenumbers
besideeachsimulationdatapointaretherichsolutionCO
2
loadingvalues,whicharehigherthan
correspondingrichloadingsforMDEA.KDiMGlyishardertostripthanMDEAbecauseforthesame
loadingvaluetheCO
2
vaporpressureislower.Inotherwords,becauseofitshigherpH(e.g.,12.05for
54.25wt%KDiMGlyversus11.72for50wt%MDEA,bothat26.5C)KDiMGlyhashigheraffinityforCO
2
thanMDEAdoes.TheleansolutionloadingforpiperazineactivatedMDEAistypically0.003butfor
KDiMGlyundersimilarconditionsitis0.13;thequestioniswhetheradifferenceof0.13loadingunits
makesaKDiMGlysolutionmorecorrosivethanMDEAunderthesameconditions.Becauseitis
bicarbonatethatisresponsibleforcorrosion,theanswerisprobablyyes,andsomestainlessmetallurgy
invulnerablesectionsofprocessequipmentandpipingwouldbeprudent.However,thereisanother
significantbenefittobegainedfromusingpiperazinepromotedKDiMGly.
Basis54.25wt%DiMGly
Basis50wt%MDEA
Figure7 EffectofPiperazineConcentrationonCO
2
LevelinTreatedSyngas
Figure8 EffectofTotalAmineStrengthonCO
2
LevelinTreatedSyngasandonViscosityofKDiMGly
EffectofTreatingTemperatureUsingKDiMGly
AsshowninFigure9,highersolventtemperatures(lowerviscositysolvent)leadtosignificant
improvementstotheresidualCO
2
concentrationinthetreatedgas.UnliketheMDEAbasedsolvent,
thereisnoprecipitouslossofperformancewhenthetemperaturereacheshighvalues.Instead,thereis
abroadminimuminthetreatingperformancecurve.Thisresultsfromthecompetingeffectsof
increasingabsorptionrates(fromdiminishedsolventviscosity),versusincreasingequilibriumCO
2
vapor
pressure(reduceddrivingforceforabsorption)withhighertemperatures.Solventtemperatures
anywherebetween35and85Cpermitverysatisfactorytreating;however,theabilitytouseahigh
temperaturesolventofferspotentialadvantages.
Figure9 EffectofSolventTemperatureonCO
2
LevelinTreatedSyngasandonKDiMGlyViscosity
AdvantagestoTreatingwithHotSolvent
Figure10showshowleanaminetemperatureaffectsthetemperatureoftherichsolvent
leavingtheabsorber.Hereonecanseethatinthepresentcase,foraleanaminetemperatureof85C,
therichaminewillbeatnearly105C.Thismeansthattherichsolventrequiresnopreheatingtoensure
itentersthestripperatareasonabletemperature.Thecrossexchangercanbecompletelyeliminated
fromtheplant,andthiswouldbepossibleevenwithaleansolventtemperatureof60Cwherethebest
CO
2
removalisachieved.TheabilitytorunhothasacertainparallelwiththeHotPotprocessbutdoes
Figure10 EffectofLeanSolventTemperatureonRichAmineTemperature
notneedasemileanprocessflowsheetconfiguration.Instead,theprocessflowsheetisevensimpler
thanforconventionalaminetreating.
PiperazinepromotedKDiMGlyappearstobeaviableoptionforCO
2
removalfromammonia
syngas.Itsaquestionofwhetherthecostofbettermetallurgy(whichmayalreadyexistinapiperazine
promotedMDEAplant)exceedsthebenefitsofloweraminelosses,smallerheatexchangers,andthe
eliminationoftheusuallyquitelargecrossexchanger.
SUMMARY
Usingminimalnewdata(somevaporliquidequilibriumandpHdatafordeterminingamine
dissociationconstantstogetherwithestimatedsolutionproperties),arealmassandheattransferrate
basedaminesimulatorallowstheconstructionofavirtualplantinsoftware.Thevirtualplantis
extraordinaryversatileandcapableofaccuratelypredictingtheperformanceofproposedunits,without
pilotplantingorthegatheringoffielddata,i.e.,veryinexpensively.Masstransferratebasedmodeling
hasbeenaroundsincethemid80sandisafairlymaturetechnology.Thegastreatingindustryisnow
rapidlymovingawayfromthetriedandtrue,butantiquated,equilibriumstageapproach,withitsneed
forengineerguessedefficienciesandtransferunitheights.
