Académique Documents
Professionnel Documents
Culture Documents
14361332
Transféré par
Houcine BendaoudDescription originale:
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
14361332
Transféré par
Houcine BendaoudDroits d'auteur :
Formats disponibles
Analytica Chimica Acta 479 (2003) 173184
Optimisation of a ow injection system with electrochemical detection using the desirability function Application to the determination of hydroquinone in cosmetics
M.E. Rueda a , L.A. Sarabia b, , A. Herrero a , M.C. Ortiz a
b a Department of Chemistry, Faculty of Sciences, University of Burgos, Plaza Misael Bauelos s/n, Burgos 09001, Spain Department of Mathematics and Computation, Faculty of Sciences, University of Burgos, Plaza Misael Bauelos s/n, Burgos 09001, Spain
Received 27 March 2002; received in revised form 28 November 2002; accepted 29 November 2002
Abstract A procedure is proposed in which the determination of hydroquinone using a ow injection system with electrochemical detection is described. Size and coefcient of variation of the signal are optimised by a desirability function and a central composite design. The robustness of the optimum reached in the optimisation step is evaluated by means a PlackettBurman design. The optimised FIA system is able to determine hydroquinone with a minimum detectable net concentration of 10 g l1 with a false positive probability of 0.05 and a false negative probability less than 0.05. In samples of bleaching cream, the proposed procedure has a recovery of 102.2% with standard deviation of 4.4% and a relative error of 6.2%. 2002 Elsevier Science B.V. All rights reserved.
Keywords: FIA; Electrochemical detection; Optimisation; Experimental design; Canonical analysis; Desirability functions; Robustness; Hydroquinone; Bleaching cream
1. Introduction In routine analysis ow injection systems are interesting because of their multiple advantages, that include the fast response, low-cost instrumentation, reproducible and accurate results, in addition to the large number of samples which can be analysed per unit of time. The combination of this technique with the electrochemical detection signicantly increases the advantages offered by these systems which are at the same time highly selective and sensitive, therefore low detection limits can be achieved.
Corresponding author. Fax: +34-947258831. E-mail address: lsarabia@ubu.es (L.A. Sarabia).
Several attempts have been made to theoretically establish the functional relation existing between the prole of a signal recorded in FIA and the experimental parameters which affect the system [1]. The theoretical study of the behaviour of a sample injected in a ow is difcult to predict, since it is not easy to foresee the contribution to the dispersion of the elements which make up the system. Most theoretical models have certain restrictions which make it unviable to be applied to ordinary FIA systems. Most of the processes studied in the laboratory are the result of the inuence and interaction of several experimental factors. Traditionally the study of the inuence of these factors is done by modifying one factor at a time. This univariate analysis means carrying out a large number of experiments, but it does not
0003-2670/03/$ see front matter 2002 Elsevier Science B.V. All rights reserved. doi:10.1016/S0003-2670(02)01542-8
174
M.E. Rueda et al. / Analytica Chimica Acta 479 (2003) 173184
allow the statistical interpretation of results and does not detect the interactions between factors, which can often lead to a mistaken interpretation of results. Experimental design methodology makes a joint study of the relation between a number of factors or experimental variables and a specic response when the mathematical model which describes the phenomenon is unknown. The designed experiments [2] allow one, through the controlled modication of the experimental variables, to study the effect of a variation of the levels of experimental variables on the response. The knowledge of the problem, the adequate selection of variables and the establishment of the objectives and aims of the analysis are essential if one is to perform an efcient experiment [3], in other words, to select a good design. The main aim of optimisation is to nd the experimental conditions which give the best response. Response surface methodology [46] is an area of the experimental design that deals with the optimisation and modelling of a system. Sometimes the response optimisation let us to study the sensitivity of the optimum to small changes in the experimental variables. This methodology was introduced by Box and Wilson [7] at the beginning of the 1950s. It is increasingly common to nd examples in the bibliography of the application of experimental design methodology in the optimisation of ow injection systems. The simplex method and its variants are the most popular for optimisation [8,9] preceded at times by the prior selection of the most signicant factors. The kind and number of factors studied, and the choice of response is different in each particular case depending on the nal objective of the analysis. Several authors [1012] have studied the effect of FIA system parameters as carrier ow rate, sample volume and reactor length, etc. The study of chemical factors such as the composition of the carrier solution, pH, concentration of reagents, is also usual [13,14], also together with the parameters of FIA system [8]. The response selection is a critical stage in the optimisation. It is common to optimise parameters such as the peak height or area, although other authors, such as Janse et al. [15] use criteria as the signal/noise ratio, the correlation coefcient or other more complex functions [16] which indirectly evaluate the sensitivity, precision, sample throughput or cost in a ow injection system. Also the Pareto optimality concept
was applied to make a compromise between residence time and the peak height [12] or a linear combination of them [14]. The hydroquinone has a simple and well-known oxidation mechanism and is commonly used in electrochemistry as a test analyte to validate new electroanalytical methods. Hydroquinone is a phenolic compound which is important in a wide number of biological and industrial processes (coal-tar production, paper manufacturing or in the developing process in photography), and in the aquatic environment, it is considered an important xenobiotic micropollutant. Thus, hydroquinone and its reaction products have been determined by several analytical techniques in cosmetics, hair products and pharmaceutical preparations, in air samples and in different biological uids to determine its possible carcinogenic effect. The determination and quantication of this analyte may be done using different techniques, such as HPLC [17,18] with different detectors, ow injection analysis [19], kinetic spectrophotometry [20,21], GC/MS chromatography [22,23], differential pulse voltammetry [24], based on the BelousovZhabotinski oscillating chemical reaction [25,26] or with biosensors [27] amongst others. The detection limits found in the literature for hydroquinone vary in a wide range of concentrations depending on the technique used, although the lowest limits are achieved with electrochemical detection systems. Fang et al. [28] describe a system of analysis by ow injection with amperometric detection that reaches a detection limit of 22 g l1 for hydroquinone. This limit descends to 0.05 g l1 with capillary electrophoresis or liquid chromatography with electrochemical detection, although in none of the cases is the probability of false negative evaluated, as required in the ISO 11843 [29] and by the IUPAC [30]. This work describes the optimisation of a ow injection system with electrochemical detection for the determination of hydroquinone. In general, the optimisation of an analytical signal implies solving a conict between signal size and its precision since normally by modifying the experimental parameters in order to get a greater signal, its variance is also increased. This is the key problem for the quality of the measurement of an analytical signal. This problem, the joint optimisation of contradictory responses, has been given a great deal of attention as the key to the quality of
M.E. Rueda et al. / Analytica Chimica Acta 479 (2003) 173184
175
a product, process or service. For these tasks, there are basically two approaches to simultaneously optimise several responses: to optimise the signalnoise Taguchi function or the desirability function. In the case of chemical analysis, the analyst has additional information about the desired values in which the variability of the signal must be maintained. In order to explicitly use this information, this paper proposes the optimisation of an overall desirability function of the size and the coefcient of variation (CV) of the signal. Using the response surface methodology and a central composite design, the values of three experimental factors (ow rate, conditioning potential and analytical cell potential) which optimise the desirability has been determined.
