Académique Documents
Professionnel Documents
Culture Documents
Garcea 2009 Liver Failure After Resection
Transféré par
flowptzCopyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Garcea 2009 Liver Failure After Resection
Transféré par
flowptzDroits d'auteur :
Formats disponibles
J Hepatobiliary Pancreat Surg (2009) 16:145155 DOI 10.
1007/s00534-008-0017-y
REVIEW ARTICLE
Liver failure after major hepatic resection
Giuseppe Garcea G. J. Maddern
Received: 17 August 2008 / Accepted: 19 September 2008 / Published online: 26 December 2008 Springer 2008
Abstract Introduction The consequence of excessive liver resection is the inexorable development of progressive liver failure characterised by the typical stigmata associated with this condition, including worsening coagulopathy, hyperbilirubinaemia and encephalopathy. The focus of this review will be to investigate factors contributing to hepatocyte loss and impaired regeneration. Methods A literature search was undertaken of Pubmed and related search engines, examining for articles relating to hepatic failure following major hepatectomy. Results In spite of improvements in adjuvant chemotherapy and increasing surgical condence and expertise, the parameters determining how much liver can be resected have remained largely unchanged. A number of preoperative, intraoperative and post-operative factors all contribute to the likelihood of liver failure after surgery. Conclusions Given the magnitude of the surgery, mortality and morbidity rates are extremely good. Careful patient selection and preservation of an obligate volume of remnant liver is essential. Modiable causes of hepatic failure include avoidance of sepsis, drainage of cholestasis with restoration of enteric bile salts and judicious use of portal triad inow occlusion intra-operatively. Avoidance of postoperative sepsis is most likely to be achieved by patient selection, meticulous intra-operative technique and postoperative care. Modulation of portal vein pressures postoperatively may further help reduce the risk of liver failure.
Keywords
Liver failure Liver resection
Introduction Liver resection is the accepted gold standard of treatment for liver tumours. Unfortunately, only 1020% of patients with colorectal liver metastases are candidates for hepatic resection [1]. The resectability rate for hepatocellular carcinoma is about 2030% in normal livers, but reduced in patients with cirrhotic liver [2, 3]. Hence, in any cohort of patients with primary or secondary tumours, most will be unsuitable for curative resection due to the presence of extrahepatic disease, anatomical distribution of their lesions or tumour burden. The aim of liver resection is to remove all macroscopic disease (with negative resection margins) and leave sufcient functioning liver [4] with preservation of vascular inow and outow. The acceptable residual functioning volume should be approximately 20% of the standard liver volume or the equivalent of a minimum of two segments [4]. In patients without normal liver parenchyma, this obligate functional volume has been estimated to range from 30 to 60% in patients with chemotherapy steatosis or hepatitis, and from 40 to 70% in cases of cirrhosis [5]. In spite of improvements in adjuvant chemotherapy and increasing surgical condence and expertise, the parameters determining how much liver can be resected have remained largely unchanged. Liver failure The consequence of excessive liver resection is the inexorable development of progressive liver failure characterised by the typical stigmata associated with this condition, including worsening coagulopathy, hyperbilirubinaemia and encephalopathy. This rarely occurs in isolation and is often coupled with failure of multiple organs and/or features of sepsis.
G. Garcea (&) G. J. Maddern Department of Hepatobiliary and Upper Gastrointestinal Surgery, The Queen Elizabeth Hospital, 28 Woodville Road, Adelaide, SA 5011, Australia e-mail: gg43@le.ac.uk
123
146
J Hepatobiliary Pancreat Surg (2009) 16:145155
Whilst fulminant liver failure is probably easily diagnosed, the true contribution of milder forms of liver dysfunction to mortality post-operatively may be harder to assess and accurately quantify. Clinically, a mild derangement in liver function is very common after extended liver resection and generally resolves within 6 or 7 days postoperatively. Lack of resolution of this mild dysfunction may herald the insidious onset of liver failure. Attempts to classify histological changes following surgery-induced hepatic failure have revealed interesting results, albeit from a small number of patients (n = 7) [6]. From these clinical ndings, liver failure could be dened as either cholestatic (characterised by regeneration of hepatocytes and brosis) or nonregenerative (characterised by pronounced apoptosis of hepatocytes) [6]. The incidence of liver failure after major hepatic resection ranges from 0 to 30%; however, the lack of a standardised denition of liver failure makes comparison of reported incidence between centres difcult [7]. Liver failure would appear to be a major contributor to post-operative mortality, being implicated in 1875% of cases [810].
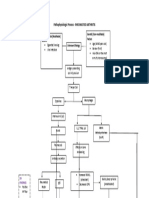
Liver regeneration and liver failure Normal mechanism of liver regeneration The liver is unique amongst all other body organs in its ability to regenerate fully after extensive liver damage, either due to resection or secondary to drug-induced/viralinduced damage. Following partial hepatectomy, 95% of the normally quiescent liver cells re-enter the cell cycle, with an increase in DNA synthesis that peaks at 24 h following injury [11]. Induction of DNA synthesis in other liver cells occurs later, at 48 h for Kupffer and stellate cells and 96 h for endothelial cells [11]. Clinical studies suggest that regeneration is evident within 2 weeks following resection and is complete at 3 months following resection [12, 13]. Hepatocyte activation requires their priming by inammatory cytokines, such as interleukin 6 (IL-6) and tumour necrosis factor alpha (TNF-a) (Fig. 1). These cytokines are released by Kupffer cells in response to portalsystem-carried factors such as lipopolysaccharide (LPS).
Fig. 1 Overview of the mechanism of liver regeneration following hepatectomy
123
J Hepatobiliary Pancreat Surg (2009) 16:145155
147
Once primed, hepatocytes respond to a number of growth factors including hepatocyte growth factor (HGF) released by stellate cells. HGF release by stellate cells occurs by cleavage of pro-HGF by proteases such as urokinase-type plasminogen activator (uPA). In addition, other trophic factors from different sources (Fig. 1) also act on the primed hepatocytes, moving them from G0 to S-phases of the cell cycle. IL-6, if administered in adequate concentrations, can directly stimulate hepatocyte proliferation, even in the absence of other growth factors [14]. The synthesis of transforming growth factor beta (TGF-b) (which acts in an inhibitory fashion on hepatocyte proliferation) is blocked in the early stages of hepatocyte proliferation, but is eventually restored, bringing an end to hepatocyte regeneration [14]. In addition to promoting cell growth, the cytokine pathway (specically the production of IL-6) also inhibits liver apoptosis (Fig. 1), thus further protecting the postoperative liver. Inducible nitric oxide is released by hepatocytes in response to cytokine release from Kupffer cells and may act in suppressing the inhibition of HGF-induced cyclin D1 and D2 expression [15]. In iNOS gene knockout murine models, hepatectomy has been found to result in hepatic failure characterised by marked apoptosis of hepatocytes [16]. Re-establishment of normal liver architecture is achieved by stellate cells, in conjunction with proteins such as connexin-32 and keratin-8, which are involved in the production of an extra-cellular matrix approximately 4 days following liver damage [11]. Correlation between liver regeneration and the failing liver If enough viable liver remains to support bodily functions post-operatively, then regeneration (or the lack of it) should not have an impact on subsequent function of the organ. However, this would not be the case in the present understanding of liver failure following hepatectomy, where a lack of liver regeneration is frequently linked with the development of liver failure. This nding could be explained by postulating that the 20% rule, dening the obligate mass of liver tissue remaining post-hepatectomy, is dependent on liver regeneration in order to preserve long-term homeostatic function. Alternatively, the lack of regeneration found in failing livers may be an index of excessive resection rather than a contributory cause of failure. On-going loss of hepatocytes as a consequence of surgery may mean liver regeneration is essential in order to continue metabolic activity, in spite of the initial volume of liver remnant being adequate. As will be discussed subsequently in this review, physiological and blood-ow-related changes contribute to post-hepatectomy hepatocyte loss, which may be exacerbated by other factors such as sepsis. Marked apoptosis has been described following hepatectomy in
animal models, further contributing to hepatocyte loss [17]. The disturbance of this balance between immediate/on-going hepatocyte loss and replacement explains the insidious nature of evolving liver failure following major hepatectomy. Hence, once the initial liver injury (i.e. resection) has been sustained, the subsequent recovery of liver function is dependent on a number of patient-related operative and postoperative factors (Fig. 2). The focus of this review will be to investigate factors contributing to hepatocyte loss and impaired regeneration in patients undergoing hepatectomy and how a better understanding of this interplay can be used to optimise outcome after extended liver resection.