ThiscontributionhasshowthatwithpiperazineactivatedMDEA:
Onemustbecarefultomonitorsolutioncompositionandcontrolthepiperazinelevel
becauseperformanceisquitesensitivetothisparameter,
Themaximofalwaysusing50wt%totalaminesolventisfalse,andsignificant
improvementstotreatinglevelscanberealizedbyoperatingatlowerconcentrations.The
50wt%ruleshouldbeexaminedineachandeverycase,
Whentreatinggoalsarenotbeingmet,usingacolderaminemaybeastepinthewrong
direction.
SolutionviscosityhasaprofoundeffectonCO
2
removalratesandwhatevercanbedoneto
loweritsvalue,consistentwithotheroperatingconstraints,willbebeneficial.
WithKDiMGlythereispotentialto:
Reduceheatexchangersurfaceareas.
Eliminatethecrossexchangeraltogether.
Lowerpotentialaminevaporizationlosses.
Perhapsuseamorerobustamineasthebaseforthesolvent.
Butwhenallissaidanddone,therealkeytounderstandingandimprovingplantperformanceisthe
availabilityanduseofarealmasstransferratebasedsimulator.
REFERENCES
Wagner,R.,U.LichtfersandV.Schuda,RemovalofCarbonDioxidefromCombustionExhaustGases,
PatentApplicationUS2009/0320682A1,31December2009.
Vous aimerez peut-être aussi
- CO2 by MDEADocument6 pagesCO2 by MDEAFadillah Akhbar MarshaPas encore d'évaluation
- s11814 019 0296 9 PDFDocument2 pagess11814 019 0296 9 PDFmppatilmayur1679Pas encore d'évaluation
- Amine UnitDocument69 pagesAmine UnitISEDAC21Pas encore d'évaluation
- 1 s2.0 S1110062115300404 MainDocument12 pages1 s2.0 S1110062115300404 MainMuhammad Aizuddin Zainal AbidinPas encore d'évaluation
- Mathematical Modeling, Parametric Estimation, and Operational Control For Natural Gas Sweetening ProcessesDocument18 pagesMathematical Modeling, Parametric Estimation, and Operational Control For Natural Gas Sweetening Processesmohsen ranjbarPas encore d'évaluation
- 1 s2.0 S1876610213003305 MainDocument10 pages1 s2.0 S1876610213003305 MainDeva AfrgPas encore d'évaluation
- Design Guidelines For Amine PlantsDocument21 pagesDesign Guidelines For Amine Plantsargacho100% (2)
- Simulation and Optimization of Gas Sweetening ProcessDocument20 pagesSimulation and Optimization of Gas Sweetening ProcessAhmed ShaepPas encore d'évaluation
- Sanchez Fernandez2014Document12 pagesSanchez Fernandez2014mppatilmayurPas encore d'évaluation
- Evaluation of Amine-Blend Solvent Systems For CO Post-Combustion Capture ApplicationsDocument8 pagesEvaluation of Amine-Blend Solvent Systems For CO Post-Combustion Capture ApplicationsaziziPas encore d'évaluation
- Kinetics of CO Absorption Into A Novel 1-Diethylamino-2-Propanol Solvent Using Stopped-Flow TechniqueDocument9 pagesKinetics of CO Absorption Into A Novel 1-Diethylamino-2-Propanol Solvent Using Stopped-Flow TechniqueYatindra AgrawalPas encore d'évaluation
- Comparison Study of Activators Performance For MDEA Solution of Acid Gases Capturing From Natural Gas Simulation-Based On A Real PlantDocument32 pagesComparison Study of Activators Performance For MDEA Solution of Acid Gases Capturing From Natural Gas Simulation-Based On A Real PlantYogesh PatilPas encore d'évaluation
- Amine Sweetening: Diego Alejandro Ayala Gómez 2154120 Jeison Stiven Villota Narváez 2155605 Gas Engineering 2020Document35 pagesAmine Sweetening: Diego Alejandro Ayala Gómez 2154120 Jeison Stiven Villota Narváez 2155605 Gas Engineering 2020jeison villotaPas encore d'évaluation