2. Experimental 2.1. FIA manifold All the measurements were carried out using the ow injection system with electrochemical detection shown in Fig. 1. This is basically made up of a solvents kit with helium sparge which allows the elimination from the system of gases dissolved in the carrier solution which could react and cause noise and/or drifting in the base line. It also has an isocratic Perkin-Elmer
series 200 LC pump, a Rheodyne syringe loading sample injector model 7725i with a 20 l loop and an ESA Coulochem II 5200A electrochemical detector with a 5021 conditioning cell and a 5011 high analytical cell. The conditioning cell function is to eliminate from the system any possible interference, improving the selectivity of the analysis. The analytical cell consists of two working porous graphite electrodes with high electrolytic efciency. Both electrodes have a very small hydrogen/palladium reference electrode that is placed in close proximity to the working and counter electrode. Other details about this electrochemical system can be seen in [31]. The rst working electrode of the analytical cell was used in amperometric mode, that is, at xed potential. The second electrode has not been used in the analysis. A Crison micro-pH-2002 pH-meter was used for pH measurements, and a vibrating shaker ZX3 from Velp Cientica for the real sample stirring. The total length of tubes were 150 cm with 0.5 mm inside diameter. The cell potentials were changed in the optimisation step. 2.2. Reagents The hydroquinone used was of analytical reagent grade (minimum 99%, Merck, Darmstadt, Germany). The stock solutions (100 mg l1 ) were prepared beforehand by dissolving 10 mg of hydroquinone in
Fig. 1. Scheme of the ow injection system with electrochemical detection used for the determination of hydroquinone.
176
M.E. Rueda et al. / Analytica Chimica Acta 479 (2003) 173184
0.1 l of gradient grade for liquid chromatography methanol (minimum 99.9%, Merck, Darmstadt, Germany). The sample solutions were obtained by diluting the stock solutions with the carrier solution that was prepared by mixing acetic/acetate buffer and methanol in the proportion 85:15. The acetic/acetate buffer (pH = 4.8) was prepared by mixing 41.5 ml of PA grade acetic acid 2 M (minimum 99.8%, PanReac, Barcelona, Spain) and 58.5 ml of a PA sodium acetate 2 M (minimum 99%, Merck, Darmstadt, Germany) diluted to 1000 ml with water. The water used in all cases was puried with a Milli-Q water purication system (Millipore). The determination of hydroquinone in cosmetics was performed on the bleaching cream Licostrat gel with a nominal concentration of hydroquinone of 2%, made by Industrial Farmacutica Cantabria SA, Spain. 2.3. Experimental procedure First the carrier solution was degassed by sparging with helium for 10 min, then the ow rate and different parameters relative to the electrochemical detection at constant potential (conditioning cell potential, analytical cell potential, sensitivity and signal output) were selected. Afterwards, the electrode equilibration was allowed for a few minutes and the stability of the base line evaluated. Then the sample (20 l) was injected and the peaks in the obtained agram were quantied. The experimental procedure used for the analysis of bleaching cream was that described by Wang [32]. Fifty milligrams of sample was accurately weighed into a glass centrifuge tube, 10 ml of methanol was added and the tube was heated at 40 C in a water-bath with stirring until sample dissolution was complete. After cooling and centrifugation, the supernatant was transferred into a 10 ml calibrated ask and made up to volume with methanol. Because of the high concentration of hydroquinone present in the cream the solution had to be diluted. This was done by taking 100 l of the previous solution and making it up to a volume of 25 ml with the carrier solution prepared for the analysis. Finally, 20 l of the solution were injected into the FIA system and the obtained agram was quantied. The adjustment and optimisation of the response surfaces for the desirability function were done with NemrodW et al. [33]. Progress [34] and Statgraphics
[35] were used for the statistical analysis of the calibrations and Detarchi [36] to calculate the minimum detectable net concentration. 3. Results and discussion 3.1. Optimisation In the optimisation step, a central composite design with seven replicates at the central point was used to determine the effect of three experimental factors on two responses. The selection of the extreme levels of the factors in the experimental design was done by previous experiments, guidance given by the manufacturers and by the technical limitations of the system. The studied experimental factors were: (i) The carrier solution ow rate ( ), an hydrodynamic factor which inuences the dispersion of the sample injected. (ii) The conditioning cell potential (Ea ), which inuences the selectivity of the analysis and whose function is to eliminate, by selection of an adequate oxidation or reduction potential, the different species which can be found dissolved in the sample and interfere. (iii) The working electrode potential (EL1 ) of the analytical cell, which enables improvement in the sensitivity of the analysis. The aim of the analysis was to maximise the peak height, estimated for the average value of n signals (ci ) obtained under the same experimental conditions, and to minimise the relative variability estimated for the coefcient of variation of these signals. This is usual in chemical analysis in order to increasing the sensitivity and reducing the variability, i.e. to reach better detection limits and more precise determinations. A constant amount of hydroquinone solution (0.1 mg l1 ) was injected ve times in each of the experimental conditions of the central composite design shown in Table 1. The design is a central composite one with axial point spacing = 2 and Nc = 7 centre points, that has a D-efciency, variance ination factors and maximum of variance function comparable or superior to that of an standard central composite for three factors ( = 1.68 and Nc = 6). The design is very near rotatable (its Khuri index is 97.31%).
