Académique Documents
Professionnel Documents
Culture Documents
Anne A Ling and Normalizing of Steel
Transféré par
Tareef HashDescription originale:
Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Anne A Ling and Normalizing of Steel
Transféré par
Tareef HashDroits d'auteur :
Formats disponibles
Annealing and normalizing of steel
Objective:To study the effect of the cooling rate and the amount of alloying elements on the micro structure and the mechanical properties ( Rockwell hardness ) of medium carbon steel.
Background:Full annealing is the process of slowly raising the temperature about 50 C (90 F) above the Austenitic temperature line A3 in the case of Hypoeutectoid steels (steels with < 0.77% Carbon) and 50 C (90 F) into the Austenite-Cementite region in the case of Hypereutectoid steels (steels with > 0.77% Carbon).
It is held at this temperature for sufficient time for all the material to transform into Austenite or Austenite-Cementite as the case may be. It is then slowly cooled at the rate of about 20 C/hr (36 F/hr) in a furnace to about 50 C (90 F) into the Ferrite-Cementite range. At this point, it can be cooled in room temperature air with natural convection.
The grain structure has coarse Pearlite with ferrite or Cementite (depending on whether hypo or hyper eutectoid). The steel becomes soft and ductile.
Normalizing is the process of raising the temperature to over 60 C (108 F), above line A3 or line ACM fully into the Austenite range. It is held at this temperature to fully convert the structure into Austenite, and then removed form the furnace and cooled at room temperature under natural convection. This results in a grain structure of fine Pearlite with excess of Ferrite or Cementite. The resulting material is soft; the degree of softness depends on the actual ambient conditions of cooling. This process is considerably cheaper than full annealing since there is not the added cost of controlled furnace cooling. The main difference between full annealing and normalizing is that fully annealed parts are uniform in softness (and machinablilty) throughout the entire part; since the entire part is exposed to the controlled furnace cooling. In the case of the normalized part, depending on the part geometry, the cooling is non-uniform resulting in non-uniform material properties across the part. This may not be desirable if further machining is desired, since it makes the machining job somewhat unpredictable. In such a case it is better to do full annealing. Process Annealing is used to treat work-hardened parts made out of lowCarbon steels (< 0.25% Carbon). This allows the parts to be soft enough to undergo further cold working without fracturing. Process annealing is done by raising the temperature to just below the Ferrite-Austenite region, line A1on the diagram. This temperature is about 727 C (1341 F) so heating it to about 700 C (1292 F) should suffice. This is held long enough to allow recrystallization of the ferrite phase, and then cooled in still air. Since the material stays in the same phase through out the process, the only change that occurs is the size, shape and distribution of the grain structure. This process is cheaper than either full annealing or normalizing since the material is not heated to a very high temperature or cooled in a furnace.
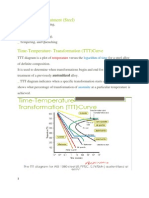
The Iron Carbon Equilibrium Diagram :Pure iron is a relatively soft, ductile low strength metal with few practical engineering applications. The addition of Carbon to pure iron increases strength and hardenability to useful levels. However it decreases ductility. The addition of Carbon influences the allotropic changes discussed in the last section. Since mechanical behaviour is directly related to the phases present it is important to study these phases and how they are influenced by temperature. A study of the IronCarbon phase diagram is used for this purpose. Figure 1 is an Iron - Carbon diagram showing the phases present in any alloy containing up to 6% Carbon. As you can see a eutectic point also exists in this diagram
Ferrite Known as -iron Pure iron at room temperature Body-centered cubic structure BCC. Austenite Known as -iron 910C Face-centered cubic FCC. Much softer than ferrite More easily worked
Pearlite (Perlite) Pearly luster in the microscope Interference of light in its regular layers Most common constituent of steel Gives steel most of its strength layered mixture of ferrite and cementite of average composition 0.83% carbon Cementite Hard, brittle, white melts at 1837C density of 7.4 g/cc On the phase diagram, cementite corresponds to a vertical line at 6.7% C Engineers only care about compounds with less carbon
Materials:A stock bar of plain carbon steel AISI 1040 and a stock of low alloy steel AISI 4340. They both have the same carbon content, about 0.4%, but the second contains other alloying elements such as molybdenum, nickel, and chromium.