- Improve-Your-Gas-Plant's-Performance-in-the Middle East-Part-I-The-Amine-PlantDocument17 pagesImprove-Your-Gas-Plant's-Performance-in-the Middle East-Part-I-The-Amine-PlantAnggun RushPas encore d'évaluation
- Acs Iecr 7b02744Document12 pagesAcs Iecr 7b02744Bikash MondalPas encore d'évaluation
- The Use of MDEA and Mixtures of Amines For Bulk CO2 RemovalDocument9 pagesThe Use of MDEA and Mixtures of Amines For Bulk CO2 RemovalTrùm Dầu Mỏ BkPas encore d'évaluation
- Hysys PromDocument28 pagesHysys PromBashar Al ZoobaidiPas encore d'évaluation
- Natural Gas Sweetening & Effect of Decline PressureDocument29 pagesNatural Gas Sweetening & Effect of Decline Pressureromdhan88Pas encore d'évaluation
- Applied Energy: Contents Lists Available atDocument13 pagesApplied Energy: Contents Lists Available atKhánh TrangPas encore d'évaluation
- Gas Sweetening Process PDFDocument57 pagesGas Sweetening Process PDFrcalaforraPas encore d'évaluation
- 1999 XD PengDocument8 pages1999 XD PengLeonard SaftaPas encore d'évaluation
- Hydrazine Formate ReductionsDocument3 pagesHydrazine Formate ReductionsPedro Mendonca100% (1)
- Thermal Stability of Polyethylenimine Based Carbon Dioxide Adsorbents and Its Influence On Selection of Regeneration StrategiesDocument9 pagesThermal Stability of Polyethylenimine Based Carbon Dioxide Adsorbents and Its Influence On Selection of Regeneration StrategiesJaancaarloDiiazPas encore d'évaluation
- 5 .1-S2.0-S0016236122016763-MainDocument13 pages5 .1-S2.0-S0016236122016763-Mainmohamed magedPas encore d'évaluation
- Sustainability 12 08524Document13 pagesSustainability 12 08524Ainul YaqinPas encore d'évaluation
- Recovery Enhancement of Liquid HydrocarbDocument9 pagesRecovery Enhancement of Liquid Hydrocarbsarkmank1Pas encore d'évaluation
- A Comparison of Physical Solvents For Acid Gas Removal PDFDocument10 pagesA Comparison of Physical Solvents For Acid Gas Removal PDFVirnia PatziPas encore d'évaluation
- Absorption of CO2 in Aqueous DiglycolamineDocument10 pagesAbsorption of CO2 in Aqueous DiglycolamineLê Vinh HảoPas encore d'évaluation
- A Tailored Multi-Functional Catalyst For Ultra-Ef Ficient Styrene Production Under A Cyclic Redox SchemeDocument11 pagesA Tailored Multi-Functional Catalyst For Ultra-Ef Ficient Styrene Production Under A Cyclic Redox SchemedeboPas encore d'évaluation
- Wet Air Oxidation and Catalytic Wet Air Oxidation For Refinery Spent Caustic DegradationDocument7 pagesWet Air Oxidation and Catalytic Wet Air Oxidation For Refinery Spent Caustic DegradationSudeep MukherjeePas encore d'évaluation
- Chem Eng Technol - 2015 - Wen - Comparison of Overall Gas Phase Mass Transfer Coefficient For CO2 Absorption BetweenDocument9 pagesChem Eng Technol - 2015 - Wen - Comparison of Overall Gas Phase Mass Transfer Coefficient For CO2 Absorption BetweenAbhi SinghPas encore d'évaluation
- The Activator Mechanism of Piperazine in Aqueous Methyldiethanolamine SolutionsDocument10 pagesThe Activator Mechanism of Piperazine in Aqueous Methyldiethanolamine SolutionsSimone SanPas encore d'évaluation
- Gas SweeteningDocument3 pagesGas SweeteningAleem AhmedPas encore d'évaluation
- Granular Activated Carbon Supplementation Enhances Anaerobic Digestion of Lipid-Rich WastewatersDocument13 pagesGranular Activated Carbon Supplementation Enhances Anaerobic Digestion of Lipid-Rich WastewatersJoël KoffiPas encore d'évaluation