M.E. Rueda et al. / Analytica Chimica Acta 479 (2003) 173184 Table 1 Design matrix, experimental matrix and experimental responses of the experimental design Run Design matrix (codied variables) x1 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 1 1 1 1 1 1 1 1 2 2 0 0 0 0 0 0 0 0 0 0 0 x2 1 1 1 1 1 1 1 1 0 0 2 2 0 0 0 0 0 0 0 0 0 x3 1 1 1 1 1 1 1 1 0 0 0 0 2 2 0 0 0 0 0 0 0 Experimental matrix (real variables) u1 = 0.5 1 0.5 1 0.5 1 0.5 1 0.25 1.25 0.75 0.75 0.75 0.75 0.75 0.75 0.75 0.75 0.75 0.75 0.75 (ml min1 ) u2 = Ea (mV) 150 150 50 50 150 150 50 50 100 100 200 0 100 100 100 100 100 100 100 100 100 u3 = EL1 (mV) 50 50 50 50 300 300 300 300 175 175 175 175 75 425 175 175 175 175 175 175 175 Experimental responses Peak height (A) 1.39 1.71 1.28 1.73 1.37 1.97 1.20 1.91 0.84 1.81 1.64 1.40 1.36 1.69 1.53 1.48 1.48 1.65 1.51 1.39 1.43
177
CV% 1.95 0.52 1.76 0.58 2.24 0.43 0.79 0.63 3.07 2.06 0.69 0.34 2.05 0.58 0.60 0.70 0.56 0.30 1.09 1.35 0.52
The peak height shows the mean height of ve replicates.
The use of additional centre runs bring stability in variance function and produces important pure error degrees of freedom. 3.1.1. Determination of the maximum for the peak height In the rst step, a complete second-order model has been tted to the experimental data obtained for the peak height. An exhaustive analysis of the variance (ANOVA) allows one to accept that the tted surface model adequately describes the peak height in the experimental domain, is highly signicant and has not lack of t at a signicance level of 5% (R 2 = 0.93). The coefcients of the tted model are shown in Table 2, the more signicant being b1 and b3 , and to a lesser extent b2 and b13 (both P-values are close to 0.05). On the other hand, the critical point of the tted second-order surface is 0.00, 0.39 and 3.61 for ow rate, conditioning cell potential and working electrode potential, respectively, which is outside the experimental domain since its distance from the centre of the domain is 3.64, >2 which is that anal-
ysed. In addition, analysis of the Hessian at this point shows that it is a saddle point ([5,6] show a detailed characterisation of stationary points). Consequently, it is necessary to determine the optimum path of the response surface by tracing spherical surfaces, centred on the central point of the domain and with growing radius, and calculating on each of
Table 2 Estimates and statistics of the coefcients from the tted response surface for peak height Coefcient b0 b1 : b2 : E a b3 : EL1 b11 b22 b33 b12 b13 b23 1.51 0.25 0.05 0.06 0.03 0.02 0.02 0.03 0.07 0.02 Standard deviation 0.04 0.02 0.02 0.02 0.02 0.02 0.02 0.03 0.03 0.03 texp 43.23 10.65 2.08 2.70 1.76 0.89 0.96 0.95 1.98 0.51 P-value <104 <104 0.06 0.02 0.10 0.40 0.36 0.37 0.07 0.63
Coefcients are written in codied variables.
178
M.E. Rueda et al. / Analytica Chimica Acta 479 (2003) 173184 Table 3 Estimates and statistics of the coefcients from the tted response surface for CV% Coefcient Standard deviation 0.15 0.10 0.10 0.10 0.08 0.08 0.08 0.15 0.15 0.15 texp P-value
b0 b1 : b2 : E a b3 : EL1 b11 b22 b33 b12 b13 b23
0.71 0.41 0.13 0.23 0.44 0.07 0.13 0.24 0.08 0.14
4.57 3.96 1.25 2.20 5.44 0.87 1.59 1.61 0.54 0.95
8.6 104 2.3 103 0.236 0.048 2.4 104 0.406 0.136 0.132 0.603 0.365
Coefcients are written in codied variables.