Energy charge following hepatic resection Failure to regenerate occurs once the remnant volume of liver falls below a certain threshold. The rate of hepatic metabolism, or the so-called energy charge, has been shown to decrease following partial hepatectomy [18]. The energy charge also relates to the volume of remaining liver, and once a certain threshold of volume is reached, regeneration ceases as the energy demands of other metabolic process (such as gluconeogenesis) take precedence [19]. Arterial ketone body ratio offers an index of the mitochondrial adenylate charge of the liver, and this has been used to predict the prognosis of patients undergoing hepatic resection [20, 21]. Preservation of energy metabolism has been shown to increase survival probability in small-for-size liver grafts and following hepatectomy in animal models [2224]. Preserving liver remnant may be achieved by selective portal vein embolisation to the segments of liver planned for resection, resulting in hypertrophy of the future remnant liver volume. Portal vein embolisation may be achieved preoperatively using radiological means or as part of a staged liver resection. Portal vein embolisation has been shown to increase liver volume by 816%, although this increase is dependent on underlying liver function [2528]. Two-stage liver resection involves removing disease from one lobe, allowing the liver to regenerate, and then undertaking a second resection to clear any remaining disease. In this manner, the critical threshold for obligate remaining liver is never reached. Unfortunately, disease progression between intervening resections is a signicant risk and has been reported to be as high 46.7% [28].
Haemodynamics following hepatectomy Normal liver ow The liver receives a dual blood supply from both the hepatic artery and the portal vein, with the contribution of
123
148 Fig. 2 Interplay of patientrelated intraoperative and postoperative factors in the development of liver failure post-hepatectomy
J Hepatobiliary Pancreat Surg (2009) 16:145155
blood ow being widely accepted as 20 and 80% respectively. Following portal vein embolisation, a phenomenon known as the hepatic arterial buffer response results in an increased arterial ow to the embolised and non-embolised lobe (due to an adenosine-mediated response system) [29]. Since the return from the distal portal circulation is unchanged and more blood ow is now channeled down the remaining portal vein branch, ow to the non-embolised lobe also increases [30] (Fig. 3). Portal ow following hepatectomy Following hepatectomy, the reduction in liver size, and as a consequence, vascular capacity, result in a marked decrease in portal vein ow, an increase in hepatic artery resistance and an increase in portal vein pressure [31]. The subsequent increase in shear stress in liver sinusoids is an important initiating factor in liver regeneration and likewise a reduction in shear stress may contribute to liver atrophy [32, 33]. Hepatocytes are surrounded by a threedimensional network of vessels which are fenestrated (the liver sieve) and thus are directly exposed to portal pressure via these sieve plates. The lack of hepatocyte regeneration in patients with portal hypertension would seem at odds with this model of shear stress. This could be explained by the loss of sieve plates in cirrhotic patients blocking the stimulus of raised portal pressure on hepatocytes [32] or by excessive brosis limiting the regeneration of hepatocytes [32].
Shear stress and liver damage Whilst shear stress is an important component of liver regeneration, excessive pressures may result in microcirculatory collapse and subsequent hepatocyte necrosis. These changes are frequently found with hepatectomies involving resection of up to 90% of liver [34]. The reduction in portal vein ow accompanying the dramatic rise in portal pressure further contributes to reduced regeneration. These observations have been reported in small-for-size liver transplants where severe ischaemic changes with sinusoidal congestion have been found in association with increased portal vein pressure [3537]. Attempts at reducing portal vein pressure have included portal-systemic shunts and splenectomy [38]. Control of portal pressure has been shown to improve the survival of small-for-size grafts in a number of studies [3941]. In animal models of partial hepatectomy, porto-caval shunting has resulted in a reduced rate of hepatic necrosis and reduced apoptotic index in the shunted animals [42, 43]. In spite of these encouraging results, a marked delay in liver regeneration has also been reported to be associated with porto-caval shunts [42, 44]. This could be explained by an over-reduction of portal shear stress or by diversion of hepatotrophic factors into the systemic circulation as a consequence systemic shunting. As discussed previously, important trophic factors are carried in the portal circulation, which are also required for liver regeneration following hepatectomy. Liver atrophy is a well-recognised
123
J Hepatobiliary Pancreat Surg (2009) 16:145155
149
Fig. 3 Normal hepatic inow, demonstrating the hepatic arterial buffer response (gure adapted from Ref. [103])
complication of porto-systemic shunting, in cirrhotic livers at least, and may limit the use of such shunts outside of an experimental setting. There is, however, animal model evidence of non-portal growth factors inuencing liver regeneration [45] and of complete liver regeneration being possible, even with porto-caval shunting [46]. In addition, the use of mesocaval shunts may be a satisfactory compromise by reducing excessive shear stress, whilst maintaining gastroduodenosplenopancreatic venous return to the liver and thus preserving hepatotrophic inow [47]. Alternatively, pharmacological control of portal pressure may provide short-term post-operative control of portal pressure in major hepatectomies, which can be ceased when necessary to allow normal regeneration subsequently.
Intraoperative and post-operative ischaemia A variety of techniques have been adopted during liver resection to help reduce the degree of blood loss intraoperatively. The Pringle manoeuvre has been widely adopted
and involves clamping the hepatic inow usually intermittently or up to 1 h continuously. Although blood loss has been shown to be reduced by this method [48], bleeding may still occur from the hepatic veins, and for this reason, some centers have advocated total vascular exclusion of the liver (clamping of the supra and infra hepatic inferior vena cava coupled with Pringle occlusion) during resection to create a completely bloodless eld. The liver appears remarkably tolerant to even prolonged periods of ischaemia, and for the most part, neither the Pringle nor total vascular exclusion (TVE) appears to cause any permanent damage to hepatic tissue, with any histological changes observed rapidly reversing on re-perfusion [49]. Ischaemic reperfusion has been advocated as a means of reducing the deleterious effect of ischaemia reperfusion on the liver. This involves a short period of ischaemia (usually about 5 min) followed by up to 30 min of reperfusion. Following this, Pringle clamping can be employed either continuously or in intermittent cycles. Ischaemic preconditioning (IP) has been shown to decrease the severity of liver necrosis [50], exhibit an anti-apoptotic effect [51],
123
150
J Hepatobiliary Pancreat Surg (2009) 16:145155
preserve liver microcirculation [52], and improve survival rates following hepatectomy [53]. More recently IP has been described as promoting liver regeneration via the upregulation of cytokines such as TNF-a and IL-6, and the down-regulation of TGF-b [54]. For liver resections of the magnitude where liver failure post-operatively is a signicant risk, the impact of IP may not be as benecial. There is evidence that in animals undergoing 90% hepatectomy, IP may serve to impair liver regeneration [55]. In addition, a number of deleterious effects have been associated with hepatic inow occlusion including bacterial translocation of gut organisms and elevated endotoxins in the portal system [56]. As will be discussed in the following section, bacteraemia together with associated sepsis is a frequent association with postoperative liver failure and prolonged inow occlusion may exacerbate this. Finally, prolonged hypotension can adversely affect liver function, as evidenced by necrosis, bile plugging and inammatory cell inltrates in conjunction with hyperbilirubinaemia [57]. Post-operative hypotension (whether due to bleeding, sepsis or increased inotropic requirements) may signicantly prolong hepatic ischaemia, especially when prolonged Pringle clamping has been applied, and result in post-operative liver failure. Thus, although TVE and Pringle clamping are relatively safe to employ, their use may combine with other factors also contributing to liver hypoperfusion, resulting in signicant liver dysfunction. This may only be partially ameliorated by IP in the context of extended hepatectomies (Fig. 4).