- Homogeneous 1Document6 pagesHomogeneous 1HIRAL PANDYAPas encore d'évaluation
- Overcoming Thermodynamic Limitations in Dimethyl CDocument7 pagesOvercoming Thermodynamic Limitations in Dimethyl CKaleemPas encore d'évaluation
- Kinetics Study and Simulation of CO 2 Absorption Into Mixed Aqueous Solutions of Methyldiethanolamine and HexylamineDocument11 pagesKinetics Study and Simulation of CO 2 Absorption Into Mixed Aqueous Solutions of Methyldiethanolamine and HexylamineKarla FelixPas encore d'évaluation
- Lin 2014Document7 pagesLin 2014mppatilmayurPas encore d'évaluation
- Green Diesel - A Second Generation BiofuelDocument11 pagesGreen Diesel - A Second Generation BiofuelChatkamol KaewbuddeePas encore d'évaluation
- Investigation of Mixed Amine Solution in Gas Sweetening PlantDocument5 pagesInvestigation of Mixed Amine Solution in Gas Sweetening PlantRenan Villalobos JimenezPas encore d'évaluation
- Tecnologías de Endulzamiento de Gas AmargoDocument17 pagesTecnologías de Endulzamiento de Gas AmargoKarla Johanna Tejeda AlvarezPas encore d'évaluation
- A Comparison of Physical Solvents For Acid Gas RemovalDocument14 pagesA Comparison of Physical Solvents For Acid Gas Removalkaaskopdawie5755Pas encore d'évaluation
- Gas Pre TreatmentDocument12 pagesGas Pre TreatmentAnonymous bHh1L1100% (4)
- GPC3 - CO2 Capture Using An Aqueous Formulated Solvent - Published - June 2015Document9 pagesGPC3 - CO2 Capture Using An Aqueous Formulated Solvent - Published - June 2015Rahul BhosalePas encore d'évaluation
- J. Chem. ThermodynamicsDocument6 pagesJ. Chem. ThermodynamicsSam BanPas encore d'évaluation
- Carbon Dioxide Solubility in Mixtures of MethyldieDocument72 pagesCarbon Dioxide Solubility in Mixtures of MethyldiePaula Estefania Urrego VillalobosPas encore d'évaluation
- Process Simulation of Dehydration Unit For The Comparative Analysis of Natural Gas Processing and Carbon Capture ApplicationDocument40 pagesProcess Simulation of Dehydration Unit For The Comparative Analysis of Natural Gas Processing and Carbon Capture ApplicationeduryuPas encore d'évaluation
- A Project On Dehydration of NG New - REVIEWDocument11 pagesA Project On Dehydration of NG New - REVIEWZayn AhmedPas encore d'évaluation
- Samanta I Bandyopadhyay - 2009 - Absorption of Carbon Dioxide Into Aqueous SolutionDocument10 pagesSamanta I Bandyopadhyay - 2009 - Absorption of Carbon Dioxide Into Aqueous Solutionantrios123Pas encore d'évaluation
- International Journal of Greenhouse Gas Control: Sukanta Kumar Dash, Syamalendu S. BandyopadhyayDocument11 pagesInternational Journal of Greenhouse Gas Control: Sukanta Kumar Dash, Syamalendu S. BandyopadhyayYogesh PatilPas encore d'évaluation
- Rhodium Catalyzed Hydroformylation - CH 07Document14 pagesRhodium Catalyzed Hydroformylation - CH 07maildesantiagoPas encore d'évaluation
- Efficient Utilization of Hydrocarbon Mixture To Produce AromaticsDocument12 pagesEfficient Utilization of Hydrocarbon Mixture To Produce AromaticsBoby SixkillersPas encore d'évaluation
- Selective Extraction of Neutral Nitrogen Compounds Found in Diesel Feed byDocument8 pagesSelective Extraction of Neutral Nitrogen Compounds Found in Diesel Feed byJohnSmithPas encore d'évaluation