tive to variations in ow rate and working electrode potential than to changes of the conditioning cell potential. To reach the maximum, the rst two factors must have higher values while the conditioning potential must tend towards less positive values. For small values, close to the minimum peak height, only the ow rate is inuential while the analytical potential loses importance. 3.1.2. Determination of the minimum for the CV% The same analysis has been carried out in this case, the quadratic model tted being also adequate to describe the variability of the CV% in the experimental domain (R 2 = 0.85). As can be seen in Table 3, that shows the coefcients of the tted model, at a level of signicance of 0.05, the coefcient b1 for the ow rate, its square b11 and the coefcient b3 for the working electrode potential, are signicant. The analysis of these coefcients does not give an indication of how to reach the minimum. The only critical point of the tted surface has coordinates 0.55, 0.46 and 0.47 and is at a distance of 0.73 from the centre, within the experimental domain, but it is a saddle point. Again the optimum, in this case the minimum of the CV%, is to be found on the boundary of the experimental domain. The study of the optimum path of the response surface is shown in Fig. 3a. The minimum transformed into real variables is reached (Fig. 3b), for 0.75 ml min1 , 12.5 and 275 mV for ow rate, conditioning potential and analytical potential, respectively. It is highly sensitive to the variation of
Fig. 2. (a) Optimum path of the response surface tted to the peak height where ordinates represent the response reached on the built spheres for radius R indicated in abscissas; (b) coordinates of the points of plot (a) for each factor in codied variables. The right part of both plots refers to the maximisation of the response; the left part of the plots refers to the minimisation of the response.
these the maximum and minimum of the response surface [5,6]. It is clear that in the optimum path of the surface response shown in Fig. 2a, the maximum peak height, 2.05 A, is reached at the boundary of the experimental domain, at distance 2. The coordinates for the maximum, Fig. 2b, transformed into real variables correspond to a ow rate, a conditioning potential and a working electrode potential of 1.11 ml min1 , 110 and 337 mV, respectively. In addition, this gure shows that close to the maximum, or to distances close to 2 from the centre of the experimental domain, the peak height is more sensi-
M.E. Rueda et al. / Analytica Chimica Acta 479 (2003) 173184
179
same direction, towards more positive values, but in a greater quantity to reach the maximum peak height, 337 mV, than to reach the minimum variation coefcient, 275 mV. It is therefore necessary to reach a compromise solution between the two responses. 3.1.3. Multi-response analysis: desirability function When several responses are evaluated in an experimental design it is unlikely that the optimum points reached individually for each factor coincide in all the cases. Faced with this situation, it is necessary to look for a compromise zone where all the experimental responses full the specications imposed by the researcher to achieve the aims proposed. In our case, the objective is to achieve a maximum mean peak height (maximum sensitivity) and minimum CV%, in other words, greater relative precision. This problem, known in the literature as multi-objective optimisation can be approached with the preference-based procedure thought up by Harrington [37] in 1965 and Derringer and Suich [38] in 1980. Based on the expertise information, a desirability function [3,39,40] is constructed for each response, c and CV%. This individual desirability function is a continuous function chosen from among a family of linear or exponential functions (see [5,6] for more details) and varies from zero, undesirable response, to 100, the response is optimal. Based on these individual functions the overall desirability function is constructed, which will be optimised. The overall desirability function D is dened as the weighted geometric average of n individual desirability functions. D=
n
Fig. 3. (a) Representation of the optimum path of the response surface tted for the CV%; (b) coordinates of the points of plot (a) for each factor in codied variables. The right part of both plots refers to the maximisation of the response; the left part of the plots refers to the minimisation of the response.
the conditioning potential, less so to the change in the analytical potential and practically insensitive to variations in the ow rate. With regard to the optimisation of the maximum peak height, it is clear that the experimental conditions are contradictory: the ow must be high, 1.11 ml min1 , to obtain a greater peak height but it must be maintained around the central point of the design, 0.75 ml min1 to obtain a minimum CV%. The opposite happens with the conditioning potential, which must take a value close to the central value 110 mV to maximise the height, but must be 12.5 mV, a value close to the maximum of the design, in order to minimise the variation coefcient. The working electrode potential, must move in the
d1 1 d 2 2 d n n
(1)
where pi is the weighting of the i-th response normalised so that n i =1 pi /n = 1. Through the individual functions the analyst introduces the specications that each response must full and through the weighting the relative importance given to each of them. Furthermore, the overall desirability function is not derivable, so the optimisation process must use a method which is free of derivatives, such as the simplex method, a genetic algorithm or a simulated annealing algorithm which is that used in NemrodW et al. [33]. In this work, the linear desirability functions, dened by the following specications, were chosen:
180
M.E. Rueda et al. / Analytica Chimica Acta 479 (2003) 173184
(i) In the case of peak height, c , values below 1.5 A are not acceptable (zero desirability), while values above 1.8 A are optimal (desirability 100). Between these two values the desirability function varies linearly. (ii) The CV% must not take values above 2.5%, above which the desirability is equal to 0 while values below 1.5% are optimal (desirability 100). As for the peak height the function varies linearly between these two values. In addition, for the overall desirability function D, equal weights were given, since both responses are considered equally important, that is p1 = p2 = 1 in Eq. (1). The overall desirability function gives an optimum of coordinates 1.25, 0.32 and 0.73 in codied variables, that is, 1.06 ml min1 , 116 and 266 mV in real variables. At this point, the peak height is equal to 1.9 A and the CV% equal to 0.8%. This solution fulls all the restrictions imposed to construct the individual functions and to reach a global and individual desirability of 100. On the other hand, the analysis of the sensitivity of this maximum to variations in the experimental conditions cannot be done by means of the optimum path or similar techniques such as analysis of the curvature of D around the maximum because function D is not derivable. This means that one has to vary each coordinate of the optimum and calculate the global desirability. When this variation is in a radius of 0.1, in codied variables, values of D = 100 were obtained at the points corresponding to a complete factorial design centred around the optimum. This points out that the maximum is insensitive to small variations in the studied experimental variables. The graphic study of the variability of the global desirability function is in Fig. 4, that corresponds to the three-dimensional representations of the global function in the space of the different variables. Although these representations are only partial, since in each case one of the three factors must remain constant, they conrm that in the region around the optimum only valid results (i.e. D = 100) are obtained, that is, it is not very sensitive to small accidental changes in the analysed factors. Table 4 shows the numerical results and clearly points out the conict between the
Fig. 4. Three-dimensional representations of the global desirability function in the space of the variables vs. Ea (plot a), vs. EL1 (plot b) and Ea vs. EL1 (plot c). In each case, the variable not represented remains constant with the value corresponding to the optimum of the global function, i.e. EL1 = 266 mV in the rst case, Ea = 116 mV in the second case and = 1.06 ml min1 in the third case.