Fig. 5 Effect of sepsis on liver function and regeneration after hepatectomy
cause of post-operative hypotension and in this manner may prolong hepatic ischaemia following surgery (see above). In addition, sepsis adversely affects Kupffer cell function, may increase the concentration of liver-toxic cytokines, and endotoxins released by bacteria have a direct inhibitory action on hepatocyte proliferation (Fig. 5). This complex interplay between sepsis and liver regeneration explains the frequent association of sepsis with liver failure in post-hepatectomy patients. Sepsis and Kupffer cell function Kupffer cell activation is an important component in the early initiation of liver regeneration. Leukocyte-Kupffer cell interaction is thought to trigger a local inammatory response leading to release of TNF-a and IL-6 which then act on hepatocytes leading to proliferation [58]. This interaction is thought to be mediated by an intracellular adhesion molecule known as ICAM-1, and ICAM-1-decient mice exhibit impaired liver regeneration with a concomitant decrease in TNF-a and IL-6 concentrations, following 70% hepatectomy [58]. The complement cascade is pivotal to this regeneration pathway [59]. Administration of endotoxin to hepatectomised rats results in massive necrosis and up to 50% mortality [60], possibly by interfering with Kupffer cell activation, either via the complement pathway or Kupffer cell-leukocyte interaction. Following large volume hepatectomy, Kupffer cell numbers are reduced and hence the rapid clearance of bacteria from blood (one of the predominant roles of Kupffer cells) is diminished. Dysfunction of Kupffer cell activity may persist for up 2 weeks following hepatectomy [61]. As a result, impaired clearance of blood-borne enteric bacteria and their associated endotoxins make hepatectomised animal models prone to the rapid development of multi-organ failure in the presence of sepsis
Sepsis Sepsis affects post-operative liver function and regeneration in a number of different ways. Sepsis is an important
Fig. 4 Shear stress and hepatocyte regeneration
123
J Hepatobiliary Pancreat Surg (2009) 16:145155
151
[62]. Translocation of enteric organisms following hepatectomy is well documented and the application of Pringle clamping may be, in part, responsible [56]. As a result, hepatectomy results in a proclivity for rapid, overwhelming sepsis leading to multi-organ failure, whilst in turn, sepsis acts to diminish the regenerative ability of the liver. Sepsis and circulating TNF-a One of the earliest TNF-a mediated event within hepatocytes is activation of NF-kappaB (the main gateway to proinammatory cytokine pathways). In addition to exerting a proliferative effect on hepatocytes, NF-kappaB may also induce apoptosis [63]. In the context of liver resection, the proliferative pathway appears to predominate [63]. Excessive circulating levels of TNF-a after massive hepatectomy may contribute to liver failure and death, and suppression of TNF-a has been shown to improve survival [64]. Sepsis is a further stimulus for elevation of TNF-a, and whilst not yet proven, this may serve as a further mechanism contributing to on-going liver damage and inhibition of liver regeneration. Endotoxins and hepatocytes Endotoxin release from blood-borne bacteria appears to have a direct action on hepatocytes resulting in decreased mitochondrial function and impaired bile salt excretion [6567]. These effects appear to be mediated independently of changes in cardiovascular status. Endotoxin treatment has been found to inhibit liver regeneration by suppression of proliferative inhibitory pathways via upregulation of TGF-b [67], leading to hepatocyte apoptosis and perisinusoidal brosis [68].
Fig. 6 Effect of cholestasis on liver function and regeneration
Cholestasis and restricted portal venous ow The portal vein, hepatic artery and bile duct are enclosed in a sheath of tissue known as the Glissonian capsule, with a limited amount of space within called the space of Mall (Fig. 3). Dilatation of the biliary tract reduces the volume within this space leading to reduction in portal venous ow, accompanied by an increase in hepatic arterial ow (hepatic buffer response) [71]. The reduction in portal venous ow is further exacerbated by hepatectomy, which may contribute to impaired regeneration post-surgery. In addition, portal-systemic shunting accompanying obstructive jaundice may further reduce portal venous ow [72]. Impaired liver regeneration and induction of apoptosis Hepatectomy in the presence of cholestasis has been found to signicantly inhibit liver regeneration and the expression of c-myc, which normally precedes the rst wave of mitosis [73, 74], and to decrease the expression of transcription factors involved in hepatocyte proliferation such as cyclin E [74]. Other liver-regeneration cytokines, such as epidermal growth factor and IL-6, are also depressed following hepatectomy in animal models with obstructive jaundice [72, 75, 76]. High levels of bile salts are associated with increased hepatocyte apoptosis [77], possibly via a FAS-dependent mechanism [78]. Interruption of enterohepatic circulation Bile salts within the small bowel lumen have an important function in maintaining bowel integrity and prevention of bacterial translocation [79]. In the presence of obstructive jaundice, the normal enterohepatic circulation is interrupted and so portal bacteraemia may be present, increasing the risk of septic complications following hepatectomy.
Cholestasis Obstructive jaundice is a ubiquitous presenting sign in patients with hilar cholangiocarcinomas. Preoperative biliary drainage of the future liver remnant has been advocated by some centres in order to optimise patients prior to surgery [69]. However, major hepatectomy in the absence of preoperative drainage has been described with acceptable mortality rates [70]. Drainage may comprise internal stenting using a plastic endoprosthesis (with subsequent inammatory changes making hilar dissection and delineation between normal and malignant tissue problematic) or external biliary drainage, with diversion of bile ow extra-hepatically. Cholestasis and diversion of biliary ow present particular problems and risks in patients facing major hepatectomies (Fig. 6).
123
152
J Hepatobiliary Pancreat Surg (2009) 16:145155
Supplementation with bile salts has been show to improve intestinal barrier function [80]. External biliary drainage (whilst reducing blood levels of bilirubin) still results in diversion of bile salts outside of the gut lumen and this may account for reduced liver regeneration in rats undergoing hepatectomy with external drains [81]. Liver regeneration has been found to be preserved with internal drainage [82] which therefore may be preferable to external drainage. However, in the context of hilar malignancies (where multiple biliary drainages may be required) endoscopic internal drainage can be difcult and carries with it a risk of cholangitis [83]. In addition, internal endoprostheses serve to increase the technical difculty of resection. For this reason, external biliary drainage is often preferred, but the addition of bile replacement agents may help restore gut immunity [83].
have been found to lead to severe sinusoidal dilatation and brosis in livers of some patients, which may further increase the risk of hepatic failure in these individuals undergoing resection surgery [97].