- A Second Generation Biofuel PDFDocument11 pagesA Second Generation Biofuel PDFNoviPas encore d'évaluation
- EEMPA Process 1Document9 pagesEEMPA Process 1Maythee SaisriyootPas encore d'évaluation
- Clean Energy Catalyst: Green Hydrogen Revolutionizing RefiningD'EverandClean Energy Catalyst: Green Hydrogen Revolutionizing RefiningPas encore d'évaluation
- Temperature-Responsive Polymers: Chemistry, Properties, and ApplicationsD'EverandTemperature-Responsive Polymers: Chemistry, Properties, and ApplicationsPas encore d'évaluation
- Sustainable synthesis of ciclopentene derivatives through multicomponent reactions in continuous flow regimeD'EverandSustainable synthesis of ciclopentene derivatives through multicomponent reactions in continuous flow regimePas encore d'évaluation
- Advanced Pharmaceutical analysisD'EverandAdvanced Pharmaceutical analysisÉvaluation : 4.5 sur 5 étoiles4.5/5 (2)
- Ffoorr Tthhee PpeeoopplleeDocument8 pagesFfoorr Tthhee PpeeoopplleeSyed Shah Jehan GillaniPas encore d'évaluation
- IBFT - Member Bank Account Number FormatDocument1 pageIBFT - Member Bank Account Number FormatSyed Shah Jehan GillaniPas encore d'évaluation
- Computational and Experimental Modeling of Three-Phase Slurry-Bubble Column ReactorDocument72 pagesComputational and Experimental Modeling of Three-Phase Slurry-Bubble Column ReactorSyed Shah Jehan GillaniPas encore d'évaluation
- Faisal KhanDocument10 pagesFaisal KhanSyed Shah Jehan GillaniPas encore d'évaluation
- Four Factor Formula FinalDocument37 pagesFour Factor Formula FinalSyed Shah Jehan GillaniPas encore d'évaluation
- Application Longterm NewDocument4 pagesApplication Longterm NewSyed Shah Jehan GillaniPas encore d'évaluation
- Carbon Dioxide Capture and Storage in The Nitrogen and SynGas Industries 2Document10 pagesCarbon Dioxide Capture and Storage in The Nitrogen and SynGas Industries 2Syed Shah Jehan GillaniPas encore d'évaluation
- 97 6 TocDocument10 pages97 6 TocJose Luis Gutierrez MadariagaPas encore d'évaluation
- Social Problems of AdolescentsDocument22 pagesSocial Problems of AdolescentsSyed Shah Jehan Gillani100% (1)
- 1990 Ifa Venice DybkjaerDocument25 pages1990 Ifa Venice DybkjaerSyed Shah Jehan GillaniPas encore d'évaluation
- Book 1Document3 pagesBook 1Syed Shah Jehan GillaniPas encore d'évaluation
- Social Problems of AdolescentsDocument7 pagesSocial Problems of AdolescentsSyed Shah Jehan GillaniPas encore d'évaluation
- University of Engineering & Technology, Lahore: Unofficial TranscriptDocument1 pageUniversity of Engineering & Technology, Lahore: Unofficial TranscriptSyed Shah Jehan GillaniPas encore d'évaluation
- C.R.E Copy ChartDocument5 pagesC.R.E Copy ChartSyed Shah Jehan GillaniPas encore d'évaluation
- Mass TransferDocument2 pagesMass TransferSyed Shah Jehan GillaniPas encore d'évaluation
- Lab 3 Biological MoleculesDocument6 pagesLab 3 Biological Moleculesjohn NisPas encore d'évaluation
- Lecture 6 CrystallizationDocument29 pagesLecture 6 CrystallizationRonak AdrojaPas encore d'évaluation
- Air Entraining CementDocument14 pagesAir Entraining CementBashairu WaseemPas encore d'évaluation
- Republic of The Philippines Batangas State UniversityDocument10 pagesRepublic of The Philippines Batangas State UniversityHANNAH MARIE VINOYAPas encore d'évaluation
- Tandy 4301Document2 pagesTandy 4301Kamal PriyanPas encore d'évaluation