M.E. Rueda et al. / Analytica Chimica Acta 479 (2003) 173184 Table 4 Numerical results of the optimisation process carried out taking into account different responses Optimised response Flow rate (ml min1 ) Ea (mV) EL1 (mV) Estimated values c Peak height c (A) CV% Global desirability 1.11 0.75 1.06 110.0 12.5 116.0 337 275 266 2.05 1.51 1.89 CV% 1.09 0.00 0.81 D
181
100 15 100
two responses and the usefulness of the desirability function. 3.2. Study of the robustness of the optimum of the global desirability function The robustness of an analytical method is dened [41] as its capacity to remain unchanged when there are small variations in experimental conditions, variations similar to those which could occur when the method is applied routinely in the laboratory. On the other hand, in [42] the robustness of an analytical method is dened as a measure of its capability to reproduce a series of results when the procedure is repeated under different circumstances. This second denition is more general and studies both the behaviour of the system under conditions of reproducibility (ruggedness) and its response to small modications in the experimental variables with respect to a nominal value (robustness). In order to evaluate the robustness of the experimental optimum obtained in the optimisation step, a PlackettBurman design was performed for seven factors and eight experiments [6]; the variation of the parameters, with respect to their nominal level, being 5%. The factors selected were the three factors evaluated in the optimisation step (their nominal value
xed at the global desirability function optimum) and others which remained constant during that step, such as percentage of organic solvent in the carrier solution, pH of the buffer and concentration of hydroquinone (their nominal values set at the values xed in the optimisation step, i.e. 15% of methanol, 4.8 and 0.1 mg l1 , respectively). The seventh variable was the moment in which the measurement was taken: in the morning or in the evening. Table 5 shows the levels of the different factors and the three replicates made in the nominal level of the factors to estimate the standard deviation. In each experimental point of the design ve injections were made. Fig. 5a shows the Pareto chart corresponding to the robustness analysis for the peak height where concentration of hydroquinone and pH were signicant at a condence level of 95%. In the case of the coefcient of variation (Fig. 5b), only hydroquinone concentration was signicant. An increase in hydroquinone concentration from 0.095 to 0.105 mg l1 causes an increase in peak height and a reduction of the CV%, statistically signicant in the two models. In other words, in the experimental conditions of the optimum, the analytical model is sufciently sensitive to detect a concentration change of 5 g l1 . Furthermore, for the peak height, the change from the low to the high level of pH causes a reduction in the peak height.
Table 5 Factor levels for the seven factor eight experiences PlackettBurman design for robustness study Factor A: working electrode potential (EL1 ) (mV) B: conditioning cell potential (Ea ) (mV) C: ow rate ( ) (ml min1 ) D: methanol percentage E: pH F: hydroquinone concentration (mg l1 ) G: moment High level (+) 279 110 1.11 15.75 5.04 0.105 Morning Nominal level (0) 266 116 1.06 15.00 4.8 0.100 Low level () 253 122 1.01 14.25 4.56 0.095 Evening
182
M.E. Rueda et al. / Analytica Chimica Acta 479 (2003) 173184
determines the presence or absence of the analyte by evaluating the false negative and false positive error. Thus, the peak height of nine standards (from 0.01 to 0.1 mg l1 ) was measured in the optimum conditions found in Section 3.1.3 and ve times replicated. The robust regression detected as outliers the ve replicates of 0.1 mg l1 , which were eliminated. The validated LS model tted with the rest of the data (n = 40) was Y = 0.07 + 1.73X (standard deviation of the regression, sYX = 6.0 103 ; and R 2 = 0.99). With this calibration, the detection limit obtained was 6.6 g l1 with a probability of false positive and false negative equal to 0.05, considering the mean of ve replicates as future sample. At the level of condence 0.95 the interval for the detection limit varies from 5.4 to 8.6 g l1 . The detection limit computed is below the lowest calibration standard, so the rst standard recorded, 10 g l1 , should be taken as detection limit at = 0.05 and < 0.05. 3.4. Determination of hydroquinone in cosmetics (bleaching cream) Flow injection analysis with electrochemical detection was used to determine hydroquinone in bleaching cream in the optimum experimental conditions determined in Section 3.1.3. To evaluate the viability of this determination, eight standards of hydroquinone (from 0.1 to 0.8 mg l1 ), three samples of bleaching cream and three spiked samples of cream, to which a known quantity of hydroquinone (0.4 mg l1 ) had been added to calculate the percentage of recovery, were measured. Every sample was injected ve times. The LMS regression detected as outliers the ve replicates of the standard of concentration 0.2 mg l1 , so that in the analysis the remaining 35 standard samples were used for calibration. The tted model is Y = 0.21 + 4.10X , with a standard residual deviation equal to sYX = 0.02 and a determination coefcient of 0.99. The model veries the hypotheses of homoscedasticity and normality of residuals, but the DurbinWatson independence test gives a value of the statistic 1.70, somewhat higher than the critical value 1.4 at the level 0.05 but which is acceptable. On the other hand, the calculation of the percentage of recovery was done by determining the concentration calculated for the six test samples. Thus, an average value of the concentration added is calculated, equal to
Fig. 5. Pareto chart of standardised effects for (a) peak height and (b) CV%. The threshold value at 0.95 condence level is signed by the vertical line.