Procedures:1. Cut two small pieces from the plain carbon steel stock, and two similar pieces from the low alloy steel stock. 2. Remove burrs from the samples by filling or grinding and stamp an identification code on each sample. 3. Place each sample in a small resistant furnace which is set at the proper autenitizing temperature, about 840C. Keep each specimen in the furnace half an hour after the temperature returns to its initial setting. 4. Take one plain carbon steel specimen and one low alloy steel specimen out of the furnaces and let them cool down to room temperature (place them on a refractory tile or a brick in still air). As explained in your textbook, this is called a normalizing operation. 5. Furnace cool the other two specimens (the plain carbon steel and one low alloy steel).This is achieved by shutting off the furnace while keeping its door closed and the specimen inside, thus ensuring low cooling rate, which can actually be determined. This operation is called annealing. Remember, this takes a very long time so you can let the furnace cool down overnight. 6. Measure the Rockwell hardness of each specimen. If possible, use scale B or even scale A, so that the Rockwell hardness of the two annealed and two normalized specimens would fall on the same scale, thus facilitating comparison. 7. Prepare a microsection from each specimen for metallographic examination, and take a photomicrograph for each specimen. 8. Compare the Rockwell hardness of the different specimens as well as their photomicrographs and try to draw a correlation between the microstructure and the Rockwell hardness.
Review Questions on annealing and normalizing of steel:1. What is the effect of increasing the cooling rate (from furnace cooling to air cooling) on the fineness of pearlite formed and on the amounts of the different phases of the AISI 1040 specimens and AISI 4340 specimens? 2. What is the effect of increasing the cooling rate (from furnace cooling to air cooling) on the hardness of the AISI 1040 specimens and AISI 4340 specimens? 3. Explain why it is very difficult to determine the carbon content of a normalized steel specimen from its microstructure only? 4. Estimate the amount of pearlite in each specimen and compare these amounts with the equilibrium values obtained from the iron carbon phase diagram. 5. Mention some factors that may affect the actual cooling rate of an air cooled specimen. 6. What effects do the alloying additives have on the microstructure and mechanical properties of air cooled (normalized ) steel specimens? 7. What effects do the alloying additives have on the microstructure and mechanical properties of furnace cooled (annealed) steel specimens?
Vous aimerez peut-être aussi
- BG2802 Heat Treatment and Mechanical Properties of SteelsDocument11 pagesBG2802 Heat Treatment and Mechanical Properties of SteelsVenus LimPas encore d'évaluation
- Report Heat Treatment Eng Lab 3Document7 pagesReport Heat Treatment Eng Lab 3khalifawhan43% (7)
- 3 - Heat Treatment & Engineering ApplicationDocument24 pages3 - Heat Treatment & Engineering ApplicationHussein SaeedPas encore d'évaluation
- Anealing TypesDocument29 pagesAnealing TypesPratheep AddrinPas encore d'évaluation
- Modified HT NotesDocument16 pagesModified HT NotesBeesam Ramesh KumarPas encore d'évaluation
- Engg Metallurgy Lecture 5Document54 pagesEngg Metallurgy Lecture 5Patil Sudheer GowdPas encore d'évaluation