Age and other co-morbidities A number of patient-related factors contribute to an increased probability of hepatic failure following hepatectomy. Determining their liver-specic contribution to mortality and liver dysfunction is problematic. Liver function appears to be well maintained even at extremes of age [98], however, some evidence exists that age inuences restoration of liver volume after hepatectomy in rats [99, 100], and in humans, an age of above 50 was found to negatively inuence transplanted liver volumes [101]. Likewise, diabetes has been associated with a greater risk of mortality from liver failure following liver surgery, and this could be secondary to the presence of steatotic liver in these individuals [102]. Given the complex interaction between factors contributing to liver failure after hepatectomy, it is likely that careful attention to co-morbidities with subsequent optimisation of patients is an important component in planning and undertaking major liver resections.
Cirrhosis, steatosis and the post-chemotherapy liver Patients with liver cirrhosis have an increased risk of mortality following resection, with some series reporting the risk to be as high as 20% [84]. In addition to an overall operative risk, cirrhosis results in a higher probability of liver failure and is associated with reduced regeneration following hepatectomy. Cirrhotic livers demonstrate lower levels of hepatocyte growth factor (due to a failure of conversion of the precursor to the active form) [85] and impaired transcription factors [86] leading to a reduction of DNA synthesis and lower volumes of regenerated liver [87]. Cirrhotic livers show an increased risk of ischaemiareperfusion injury, and hyperbaric oxygen administration following hepatectomy has demonstrated some value in augmenting liver function and regeneration post-hepatectomy [88]. Fibrosis leading to regional ischaemia is also thought to contribute to impaired growth and regeneration [89]. Exogenous administration of IL-6 and hepatocyte growth factor have been shown in animal models to independently improve survival and regeneration after surgery [87, 90]. Attempts to predict which cirrhotic patients are at greater risk of fulminant liver failure after hepatectomy have included the Child-Pugh classication system and functional assessment of liver-related clearance of quantiable materials such as indocyanine green, hayaluronic acid or hepatic 99 mTc-diethylenetriamine pentaacetic acid-galactosyl-human serum albumin [9193]. Steatosis of the liver is an increasingly common nding either due to life-style related factors or as a common sequel to chemotherapy for colorectal liver metastases. Steatosis is associated with a delay in regeneration, increased susceptibility to ischaemia/reperfusion injury and increased risk of trauma and bleeding following hepatectomy [9496]. Chemotherapy agents, such as oxaliplatin,
Conclusion Major liver resections have now become the accepted gold standard of treatment for a wide range of primary and secondary liver malignancies. Given the magnitude of the surgery, mortality and morbidity rates are extremely good; however, a small but signicant number of individuals will succumb to liver failure in the immediate post-operative period. Careful patient selection and preservation of an obligate volume of remnant liver is essential. Modiable causes of hepatic failure include avoidance of sepsis, drainage of cholestasis with restoration of enteric bile salts and judicious use of portal triad inow occlusion intraoperatively. Avoidance of post-operative sepsis is most likely to be achieved by patient selection, meticulous intraoperative technique and post-operative care. Modulation of portal vein pressures post-operatively may further help reduce the risk of liver failure.
References
1. Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg. 1995;19(1):5971.
123
J Hepatobiliary Pancreat Surg (2009) 16:145155 2. Farmer DG, Rosove MH, Shaked A, Busuttil RW. Current treatment modalities for hepatocellular carcinoma. Ann Surg. 1994;219:23647. 3. Tranberg KG. Percutaneous ablation of liver tumours. Best Prac Res Clin Gastroenterol. 2004;18:12545. 4. Garden OJ, Rees M, Poston GJ, Mirza D, Saunders M, Ledermann J, et al. Guidelines for resection of colorectal cancer liver metastases. Gut. 2008;55:iii18. 5. Pawlik TM, Schulick RD, Choti MA. Expanding criteria for resectability of colorectal liver metastases. Oncologist. 2008;13: 5164. 6. Takeda K, Togo S, Kunhiro O, Fujii Y, Kuroswa H, Tanaka K, et al. Clinicohistological features of liver failure after excessive hepatectomy. Hepatogastroenterology. 2002;49:3548. 7. van den Broek MA, Damink SW, Dejong CH, Lang H, Malago M, Jalan R, et al. Liver failure after partial hepatic resection: denition, pathophysiology, risk factors and treatment. Liver Int. 2008;28:76780. 8. Detroz B, Sugarbaker PH, Knol JA, Petrelli N, Hughes KS. Causes of death in patients undergoing liver surgery. Cancer Treat Res. 1994;69:24157. 9. Bolder U, Brune A, Schmidt S, Tacke J, Jauch KW, Lohein D. Preoperative assessment of mortality risk in hepatic resection by clinical variables: a multivariate analysis. Liver Transpl Surg. 1999;5:22737. 10. Simmonds PC, Primrose JN, Colquitt JL, Garden OJ, Poston GJ, Rees M. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer. 2006;94:98299. 11. Taub R. Liver regeneration: from myth to mechanism. Nature. 2004;5:83647. 12. Yamanaka N, Okamoto E, Kawamura E, Kato T, Oriyama T, Fujimoto J. Dynamics of normal and injured human liver regeneration after hepatectomy as assessed on the basis of computed tomography and liver function. Hepatology. 1993;18:7985. 13. Nagasue N, Yukaya H, Ogawa Y, Kohno H, Nakamura T. Human liver regeneration after major hepatic resection. Ann Surg. 1987;206:309. 14. Zimmers TA, McKillop IH, Pierce RH, Yoo J, Koniaris LG. Massive liver growth in mice induced by systemic interleukin-6 administration. Hepatology. 2003;38:32634. 15. Garcia-Trevijano ER, Martinez-Chantarv ML, Latasa MU, Mato JM, Avila MA. NO sensitises rat hepatocytes to proliferation by modifying S-adenosylmethionine levels. Gastroenterology. 2002;122:135563. 16. Rai RM, Lee FY, Rosen A, Yang SQ, Lin HZ, Koteish A. Impaired liver regeneration in inducible nitric oxide synthases decient mice. Proc Natl Acad Sci USA. 1998;95:1382934. 17. Sakamoto T, Liu Z, Murase N, Ezure T, Yokomuro S, Poli V. Mitosis and apoptosis in the liver of interleukin-6-decient mice after partial hepatectomy. Hepatology. 1999;29:40311. 18. Kooby DA, Zakian KL, Challa SN, Matei C, Petrowsky H, Yoo HH. Use of phosphorus-31 nuclear magnetic resonance spectroscopy to determine safe timing of chemotherapy after hepatic resection. Cancer Res. 2000;60:38006. 19. Ozawa K, Yamada T, Ukikusa M. Mitochondrial phosphorylative activity and DNA synthesis in regenerating liver of diabetic rats. J Surg Res. 1981;31:3845. 20. Saibara T, Onishi S, Maeda T, Yamamoto Y. Arterial blood ketone body ratio as a possible indicator for predicting fulminant hepatitis in patients with acute hepatitis. Liver. 1992;12:3926. 21. Asano M, Ozawa K, Tobe T. Postoperative prognosis as related to blood ketone body ratios in hepatectomized patients. Eur J Surg Res. 1983;15:30211. 22. Kerem M, Berdirli A, Oruoglu E, Deniz K, Turkozkahn N, Pasaglu H, et al. Ischemic preconditioning improves liver