3.3. Determination of the minimum detectable net concentration of hydroquinone according to IUPAC and ISO norm 11843 The minimum detectable net concentration expressed as a concentration xd or a quantity qd , is obtained from the lowest measurement yd which can be distinguished from the blank signal with a xed probability of false positive and false negative [29,30]. The procedure to carry out its estimate consisted [36,43] of rst calculating a univariate lineal regression model, detecting outliers by using the least median of squares (LMS) regression [34]; eliminating them and estimating the nal univariate model with the rest of the data by means of the least squares (LS) regression; and next evaluating the capability of detection by means of a NeymanPearson test, which
M.E. Rueda et al. / Analytica Chimica Acta 479 (2003) 173184
183
0.409 mg l1 with a standard deviation of 0.016 mg l1 which is equivalent to an average recovery percentage of 102.2% with a standard deviation of 4.4%, so signicantly equal to 100%. Consequently, the method is selective and there is no interference caused by the medium in which the hydroquinone is found. The nominal concentration of hydroquinone in the bleaching cream is equal to 2% in weight that is equivalent to 0.4 mg l1 . The calculated concentration was 0.382 mg l1 with a standard deviation of 0.01 mg l1 , that is equivalent to 1.91% in weight, not signicantly different to the nominal value at 0.01 level. Once the viability of the calibration for the determination of hydroquinone in bleaching cream had been tested, a new determination was done with a single injection and a calibration from 0.05 to 0.50 mg l1 with three replicates, at the extremes, and at the central point. The LMS regression detects as outliers the standards with concentrations above 0.30 mg l1 , which elimination led to the LS model Y = 0.06 + 1.61X (sYX = 0.01 and R 2 = 0.99) that was suitable validated. After adequate dilution, the quantity of hydroquinone determined by ve replicates was 0.098 mg l1 , with a standard deviation of 0.006 mg l1 . This value is signicantly equal to the value declared by the manufacturer which after dilution is 0.10 mg l1 as several statistic test shows at a level of signicance of 5%. Also these values are in agreement with those obtained in the previous analysis, but in this case less experimental effort is implied.
the method is sufciently sensitive to variations in concentration of hydroquinone of 5 g l1 with respect to a value of 100 g l1 . The pH of the carrier solution in the average height response must be carefully controlled in the routine analysis of hydroquinone using this technique. The selectivity of the procedure is proven for the determination of hydroquinone in bleaching cream because the recovery is signicantly equal to 100% and determinations with a relative error of 6.2% are obtained implied.
Acknowledgements Authors thank the Ministerio de Ciencia y Tecnolog a (Project BQU2000-0863) and the Junta de Castilla y Len (Project BU15/01) for nancial support. References
[1] M. Valcarcel, M.D. Luque de Castro, Flow Injection Analysis, Principles and Applications, Ellis Horwood, Chichester, 1987. [2] D.G. Montgomery, Design and Analysis of Experiments, fourth ed., Wiley, New York, 1997. [3] T.B. Barker, Quality by Experimental Design, second ed., Marcel-Dekker, New York, 1994. [4] S.N. Deming, S.L. Morgan, Experimental Design: A Chemometric Approach, Elsevier, New York, 1987. [5] R.H. Myers, D.C. Montgomery, Response Surface Methodology, Wiley, New York, 1995. [6] G.A. Lewis, D. Mathieu, R. Phan-Tan-Luu, Pharmaceutical Experimental Design, Marcel-Dekker, New York, 1999. [7] G.E.P. Box, K.B. Wilson, J. R. Stat. Soc. B13 (1951) 1. ek, A. Svoboda, Anal. Chim. Acta 455 [8] D. at nsk, R. Karl c (2002) 103. [9] H.P. Xu, H.T. Liu, H.W. Wang, L.J. Dong, X.G. Chen, Z.D. Hu, Fresenius J. Anal. Chem. 368 (2000) 780. [10] M.F. Gin, R.L. Tuon, F.J. Krug, M.A.Z. Arruda, Anal. Chim. Acta 261 (1992) 533. [11] M.M.M.B. Duarte, G.O. Neto, L.T. Kubota, J.L.L. Filho, M.F. Pimentel, F. Lima, V. Lins, Anal. Chim. Acta 350 (1997) 353. [12] C. Vannecke, E. Van Gyseghem, M.S. Bloomeld, T. Coomber, Y. Vander Heyden, D.L. Massart, Anal. Chim. Acta 446 (2001) 413. [13] C. Vannecke, M.S. Bloomeld, Y. Vander Heyden, D.L. Massart, Anal. Chim. Acta 455 (2002) 117. [14] S. Gang, Z. Yongyao, W. Huaiwen, C. Hongli, C. Xingguo, H. Zhide, Analyst 125 (2000) 921. [15] T.A.H.M. Janse, P.F.A. Van der Wiel, G. Kateman, Anal. Chim. Acta 155 (1983) 89.