- Lab PHY 2Document16 pagesLab PHY 2Shivraj ChougulePas encore d'évaluation
- Physical Metallurgy-18 Heat Treatment of SteelDocument7 pagesPhysical Metallurgy-18 Heat Treatment of SteelDSGPas encore d'évaluation
- EAS107 Lab 1Document14 pagesEAS107 Lab 1Mohd Ashraf Mohd Ismail100% (1)
- Design For Assembly: Iit BombayDocument16 pagesDesign For Assembly: Iit Bombaytejap314Pas encore d'évaluation
- Iron Carbon DiagramDocument8 pagesIron Carbon Diagramashok pradhanPas encore d'évaluation
- 05 HeattreatmentDocument39 pages05 HeattreatmentHaerul AtamimiPas encore d'évaluation
- Lab 4 - Heat Treatment of Steels - Quenching & TemperingDocument14 pagesLab 4 - Heat Treatment of Steels - Quenching & TemperingSarah AkuteyPas encore d'évaluation
- Heat Treatment of Steels (Power Point Presentation)Document14 pagesHeat Treatment of Steels (Power Point Presentation)Armando Lopez Bond75% (4)
- Heat TreatmentDocument59 pagesHeat TreatmentINSTECH Consulting100% (1)
- Heat Treatment of Carbon SteelDocument10 pagesHeat Treatment of Carbon SteelPrasanna RajaPas encore d'évaluation
- Thermal Lab 1Document6 pagesThermal Lab 1Muhammad ZulhilmiPas encore d'évaluation
- Chemical Engineering Material Iron-Carbon-DiagramsDocument12 pagesChemical Engineering Material Iron-Carbon-Diagramsaditya_lomtePas encore d'évaluation
- Full Annealing Full Annealing Is The Process of Slowly Raising The Temperature About 50Document10 pagesFull Annealing Full Annealing Is The Process of Slowly Raising The Temperature About 50scorpionarnoldPas encore d'évaluation
- Heat TreatmentDocument8 pagesHeat TreatmentBaizura Mohd ZanPas encore d'évaluation
- Heat TreatmentDocument34 pagesHeat Treatmentrahul72Pas encore d'évaluation
- NormalizingDocument4 pagesNormalizingAbd.ul.RahmanPas encore d'évaluation
- Heat TreatmentDocument33 pagesHeat TreatmentIsrael HailuPas encore d'évaluation
- Engineering Metallurgy: Misan University-College of EngineeringDocument26 pagesEngineering Metallurgy: Misan University-College of Engineeringbone manPas encore d'évaluation
- Heat Treatment of SteelDocument59 pagesHeat Treatment of SteelNaman ShethPas encore d'évaluation
- TempleDocument17 pagesTemplesan moedanoPas encore d'évaluation
- Heat TreatmentDocument9 pagesHeat TreatmentsvsddsdsPas encore d'évaluation
- Lab Sheet 2Document4 pagesLab Sheet 2Christ LeePas encore d'évaluation
- 1 - Heat TreatmentDocument61 pages1 - Heat TreatmentMohamed Karim MohamedPas encore d'évaluation
- Carburization of SteelDocument9 pagesCarburization of SteelRtr Ahmed Abidemi CertifiedPas encore d'évaluation
- Applying Computer Simulation in Improving Heat Treating Condition of Thin High-Carbon Steel PartsDocument5 pagesApplying Computer Simulation in Improving Heat Treating Condition of Thin High-Carbon Steel PartssathishelakkiyaPas encore d'évaluation
- Heat Treatment Study On Carbon SteelDocument6 pagesHeat Treatment Study On Carbon SteelramaPas encore d'évaluation
- U1. T2. Activity 1. Heat Treatments of SteelDocument11 pagesU1. T2. Activity 1. Heat Treatments of SteelTygaPas encore d'évaluation
- Theoretical Part: Chapter-1Document10 pagesTheoretical Part: Chapter-1hayder1920Pas encore d'évaluation
- Heat Treatment Steel: ObjectDocument10 pagesHeat Treatment Steel: ObjectKetut Rizki FirmandanuPas encore d'évaluation
- Introduction To Heat TreatmentDocument10 pagesIntroduction To Heat TreatmentAzhar AliPas encore d'évaluation
- Heat Treatment ProcessDocument46 pagesHeat Treatment ProcessMallappa KomarPas encore d'évaluation