153 regeneration by sustaining energy metabolism after partial hepatectomy in rats. Liver Int. 2006;26:9949. Ma Y, Wu LW, Wu JL, Liang YJ, Zhu ZY, Hu RD, et al. Energy metabolism and survival of liver grafts from non-heart beating donor rats with warm ischaemia injury. Hepatobiliary Pancreat Dis Int. 2006;5:5215. Miyagi S, Iwane T, Akamatsu Y, Nakamura A, Sato A, Satomi S. The signicance of preserving the energy status and microcirculation in liver grafts from non-heart beating donors. Cell Transplant. 2008;17:1738. Farges O, Belghiti J, Kianmanesh R. Portal vein embolisation before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237:20817. Madoff DC, Hicks ME, Abdalla EK. Portal vein embolisation with polyvinyl alcohol particles and coils in preparation for major liver resection for hepatobiliary malignancy. Safety and effectiveness: study in 26 patients. Radiology. 2003;227:25160. Vauthey JN, Cahaui A, Do KA. Standardized measurement of the future liver remnant prior to extended liver resection. Methodology and clinical associations. Surgery. 2000;127:5129. Popescu I, David L, Brasoveanu V, Boros M, Hrehoret D. Twostage hepatectomy: an analysis of a single centers experience. Magy Seb. 2006;59:1849. Lautt WW. Mechanims and role of intrinsic regulation of hepatic arterial blood ow: hepatic arterial buffer response. Am J Physiol. 1985;249:G54956. Nagino M, Kanai M, Morioka A. Portal and arterial embolisation before extensive liver resection in patients with markedly poor functional reserve. J Vasc Interv Radiol. 2000;11:10638. Kin Y, Nimura Y, Hayakawa N, Kamiqya J, Kondo S, Nagino M. Doppler analysis of hepatic blood ow predicts liver dysfunction after major hepatectomy. World J Surg. 1994;18:1439. Sato A, Tsukada K, Hatakeyama K. Role of shear stress and immune responses in liver regeneration after a partial hepatectomy. Surg Today. 1999;29:19. Niiya T, Murakami M, Aoki T, Murai N, Shimizu Y, Kusano M. Immediate increase of portal pressure, reecting sinusoidal shear stress, induced liver regeneration after partial hepatectomy. J Hepatobiliary Pancreat Surg. 1999;6:27580. Fukauchi T, Hirosi H, Onitsuka A, Hayahsi M, Senga S, Imai N. Effects of portal-systemic shunt following 90% partial hepatectomy in rats. J Surg Res. 2000;89:12631. Kishikawa YK, Suehrio T, Niahizaki T, Shamida M, Itasaka H, Nomoto K. Partial hepatic grafting: porcine study on critical volume reduction surgery. Surgery. 1995;118:48691. Man K, Lo CM, Ng IO, Wong YC, Qin LF, Fan ST. Liver transplantation in rats using small-for-size grafts: a study of haemodynamic and morphological changes. Arch Surg. 2001;136:2805. Kiuchi T, Kasahara M, Uryuhama K, Inomata Y, Uemoto S, Asnouman K. Impact of graft mismatching on graft prognosis in liver transplantation from living donors. Transplantation. 1999;67:3217. Ito K, Ozasa H, Horkawa S. Effects of prior splenectomy on remnant liver after partial hepatectomy with Pringle maneuver in rats. Liver Int. 2005;25:43844. Asakura T, Ohkochi N, Orii T, Koyamada N, Tsukamoto S, Sato M, et al. Portal vein pressure is the key for successful liver transplantation of an extremely small graft in the pig model. Transpl Int. 2003;16:37682. Oya H, Sato S, Yamamot T, Takeishi H, Nakasuka T, Kobayshi Y, et al. Surgical procedures for decompression of excessive shear stress in small-for-size living donor liver transplantation: new hepatic vein reconstruction. Transpl Proc. 2005;37:110811. Troisi R, Riccardi S, Smeets P, Petrovic M, Van Maele G, Colle I, et al. Effects of hemi-portocaval shunts for inow modulation
23.
24.
25.
26.
27.
28.
29.
30.
31.
32.
33.
34.
35.
36.
37.
38.
39.
40.
41.
123
154 on the outcome of small-for-size grafts in living liver donor transplantation. Am J Transplant. 2005;5:1397404. Iida T, Yagi S, Taniguchi K, Hori T, Uemoto S. Improvement of morphological changes after 70% hepatectomy with portocaval shunt: preclinical study in the porcine model. J Surg Res. 2007;143:23846. Wang H, Ohkohi N, Enomoto Y, Usuda M, Miyagi S, Masuoka H, et al. Effect of portocaval shunt on residual extreme small liver after extended hepatectomy in porcine. World J Surg. 2006;30:201422. Hata Y, Yoshiawa Y, Une Y, Saasaki F, Nakajima Y, Takahashi H, et al. Liver regeneration following portacaval shunt in rats: 30 , 50 -cyclic AMP changes in plasma and liver tissue. Res Exp Med (Berl). 1992;192:1316. Griesler HP, Voohees AB, Price JB. The nonportal origin of the factors initiating hepatic regeneration. Surgery. 1979;86:2107. Guest J, Ryan CJ, Benjamin IS, Blumgart LH. Portacaval transposition and subsequent partial hepatectomy in the rat: effects on liver atrophy, hypertrophy and regenerative hyperplasia. Br J Exp Pathol. 1977;58:1406. Pouyet M, Mechet I, Paquet C, Scoazec J. Liver regeneration and haemodynamic in pigs with mesocaval shunt. J Surg Res. 2007;138:12834. Man K, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Prospective evaluation of Pringle maneuver in hepatectomy for liver tumours by a randomized study. Ann Surg. 1997;226:70411. Moussa ME, Uemoto SS, Habib NA. Effect of total vascular exclusion during liver resection on hepatic ultrastructure. Liver Transplantation. 1996;2:4617. Peralta C, Hotter G, Closa D, Prats N, Xaus C, Gelpi E, et al. The protective role of adenosine in inducing nitric oxide synthesis in rat liver ischaemia preconditioning is mediated by activation of adenosine A2 receptors. Hepatology. 1999;29:12632. Yadav SS, Sindram D, Perry DK, Clavien PA. Ischaemic preconditioning protects the mouse liver by inhibition of apoptosis through a caspase-dependent pathway. Hepatology. 1999;30:122331. Szijarto A, Hahn O, Lotz G, Schaff Z, Madarasz E, Kupcsulik PK. Effect of ischemic preconditioning on rat liver microcirculation monitored with laser Doppler owmetry. J Surg Res. 2006;131:1507. Yin DP, Sankary HN, Chong AS, Ma LL, Shen J, Foster P, et al. Protective effect of ischemic preconditioning on liver preservation-reperfusion injury in rats. Transplantation. 1998;66:1527. Gomez D, Homer-Vanniasinkam S, Graham AM, Prasad KR. Role of ischaemic preconditioning in liver regeneration following major liver resection and transplantation. World J Gastroenterol. 2007;13:65770. Eipel C, Glanemann M, Nuessler AK, Menger MD, Neuhaus P, Vollmar B. Ischemic preconditioning impairs liver regeneration in extended reduced-size livers. Ann Surg. 2005;241:47784. Filos KS, Kirkilesis I, Spilopoulou I, Scopa CD, Nikolopoulou V, Kouraklis G, et al. Bacterial translocation, endotoxaemia and apoptosis following Pringle maneuver in rats. Injury. 2004;35: 3543. Champion HR, Jones RT, Trump BF, Decker R, Wilson S, Miginski M. A clinicopathologic study of hepatic dysfunction following shock. Surg Gynecol Obstet. 1976;142:65762. Selzner N, Selzner M, Odermatt B, Tian Y, Van Rooijen N, Calvien PA. ICAM-1 triggers liver regeneration through leukocyte recruitment and Kupffer cell-dependent release of TNFalpha/IL-6 in mice. Gastroenterology. 2003;124:692700. Strey CW, Markiewski K, Mastellos D. The proinammatory mediators C3a and C5a are essential for liver regeneration. J Exp Med. 2003;198:91323. Mochita S, Ogata I, Hirata K, Ohta YSY, Fugiwara K. Provocation of massive hepatic necrosis by endotoxin after partial hepatectomy in rats. Gastroenterology. 1990;99:7717.