- Annex 2 Drawings and CatalogueDocument8 pagesAnnex 2 Drawings and CatalogueShaun DavidsPas encore d'évaluation
- Corrosion and Cracking of Weldable 13 CR Martensitic Stainless SteelsDocument75 pagesCorrosion and Cracking of Weldable 13 CR Martensitic Stainless SteelsDave M MichaelPas encore d'évaluation
- 3.1 Mass Transfer Equipment Design: H O CH Cooh CHDocument61 pages3.1 Mass Transfer Equipment Design: H O CH Cooh CHZAINOR SYAHIRA BINTI ZAINAL STUDENTPas encore d'évaluation
- Daiy Test Preparation - QuizzizDocument11 pagesDaiy Test Preparation - QuizzizJulyantho TundePas encore d'évaluation
- 2006 - Dong Et Al - Bulk and Dispersed Aqueous Phase Behavior of PhytantriolDocument7 pages2006 - Dong Et Al - Bulk and Dispersed Aqueous Phase Behavior of PhytantriolHe ZeePas encore d'évaluation
- Chlorination Guide Presentation PDFDocument168 pagesChlorination Guide Presentation PDFAmit nayakPas encore d'évaluation
- Soalan ObjektifDocument9 pagesSoalan ObjektifHairul Nizam OmarPas encore d'évaluation
- Frequently Asked Questions: GeneralDocument12 pagesFrequently Asked Questions: GeneralChoice OrganoPas encore d'évaluation
- Form 1 Chapter 1-7 Science NotesDocument11 pagesForm 1 Chapter 1-7 Science Noteswentao0420100% (1)
- 100 300 10stageDocument1 page100 300 10stagemsh16000Pas encore d'évaluation
- Phase Diagram 2Document6 pagesPhase Diagram 2Mohd AzhamPas encore d'évaluation
- Trimethylamine Oxide in Marine Products: J.S.C.I.Document7 pagesTrimethylamine Oxide in Marine Products: J.S.C.I.Veneta GizdakovaPas encore d'évaluation
- CH 2 Coulombs Law APEMDocument3 pagesCH 2 Coulombs Law APEMJames FlaughPas encore d'évaluation
- 10 Science Chemical Reactions and Equations Test 03 PDFDocument2 pages10 Science Chemical Reactions and Equations Test 03 PDFNiranjan BeheraPas encore d'évaluation
- Edwards SD TRK SD TRM Install 0957864312Document2 pagesEdwards SD TRK SD TRM Install 0957864312Anonymous PersonPas encore d'évaluation
- GATE-PYQ-Topicwise Noor Ul Huda SirDocument585 pagesGATE-PYQ-Topicwise Noor Ul Huda SirSudha balakrishnan100% (2)
- Comedk PhysicsDocument9 pagesComedk Physicssingh.krishnaanuragPas encore d'évaluation
- 2019物理習題第28回Document6 pages2019物理習題第28回廖亮鈞Pas encore d'évaluation
- Sulfuric Acid As A Dehydrating AgentDocument7 pagesSulfuric Acid As A Dehydrating AgentSidhant GakharPas encore d'évaluation
- Dark Matter PDFDocument120 pagesDark Matter PDFAswin VengatPas encore d'évaluation
- Nazis Tried To Turn Sand in To GoldDocument9 pagesNazis Tried To Turn Sand in To GoldJorge Daniel Ferreira GranadosPas encore d'évaluation
- Phys 503 Classical Mechanics I Fall 2013 Midterm Exam #3 Due Sunday, December 8 (20% of Course Grade) (At Noon in TA's Mailbox)Document4 pagesPhys 503 Classical Mechanics I Fall 2013 Midterm Exam #3 Due Sunday, December 8 (20% of Course Grade) (At Noon in TA's Mailbox)Sergio Duque MejíaPas encore d'évaluation
- Rheology and Hydraulics: Rheology Is The Science of Deformation and Flow of MatterDocument36 pagesRheology and Hydraulics: Rheology Is The Science of Deformation and Flow of Matterhassan haddadiPas encore d'évaluation
- MSDS Coconut Shell Charcoal (Indonesia Kunlun) PDFDocument11 pagesMSDS Coconut Shell Charcoal (Indonesia Kunlun) PDFGuddu YadavPas encore d'évaluation
- Sulfrex 1Document36 pagesSulfrex 1skeckdy100% (1)