4. Conclusions The usefulness of the desirability function has been proved for solving the conict between the optimisation of the size of the analytical signal and its variability by searching for the experimental conditions which maximise the former and minimise the latter. The multi-response study carried out through the desirability function permits one to arrive at a compromise solution and reach a global optimum which fulls the specications imposed by the researcher. A PlackettBurman design applied to the study of robustness shows the stability of the experimental procedure in the face of small changes which could occur in the experimental factors. This test also shows that
184
M.E. Rueda et al. / Analytica Chimica Acta 479 (2003) 173184 [30] J. Inczdy, T. Lengyed, A.M. Ure, Compendium of analytical nomenclature, Denitive Rules 1997, third ed., IUPAC, Port City Press Inc., Baltimore, MD, 2000. [31] R.W. Andrews, C. Schubert, J. Morrison, E.W. Zink, W.R. Watson, Am. Lab. October (1982) 140. [32] L.H. Wang, Analyst 120 (1995) 224. [33] D. Mathieu, J. Nony, R. Phan-Tan-Luu, NemrodW, Version 2000, LPRAI, Marseille, 2000. [34] P.J. Rousseuw, A.M. Leroy, Robust Regression and Outlier Detection, Wiley, New York, 1987. [35] StatGraphics Plus for Windows, Version 4.0, Statistical Graphics Corporation, Rockville, MD, 1994. [36] M.C. Ortiz, L.A. Sarabia, Trends Anal. Chem. 13 (1994) 1. [37] E.C. Harrington, Ind. Qual. Cont. 21 (1965) 494. [38] G. Derringer, R. Suich, J. Qual. Tech 12 (1980) 214. [39] R. Carlson, Design and Optimization in Organic Synthesis, Elsevier, New York, 1992. [40] A.I. Khuri, Statistical Design and Analysis of Industrial Experiments, in: S. Ghosh (Ed.), Marcel-Dekker, New York, 1990 (Chapter 9). [41] L.M.B.C. lvares-Ribeiro, A.A.S.C. Machado, Anal. Chim. Acta 355 (1997) 195. [42] L. Cuadros, R. Blanc, A.M. Garc a, J.M. Bosque, Chemom. Intell. Lab. Syst. 41 (1998) 57. [43] M.C. Ortiz, M.J. Arcos, J.V. Juarros, J. Lpez-Palacios, L.A. Sarabia, Anal. Chem. 65 (1993) 678.
[16] E. Vereda, A. Rios, M. Valcarcel, Anal. Chim. Acta 348 (1997) 129. [17] N.A. Penner, P.N. Nesterenko, Analyst 125 (2000) 1249. [18] N.A. Penner, P.N. Nesterenko, N.A. Rybalko, J. Anal. Chem. 56 (2001) 934. [19] G.N. Chen, J.S. Liu, J.P. Duan, H.Q. Chen, Talanta 53 (2000) 651. [20] A. Afkhami, H.A. Khatami, J. Anal. Chem. 56 (2001) 429. [21] H.A.A. Medien, A.A. Zahran, Spectrochim. Acta, Part A: Mol. Biomol. Spectrosc. 57 (2001) 2505. [22] I. MolnarPerl, S. Tisza, E. Koros, K. KurinCsorgei, I. Szalai, HRC J. High Resolut. Chromatogr. 18 (1995) 749. [23] W.A. Pryor, K. Stone, L.Y. Zang, E. Bermdez, Chem. Res. Toxicol. 11 (1998) 441. [24] R.M. Decarvalho, C. Mello, L.T. Kubota, Anal. Chim. Acta 420 (2000) 109. [25] R. Toledo, M. Silva, V.O. Khavrus, P.E. Strizhak, Analyst 125 (2000) 2118. [26] J.Z. Gao, J. Ren, W. Yang, X.H. Liu, H. Yang, Q.Z. Li, H.L. Deng, J. Elelectroanal. Chem. 520 (2002) 157. [27] O. Fatibello, I.C. Vieira, Fresenius J. Anal. Chem. 368 (2000) 338. [28] T. Fang, M. Mc Grant, D. Diamond, M.R. Smyth, Anal. Chim. Acta 305 (1995) 347. [29] ISO 11843-1, Capability of Detection, Genve, Switzerland, 1997 and ISO 11843-2, Capability of Detection, Genve, Switzerland, 2000.