J Hepatobiliary Pancreat Surg (2009) 16:145155 61. Gross K, Katz S, Dunn SP, Cikrit D, Rosentahl R, Grosfeld JL. Bacterial clearance in the intact and regenerating liver. J Pediatr Surg. 1985;20:3203. 62. Boermeester MA, Hodijik APJ, Meyer MS, Cuesta MA, Pappelmelk BJ, Wesdor RC. Liver failure induces a systemic inammatory response: prevention by recombinant N-terminal bactericidal/permeability-increasing protein. Am J Physiol. 1995;147:142840. 63. Chaisson ML, Brooling JT, Ladiges W, Tsai S, Fausto N. Hepatocyte-specic inhibition of NF-kappaB leads to apoptosis but not after partial hepatectomy. J Clin Invest. 2002;110:193202. 64. Ogata I, Yamashitia K, Horiuchi H, Okuda K, Todo S. A novel tumour necrosis factor-alpha suppressant, ONO-SM362 and promotes liver regeneration after extensive hepatectomy. Surgery. 2008;143:54555. 65. Nolan JP. Endotoxin, reticuloendothelial function and liver injury. Hepatology. 1981;1:45865. 66. Roelofsen H, van der Veere CN, Ottenhoff R, Schoemaker B, Jansen PL, Oude Elferenk RP. Decreased bilirubin transport in the perfused liver of endotoxemic rats. Gastroenterology. 1994;107:107584. 67. Akita K, Okuno M, Enya M, Imai S, Moriwaki H, Kawada N, et al. Impaired liver regeneration in mice by lipopolysaccharide via TNF-alpha/kallikrein-mediated activation of latent TGFbeta. Gastroenterology. 2002;123:35264. 68. Yoshimoto N, Togo S, Kuboto T, Kammiukai N, Saito S, Nagano Y, et al. Role of transforming growth factor-beta (TGFbeta1) in endotoxin-induced hepatic failure after extensive hepatectomy in rats. J Endotoxin Res. 2005;11:339. 69. Belghiti J, Ogata S. Preoperative optimization of the liver for resection in patients with hilar cholangiocarcinoma. HPB (Oxford). 2005;7:2523. 70. Khuntikeo N, Pugkhem A, Bhudisawasdi V, Uttarvichien T. Major hepatic resection for hilar cholangiocarcinoma without preoperative biliary drainage. Asian Pac J Cancer Prev. 2008;9: 835. 71. Kanda H, Nimura Y, Yasui A, Uematsu T, Kamiaya S, Machiki Y. Hepatic blood ow after acute biliary obstruction and drainage in conscious dogs. Hepatogastroenterology. 1996;43: 23540. 72. Baer HU, Guastella T, Wheatley AM, Zimmermann A, Blumgart LH. Acute effects of partial hepatectomy on liver blood ow in the jaundiced rat. J Hepatol. 1993;19:27782. 73. Tracy TF, Bailey PV, Goerke ME, Sotelo-Avila C, Weber TR. Cholestasis without cirrhosis alters regulatory liver gene expression and inhibits hepatic regeneration. Surgery. 1991;110: 17682. 74. Nakano K, Chiiiwa K, Tanaka K. Lower activity of CCAAT/ enhancer-binding protein and expression of cyclin E, but not cyclin D1, activation protein-1 and p21 (WAF1) after partial hepatectomy in obstructive jaundice. Bioch Biophys Res Commun. 2001;280:6405. 75. Bissig KD, Marti U, Solioz M, Forestier M, Zimmermann H, Luthi M, et al. Epidermal growth factor is decreased in liver of rats with biliary cirrhosis but does not act as a paracrine growth factor immediately after hepatectomy. J Hepatol. 2000;33: 27581. 76. Fujiwara Y, Shimada H, Yamashita Y, Adachi E, Shirabe K, Takenaka K, et al. Cytokine characteristics of jaundice in mouse liver. Cytokine. 2001;13:18891. 77. Wang DS, Dou KF, Li KZ, Gao ZQ, Song ZS, Liu ZC. Hepatocellular apoptosis after hepatectomy in obstructive jaundice in rats. World J Gastroenterol. 2003;9:273741. 78. Myoshi H, Rust C, Roberts PJ, Burgart LJ, Gores GJ. Hepatocyte apoptosis after bile duct ligation in the mouse involves Fas. Gastroenterology. 1999;117:669777.
42.
43.
44.
45. 46.
47.
48.
49.
50.
51.
52.
53.
54.
55.
56.
57.
58.
59.
60.
123
J Hepatobiliary Pancreat Surg (2009) 16:145155 79. Sano T, Ajiki T, Takeyama Y, Kuroda Y. Internal biliary drainage improves decreased number of gut mucosal T lymphocytes and MAdCAM-1 expression in jaundiced rats. Surgery. 2004;136:6939. 80. Kamiya S, Nagino M, Kanazawa H, Komatsu S, Mayumi T, Takagi K. The value of bile replacement during external biliary drainage: an analysis of intestinal permeability, integrity and microora. Ann Surg. 2004;239:5107. 81. Lyomasa S, Teraski M, Kuriki H, Nimura Y, Shionoya S, Kojima S. Decrease in regeneration capacity of rat liver after external biliary drainage. Eur J Surg Res. 1992;24:26572. 82. Suzuki H, Lyomasa S, Nimura Y, Yoshida S. Internal biliary drainage, unlike external drainage, does not suppress the regeneration of cholestatic rat liver after partial hepatectomy. Hepatology. 1994;20:131822. 83. Nagino M, Takada T, Miyazaki M, Miyakawa S, Tsukada K, Kondo S, et al. Preoperative biliary drainage for biliary tract and ampullary carcinomas. J Hepatobiliary Pancreat Surg. 2008;15: 2530. 84. Takenaka K, Kanematsu T, Fukuzawa K, Sugimachi K. Can hepatic failure after surgery for hepatocellular carcinoma in cirrhotic patients be prevented? World J Surg. 1990;14:1237. 85. Kaibori M, Inoue T, Sakakjura Y, Oda M, Nagahama T, Kwon AH. Impairment of activation of hepatocyte growth factor precursor into its mature form in rats with liver cirrhosis. J Surg Res. 2002;106:10814. 86. Zhao G, Nakano K, Chijiwa K, Ueda J, Tanaka M. Inhibited activities in CCCAAT/enhancer-binding proteins and cyclins after hepatectomy in rats with thioacetamide-induced liver cirrhosis. Biochem Biophys Res Commun. 2002;292:47481. 87. Tiberio GA, Tiberio L, Benetti A, Cervi E, Montani N, Dreano M, et al. IL-6 promotes compensatory liver regeneration in cirrhotic rat liver after partial hepatectomy. Cytokine. 2008;42: 372378. 88. Ozdogan M, Ersoy E, Dundar K, Albayrak L, Devay S, Gundogdu H. Benecial effect of hyperbaric oxygenation on liver regeneration in cirrhosis. J Surg Res. 2005;129:2604. 89. Corpechot C, Barbu V, Wendum D, Chignard N, Housset C, Poupon R. Hepatocyte growth factor and c-Met inhibition by hepatic cell hypoxia: potential mechanism for liver regeneration in experimental cirrhosis. Am J Pathol. 2002;160:61320. 90. Xue F, Takahara T, Yata Y, Kuwabara Y, Shinno E, Nonome K, et al. Hepatocyte growth factor gene therapy accelerates regeneration in cirrhotic mouse livers after hepatectomy. Gut. 2003;52:694700.