- Heat TreatmentDocument4 pagesHeat TreatmentAshish BoraPas encore d'évaluation
- Heat Treatment: ME 318 Manufacturing TechniquesDocument12 pagesHeat Treatment: ME 318 Manufacturing Techniquesmayur_mechPas encore d'évaluation
- 3 Eng Stainless Steel Tube AnnealingDocument16 pages3 Eng Stainless Steel Tube AnnealingVictor HugoPas encore d'évaluation
- Literature ReviewDocument23 pagesLiterature ReviewRISHAVPas encore d'évaluation
- Heat Treatment of SteelDocument26 pagesHeat Treatment of SteelVishal KumarPas encore d'évaluation
- Document 7Document6 pagesDocument 7Roy mugendi100% (1)
- Heat TreatmentDocument44 pagesHeat TreatmentMastram HatheshPas encore d'évaluation
- Carbon Steel NormalizingDocument2 pagesCarbon Steel NormalizingAnil S ChaudharyPas encore d'évaluation
- Iron Carbon DiagramDocument9 pagesIron Carbon DiagramNagamuthu PandianPas encore d'évaluation
- 4 - Heat TreatmentDocument29 pages4 - Heat TreatmentNomor SatuPas encore d'évaluation
- Annealing, Normalizing, Quenching, Martensitic TransformationDocument22 pagesAnnealing, Normalizing, Quenching, Martensitic TransformationAboo BackerPas encore d'évaluation
- Heat Treatment of SteelDocument7 pagesHeat Treatment of SteelmaadPas encore d'évaluation
- Course No: Experiment No:: Structural Study of Mild Steel After Heat TreatmentDocument13 pagesCourse No: Experiment No:: Structural Study of Mild Steel After Heat TreatmentRifat KhanPas encore d'évaluation
- Chapter 8 - Heat TreatmentDocument20 pagesChapter 8 - Heat TreatmentISAPas encore d'évaluation
- Steel - Macaraeg, Edward Joseph M.Document31 pagesSteel - Macaraeg, Edward Joseph M.Mark Lester LualhatiPas encore d'évaluation
- Heat TreatmentDocument40 pagesHeat TreatmentFavour LawrencePas encore d'évaluation
- Theory of Heat TreatmentDocument8 pagesTheory of Heat Treatmentayie740% (1)
- Normalization and Temper Heat Treatment On P91Document6 pagesNormalization and Temper Heat Treatment On P91Asad Bin Ala Qatari100% (2)
- Development of Bake-Hardenable Al-Killed Steel Sheet by Box Annealing ProcessDocument10 pagesDevelopment of Bake-Hardenable Al-Killed Steel Sheet by Box Annealing ProcessRamírez WillebaldoPas encore d'évaluation
- Heat Treatment Lab ReportDocument7 pagesHeat Treatment Lab Reportmuvhulawa bologo100% (1)
- Heat Treatment Part 2Document46 pagesHeat Treatment Part 2Naman DavePas encore d'évaluation
- The Working of Steel: Annealing, Heat Treating and Hardening of Carbon and Alloy SteelD'EverandThe Working of Steel: Annealing, Heat Treating and Hardening of Carbon and Alloy SteelPas encore d'évaluation
- Matlab TutorialDocument173 pagesMatlab TutorialSaurabh Tiwari100% (8)
- UCR ME SOP Manual Milling Machines v5Document12 pagesUCR ME SOP Manual Milling Machines v5Tareef HashPas encore d'évaluation
- Alnahhal Mohammed - PHD ThesisDocument192 pagesAlnahhal Mohammed - PHD ThesisTareef Hash100% (1)
- Thermodynamics II - Chapter 11 - V 1.1Document29 pagesThermodynamics II - Chapter 11 - V 1.1Tareef HashPas encore d'évaluation
- Knurling Grooving and Form TurningDocument33 pagesKnurling Grooving and Form TurningTareef Hash100% (1)
- Turning&Lathe BasicsDocument9 pagesTurning&Lathe BasicssanthoshjoysPas encore d'évaluation
- Type BOQ For Construction of 4 Units Toilet Drawing No.04Document6 pagesType BOQ For Construction of 4 Units Toilet Drawing No.04Yashika Bhathiya JayasinghePas encore d'évaluation