Vous aimerez peut-être aussi
- BiomassConversiontoFuelsandValue AddedchemicalsDocument24 pagesBiomassConversiontoFuelsandValue AddedchemicalsHoucine BendaoudPas encore d'évaluation
- Green Solvents, Potential Alternatives For Petroleum Based Products in Food Processing IndustriesDocument12 pagesGreen Solvents, Potential Alternatives For Petroleum Based Products in Food Processing IndustriesHoucine BendaoudPas encore d'évaluation
- 1 s2.0 S1631074816000059 MainDocument6 pages1 s2.0 S1631074816000059 MainHoucine BendaoudPas encore d'évaluation
- 1 s2.0 S1385894722004521 MainDocument16 pages1 s2.0 S1385894722004521 MainHoucine BendaoudPas encore d'évaluation
- Radiant Heat TransferDocument20 pagesRadiant Heat TransferAnonymous ioNuZrgPas encore d'évaluation
- Motivation Letter Sample 2Document2 pagesMotivation Letter Sample 2Houcine Bendaoud100% (3)
- Motivation Letter Sample 2Document2 pagesMotivation Letter Sample 2Houcine Bendaoud100% (3)
- Material Balance: Reactor: From The Above ReactionDocument3 pagesMaterial Balance: Reactor: From The Above ReactionHoucine BendaoudPas encore d'évaluation
- 1452641Document7 pages1452641Houcine BendaoudPas encore d'évaluation
- The in Vitro Antioxidant Activity of Different Types of Palm Dates (Phoenix Dactylifera) SyrupsDocument8 pagesThe in Vitro Antioxidant Activity of Different Types of Palm Dates (Phoenix Dactylifera) SyrupsHoucine BendaoudPas encore d'évaluation
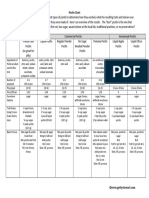
- Pectin Chart PDFDocument2 pagesPectin Chart PDFHoucine BendaoudPas encore d'évaluation
- 200 ProjectsDocument8 pages200 ProjectsHoucine BendaoudPas encore d'évaluation
- 200 ProjectsDocument8 pages200 ProjectsHoucine BendaoudPas encore d'évaluation
- Dupliant Sipam 2012Document6 pagesDupliant Sipam 2012Houcine BendaoudPas encore d'évaluation
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5794)
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (895)
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (400)
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (588)
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (74)
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (344)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (121)
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)
- Kode Simbol Rate TVDocument10 pagesKode Simbol Rate TVAndri PPas encore d'évaluation
- Total PDFDocument35 pagesTotal PDFMauricio ThanatosPas encore d'évaluation
- Install OpenERP On UbuntuDocument9 pagesInstall OpenERP On UbuntuQuynh NguyenPas encore d'évaluation
- RF Power Meter v2: Preliminary Operator's ManualDocument10 pagesRF Power Meter v2: Preliminary Operator's Manualsalvatore dalessandroPas encore d'évaluation
- GSM BSS Integration For Field Maintenance: ExercisesDocument14 pagesGSM BSS Integration For Field Maintenance: Exercisesswr cluster100% (1)
- Ram 100Document2 pagesRam 100MAT-LIONPas encore d'évaluation
- Aksesoris PumpDocument10 pagesAksesoris PumpDido AlexanPas encore d'évaluation
- HT P1200Document16 pagesHT P1200vantuyetphamPas encore d'évaluation
- Primus Iron MachineDocument67 pagesPrimus Iron MachineKonstantinos Politis100% (1)
- QAV - 1.1. Report (Sup1)Document2 pagesQAV - 1.1. Report (Sup1)Rohit SoniPas encore d'évaluation
- Thesis Process MiningDocument98 pagesThesis Process MiningRamyapremnathPas encore d'évaluation
- Troubleshooting Data: Plug Seen From The Cable SideDocument2 pagesTroubleshooting Data: Plug Seen From The Cable SideTomyPas encore d'évaluation
- 050-Itp For Installation of Air Intake Filter PDFDocument17 pages050-Itp For Installation of Air Intake Filter PDFKöksal PatanPas encore d'évaluation
- WSO&WSP Excel Shortcuts Cheat SheetsDocument7 pagesWSO&WSP Excel Shortcuts Cheat SheetsAndy ZouPas encore d'évaluation
- Absolute ValueDocument19 pagesAbsolute Valueapi-70433300Pas encore d'évaluation
- Sec 1038Document4 pagesSec 1038Lauren BowenPas encore d'évaluation
- Information Leaflet: 1. Introduction To PieasDocument4 pagesInformation Leaflet: 1. Introduction To Pieascensored126Pas encore d'évaluation
- K9900 Series Level GaugeDocument2 pagesK9900 Series Level GaugeBilly Isea DenaroPas encore d'évaluation
- UNIC Jakarta Internship ApplicationDocument4 pagesUNIC Jakarta Internship ApplicationMuhammad IkhsanPas encore d'évaluation
- Arduino Bluetooth Ralay 4chDocument5 pagesArduino Bluetooth Ralay 4chRaul Lara RochaPas encore d'évaluation
- Windchill Business Administrator's GuideDocument395 pagesWindchill Business Administrator's GuidevundavilliravindraPas encore d'évaluation
- Datasheet Borne SiemensDocument3 pagesDatasheet Borne Siemenslorentz franklinPas encore d'évaluation
- Related Learning Experience Journal: Lipa City, BatangasDocument7 pagesRelated Learning Experience Journal: Lipa City, BatangasushenPas encore d'évaluation
- Workbook Answer Key Unit 9 Useful Stuff - 59cc01951723dd7c77f5bac7 PDFDocument2 pagesWorkbook Answer Key Unit 9 Useful Stuff - 59cc01951723dd7c77f5bac7 PDFarielveron50% (2)
- The Effectiveness of Acceptance and Commitment-BasDocument18 pagesThe Effectiveness of Acceptance and Commitment-BasRaphaele ColferaiPas encore d'évaluation
- HR AuditDocument5 pagesHR AuditshanumanuranuPas encore d'évaluation
- Award Report TemplateDocument3 pagesAward Report Templatechriscivil12Pas encore d'évaluation
- L11 ImageplacementDocument2 pagesL11 ImageplacementJayram JavierPas encore d'évaluation
- Security Audit Technology Training Report To PresentDocument1 pageSecurity Audit Technology Training Report To PresentLewis Kang'araPas encore d'évaluation
- Divinity Original Sin 2 GrenadesDocument2 pagesDivinity Original Sin 2 Grenadesbeans54Pas encore d'évaluation