155 91. Caesar J, Shaldon S, Chiandussi L, Guevara L, Sherlock S. The use of indocyanine green in the measurement of hepatic blood ow and as a test of hepatic function. Clin Sci. 1961;21:4357. 92. Nanshima A, Yamaguchi H, Shibasaki S, Sawai T, Yamaguchi E, Yasutake T. Measurement of serum hyaluronic acid level during the perioperative period of liver resection for evaluation of hepatic reserve. J Gastroenterol Hepatol. 2001;16:115863. 93. Hwang EH, Taki J, Shuke E, Nakajuma K, Kinuya S, Konishi S. Preoperative assessment of residual hepatic functional reserve using 99m-TC-DTPA-galatosyl-human-serum albumin dynamic SPECT. J Nucl Med. 1999;40:164451. 94. Rao MS, Papreddy K, Abecassis M, Hashimoto T. Regeneration of liver with marked fatty changes following partial hepatectomy in rats. Dig Dis Sci. 2001;46:3542. 95. Sun CK, Zhang XY, Zimmerman A, Davis G, Wheatley AM. Effect of ischaemia-reperfusion on the microcirculation of the steatotic liver of the Zucker Rat. Transplantation. 2001;72: 162531. 96. Selzner M, Clavien PA. Failure of regeneration of the steatotic liver: disruption at two levels in the regeneration pathway. Hepatology. 2000;31:3542. 97. Arotcarena R, Cales V, Berthelemy P, Parent Y, Malet M, Etcharry F, et al. Severe sinusoidal lesions: a serious and overlooked complication of oxaliplatin-containing chemotherapy? Gastroenterol Clin Biol. 2006;30:13136. 98. Tietz NW, Shuey DF, Wekstein DR. Laboratory values in t ageing individuals: sexagenarians through centenarians. Clin Chem. 1992;38:116785. 99. Tsukamoto S, Nakata R, Kojo S. Effect of aging on rat liver regeneration after partial hepatectomy. Biochem Mol Biol Int. 1993;30:7738. 100. Beyer HS, Sherman R, Zieve L. Aging is associated with reduced liver regeneration and diminished thymidine kinase mRNA content and enzyme activity in the rat. J Lab Clin Med. 1990;117:1018. 101. Ikegami T, Nishizaki T, Yanaga K, Shjimada M, Kishikawa YK. The impact of donor age on living donor transplantation. Transplantation. 2000;70:17037. 102. Little SA, Jarnagin WR, DeMattteo RP, Blumgart LH, Fong Y. Diabetes is associated with increased preoperative mortality but equivalent long-term outcome after hepatic resection for colorectal cancer. J Gastrointest Surg. 2002;6:8894. 103. Yokoyama Y, Nagino M, Nimura Y. Mechanism of impaired hepatic regeneration in cholestatic liver. Hepatobiliary Pancreat Surg. 2007;14(2):15966.
123
Vous aimerez peut-être aussi
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (894)
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (587)
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (344)
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (119)
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (399)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2219)
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (265)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (73)
- Kushi - Macrobiotic Dietary RecommendationsDocument59 pagesKushi - Macrobiotic Dietary Recommendationstilopa100% (10)
- Wake Up Lean: 10-Day Flat Belly BlueprintDocument65 pagesWake Up Lean: 10-Day Flat Belly BlueprintElber Andrew96% (23)
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- Moeller USOLDocument8 pagesMoeller USOLflowptzPas encore d'évaluation
- Nota Kerja Pemasangan Hong Kong StyleDocument414 pagesNota Kerja Pemasangan Hong Kong StyleMohd Zamri IbrahimPas encore d'évaluation
- General SpecificationDocument327 pagesGeneral SpecificationmaymanyassinPas encore d'évaluation
- Daca - Churchil WinstonlDocument1 pageDaca - Churchil WinstonlflowptzPas encore d'évaluation
- Control Panel Comparison Chart: Feature SP4000 SP5500 SP6000 SP65 SP7000Document1 pageControl Panel Comparison Chart: Feature SP4000 SP5500 SP6000 SP65 SP7000Yew Toh TatPas encore d'évaluation
- Duffy 2007 Liver Transplant Criteria HCCDocument10 pagesDuffy 2007 Liver Transplant Criteria HCCflowptzPas encore d'évaluation
- Electronica in Imagini - Componente PasiveDocument144 pagesElectronica in Imagini - Componente Pasiveflowptz100% (1)
- K37 Formerly K32IRF - EQ00Document2 pagesK37 Formerly K32IRF - EQ00danielveigaPas encore d'évaluation
- Livraghi 2011 Treatment Opt HCCDocument8 pagesLivraghi 2011 Treatment Opt HCCflowptzPas encore d'évaluation
- Surgery in The Patient With Liver DiseaseDocument14 pagesSurgery in The Patient With Liver DiseaseflowptzPas encore d'évaluation
- Surgery in The Patient With Liver DiseaseDocument14 pagesSurgery in The Patient With Liver DiseaseflowptzPas encore d'évaluation
- Truant 2007 Remnant Liver Volume To Body Weight RatioDocument12 pagesTruant 2007 Remnant Liver Volume To Body Weight RatioflowptzPas encore d'évaluation
- Torzilli 2008 Review of Hepatectomy For Stage B and C of HCCDocument9 pagesTorzilli 2008 Review of Hepatectomy For Stage B and C of HCCflowptzPas encore d'évaluation
- Birkmayer 2011 Bariatric Surg Complications in MichiganDocument8 pagesBirkmayer 2011 Bariatric Surg Complications in MichiganflowptzPas encore d'évaluation
- Surgery in The Patient With Liver DiseaseDocument14 pagesSurgery in The Patient With Liver DiseaseflowptzPas encore d'évaluation
- Birkmayer 2011 Bariatric Surg Complications in MichiganDocument8 pagesBirkmayer 2011 Bariatric Surg Complications in MichiganflowptzPas encore d'évaluation
- Shauer 2010 Impact of GBP On Life ExpectancyDocument15 pagesShauer 2010 Impact of GBP On Life ExpectancyflowptzPas encore d'évaluation
- Nine Star Ki - Michio KushiDocument128 pagesNine Star Ki - Michio KushiMichael Kelly100% (8)
- Livraghi 2011 Treatment Opt HCCDocument8 pagesLivraghi 2011 Treatment Opt HCCflowptzPas encore d'évaluation
- Cochrane Evidence in Liver Metastases TreatmentDocument3 pagesCochrane Evidence in Liver Metastases TreatmentflowptzPas encore d'évaluation
- Chiche 2000 Liver AdenomatosisDocument8 pagesChiche 2000 Liver AdenomatosisflowptzPas encore d'évaluation