- For Email Daily Thermetrics TSTC Product BrochureDocument5 pagesFor Email Daily Thermetrics TSTC Product BrochureIlkuPas encore d'évaluation
- SVPWM PDFDocument5 pagesSVPWM PDFmauricetappaPas encore d'évaluation
- Media SchedulingDocument4 pagesMedia SchedulingShreyansh PriyamPas encore d'évaluation
- QP 4Document4 pagesQP 4Yusra RaoufPas encore d'évaluation
- Catalogue of The Herbert Allen Collection of English PorcelainDocument298 pagesCatalogue of The Herbert Allen Collection of English PorcelainPuiu Vasile ChiojdoiuPas encore d'évaluation
- VRIODocument3 pagesVRIOJane Apple BulanadiPas encore d'évaluation
- Best Practices in Developing High PotentialsDocument9 pagesBest Practices in Developing High PotentialsSuresh ShetyePas encore d'évaluation
- Annotated Portfolio - Wired EyeDocument26 pagesAnnotated Portfolio - Wired Eyeanu1905Pas encore d'évaluation
- For Exam ReviewerDocument5 pagesFor Exam ReviewerGelyn Cruz67% (3)
- Immovable Sale-Purchase (Land) ContractDocument6 pagesImmovable Sale-Purchase (Land) ContractMeta GoPas encore d'évaluation
- T R I P T I C K E T: CTRL No: Date: Vehicle/s EquipmentDocument1 pageT R I P T I C K E T: CTRL No: Date: Vehicle/s EquipmentJapCon HRPas encore d'évaluation
- Bench VortexDocument3 pagesBench VortexRio FebriantoPas encore d'évaluation
- Prepositions Below by in On To of Above at Between From/toDocument2 pagesPrepositions Below by in On To of Above at Between From/toVille VianPas encore d'évaluation
- Catalog enDocument292 pagesCatalog enSella KumarPas encore d'évaluation
- Journalism Cover Letter TemplateDocument6 pagesJournalism Cover Letter Templateafaydebwo100% (2)
- Sappi Mccoy 75 Selections From The AIGA ArchivesDocument105 pagesSappi Mccoy 75 Selections From The AIGA ArchivesSappiETCPas encore d'évaluation
- WPGPipingIndex Form 167 PDFDocument201 pagesWPGPipingIndex Form 167 PDFRaj AryanPas encore d'évaluation
- Certification DSWD Educational AssistanceDocument3 pagesCertification DSWD Educational AssistancePatoc Stand Alone Senior High School (Region VIII - Leyte)Pas encore d'évaluation
- Well Stimulation TechniquesDocument165 pagesWell Stimulation TechniquesRafael MorenoPas encore d'évaluation
- Chapter 123 RevisedDocument23 pagesChapter 123 RevisedCristy Ann BallanPas encore d'évaluation
- DevelopmentPermission Handbook T&CPDocument43 pagesDevelopmentPermission Handbook T&CPShanmukha KattaPas encore d'évaluation
- Black BookDocument28 pagesBlack Bookshubham50% (2)
- Relay Interface ModulesDocument2 pagesRelay Interface Modulesmahdi aghamohamadiPas encore d'évaluation
- ADS Chapter 303 Grants and Cooperative Agreements Non USDocument81 pagesADS Chapter 303 Grants and Cooperative Agreements Non USMartin JcPas encore d'évaluation
- Barangay Tanods and The Barangay Peace and OrderDocument25 pagesBarangay Tanods and The Barangay Peace and OrderKarla Mir74% (42)
- Marshall Baillieu: Ian Marshall Baillieu (Born 6 June 1937) Is A Former AustralianDocument3 pagesMarshall Baillieu: Ian Marshall Baillieu (Born 6 June 1937) Is A Former AustralianValenVidelaPas encore d'évaluation
- CoP - 6.0 - Emergency Management RequirementsDocument25 pagesCoP - 6.0 - Emergency Management RequirementsAnonymous y1pIqcPas encore d'évaluation
- Minas-A6 Manu e PDFDocument560 pagesMinas-A6 Manu e PDFJecson OliveiraPas encore d'évaluation
- R 18 Model B Installation of TC Auxiliary Lights and WingletsDocument29 pagesR 18 Model B Installation of TC Auxiliary Lights and WingletsAlejandro RodríguezPas encore d'évaluation