- Duffy 2007 Liver Transplant Criteria HCCDocument10 pagesDuffy 2007 Liver Transplant Criteria HCCflowptzPas encore d'évaluation
- Birkmayer 2011 Bariatric Surg Complications in MichiganDocument8 pagesBirkmayer 2011 Bariatric Surg Complications in MichiganflowptzPas encore d'évaluation
- Gillian McKeith Esti Ceea Ce MananciDocument1 pageGillian McKeith Esti Ceea Ce MananciflowptzPas encore d'évaluation
- Electronica in Imagini - Componente PasiveDocument144 pagesElectronica in Imagini - Componente Pasiveflowptz100% (1)
- Truant 2007 Remnant Liver Volume To Body Weight RatioDocument12 pagesTruant 2007 Remnant Liver Volume To Body Weight RatioflowptzPas encore d'évaluation
- Articulo Obesidad e InflamacionDocument19 pagesArticulo Obesidad e InflamacionPaulo CardosoPas encore d'évaluation
- Cytokine Expression in Dengue Fever and Dengue Hemorrhagic Fever Patients With Bleeding and Severe HepatitisDocument8 pagesCytokine Expression in Dengue Fever and Dengue Hemorrhagic Fever Patients With Bleeding and Severe HepatitisMapi M. MateoPas encore d'évaluation
- Ileus in Critical Illness Mechanisms And.11Document6 pagesIleus in Critical Illness Mechanisms And.11albimar239512Pas encore d'évaluation
- Cyclic tensile strain-induced yes-associated protein activity modulates the response of human periodontal ligament mesenchymal stromal cells to tumor necrosis factor-αDocument10 pagesCyclic tensile strain-induced yes-associated protein activity modulates the response of human periodontal ligament mesenchymal stromal cells to tumor necrosis factor-α赵忠琪Pas encore d'évaluation
- Inflammation in Fear - and Anxiety-Based Disorders - 2017Document17 pagesInflammation in Fear - and Anxiety-Based Disorders - 2017Izac OliveiraPas encore d'évaluation
- 1-S2.0-S0889159107000864-Main 12.03.12 P. M.Document6 pages1-S2.0-S0889159107000864-Main 12.03.12 P. M.DaniPas encore d'évaluation
- Serum Interleukin - 6 Level Among Sudanese Patients With Chronic Kidney DiseaseDocument3 pagesSerum Interleukin - 6 Level Among Sudanese Patients With Chronic Kidney DiseaseBIOMEDSCIDIRECT PUBLICATIONSPas encore d'évaluation
- ‘Topical Application of Avena Sativa in Managing Uremic Xerosis, Hyperpigmentation, and Pruritus among Patients with Chronic Kidney Disease: A Comprehensive Review’ Leeba L J1 , Smitha P V2 , Usharani E NDocument9 pages‘Topical Application of Avena Sativa in Managing Uremic Xerosis, Hyperpigmentation, and Pruritus among Patients with Chronic Kidney Disease: A Comprehensive Review’ Leeba L J1 , Smitha P V2 , Usharani E NeditorbijnrPas encore d'évaluation
- Pharmacological Research: Giuseppe Derosa, Pamela Maffioli, Luis E. Simental-Mendía, Simona Bo, Amirhossein SahebkarDocument11 pagesPharmacological Research: Giuseppe Derosa, Pamela Maffioli, Luis E. Simental-Mendía, Simona Bo, Amirhossein SahebkarJose Ruben Sorto BadaPas encore d'évaluation
- ARTÍCULO 10 - Application of Nanomedicine and Mesenchymal Stem Cells in Burn InjuriesDocument13 pagesARTÍCULO 10 - Application of Nanomedicine and Mesenchymal Stem Cells in Burn InjuriesPaula RPas encore d'évaluation
- Periapical Inflammatory: Responses Their ModulationDocument24 pagesPeriapical Inflammatory: Responses Their ModulationSalsabila Tri YunitaPas encore d'évaluation
- Voluntary Feed Intake in Growing-Finishing PigsDocument18 pagesVoluntary Feed Intake in Growing-Finishing PigsDiegoRodríguezSPas encore d'évaluation
- DRT2012-460419Document11 pagesDRT2012-460419Sapto SutardiPas encore d'évaluation
- Differential Release of Mast Cell Mediators and The Pathogenesis of InflammationDocument14 pagesDifferential Release of Mast Cell Mediators and The Pathogenesis of InflammationklaumrdPas encore d'évaluation
- Met 2008 0065 PDFDocument10 pagesMet 2008 0065 PDFDeedee RenovaldiPas encore d'évaluation
- COVID-19 and Autoimmune DiseasesDocument8 pagesCOVID-19 and Autoimmune DiseasesMan'SzAr'diAnSyAhPas encore d'évaluation
- Musictherapy DementiaDocument16 pagesMusictherapy DementiaConsuelo VelandiaPas encore d'évaluation
- Pathophysiology - Rheumatoid ArthritisDocument1 pagePathophysiology - Rheumatoid ArthritisAngel FiloteoPas encore d'évaluation
- Behavioral Nutrition Applied To The Treatment of oDocument46 pagesBehavioral Nutrition Applied To The Treatment of oDewi RatnasariPas encore d'évaluation
- Experimental Eye ResearchDocument6 pagesExperimental Eye ResearchdariusPas encore d'évaluation
- Free Radicals and Antioxidants in Normal PhysiologicalDocument41 pagesFree Radicals and Antioxidants in Normal PhysiologicalleosabreuPas encore d'évaluation
- Modern and Convensional Wound Dressing To Interleukin 1 and Interleukin 6 in Diabetic WoundDocument2 pagesModern and Convensional Wound Dressing To Interleukin 1 and Interleukin 6 in Diabetic WoundGilang yuanggaPas encore d'évaluation
- Review On Nature of Inter Display Between Covid 19 and Systemic DiseaseDocument9 pagesReview On Nature of Inter Display Between Covid 19 and Systemic DiseaseKhalid NPas encore d'évaluation
- UV RadiationDocument8 pagesUV RadiationDARWIN RANAPas encore d'évaluation
- IVMS - General Pathology, Inflammation NotesDocument19 pagesIVMS - General Pathology, Inflammation NotesMarc Imhotep Cray, M.D.Pas encore d'évaluation
- Snow2021 Article TocilizumabInCOVID-19AMeta-anaDocument12 pagesSnow2021 Article TocilizumabInCOVID-19AMeta-anaYajaira UgenioPas encore d'évaluation
- Pentoxifylline Influences Acute Phase Response in Acute Myocardial InfarctionDocument1 pagePentoxifylline Influences Acute Phase Response in Acute Myocardial InfarctionIoana AntonesiPas encore d'évaluation
- Cholesterol Crystals in Periapical Lesions of Root Filled TeethDocument7 pagesCholesterol Crystals in Periapical Lesions of Root Filled TeethCarlos BelsuzarriPas encore d'évaluation
- Role of Heme Oxygenase in Inflammation, Insulin-SignallingDocument18 pagesRole of Heme Oxygenase in Inflammation, Insulin-SignallingAndri Praja SatriaPas encore d'évaluation