Académique Documents
Professionnel Documents
Culture Documents
Chapter 3 - Water and The Fitness of The Environment
Transféré par
sara9990Description originale:
Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Chapter 3 - Water and The Fitness of The Environment
Transféré par
sara9990Droits d'auteur :
Formats disponibles
Life on earth probably evolved in water and Living cells are 7095% H2O.
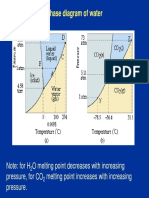
In nature, water naturally exists in all three physical states of matter, solid, liquid and gas. Water is a polar molecule. Its polar bonds and asymmetrical shape give water molecules opposite charges on opposite sides. O2 has four valence orbitals and shared electrons with hydrogen spend more time around the O2 (more electronegative), causing a weak positive charge near Hs. Each water molecule forms a maximum of 4 hydrogen bonds, which keeps water molecules further apart than they would be in the liquid state. So, water is less dense as a solid than it is as a liquid, and consequently ice floats. Heat and Temperature Kinetic energy is the energy of motion. Heat is the total kinetic energy due to molecular motion in a body of matter. Calorie (cal) is the amount of heat it takes to raise the temperature of one gram of water by one degree Celsius. 1Joule = 0.239cal Temperature is a measure of heat intensity due to the average kinetic energy of molecules in a body of matter. Scale Conversion C = 5(F-32)/9 F = 9/5C + 32 K = C + 273 Solution is a liquid that is homogenous mixture of two or more substances. Solvent dissolving agent of a solution Solute substance dissolved in a solution Aqueous solution is a solution in which water is the solvent Water is a versatile solvent owing to the polarity of the water molecule.
Hydrophilic and Hydrophobic Substances Ionic and polar substances are hydrophilic, but nonpolar compounds are hydrophobic. Molecular weight is the sum of the weight of all atoms in a molecule (in Daltons). Mole = Amount of a substance that has a mass in grams numerically equivalent to its molecular weight in Daltons. Molarity = Number of moles of solute per liter of solution. A mole of one substance has the same number of molecules as a mole of any other substance (6.02 x1023; Avogadros number). PH is a measure of the concentration of hydrogen ions. PH = -log10 [H+] mol/L The [H+] in pure water is 10-7; therefore the pH of pure water is = 7 and a lower pH always means a higher concentration of H+. Chemical equilibrium H2CO3 (carbonic acid) HCO3- (bicarbonate) + H+ Substances that prevent large sudden changes in pH are called buffers. Chemical reactions change the composition of matter. Water and the Fitness of the Environment Organisms depend on the cohesion of water molecules. Cohesion is a phenomenon of a substance being held together by hydrogen bonds. Surface tension measure of how difficult it is to stretch or break the surface of a liquid.
Vous aimerez peut-être aussi
- Lecture 02Document24 pagesLecture 02kblawan03Pas encore d'évaluation
- (A) Water and PHDocument53 pages(A) Water and PHSapana SubediPas encore d'évaluation
- Water's Polarity and Hydrogen Bonding Support LifeDocument17 pagesWater's Polarity and Hydrogen Bonding Support LifeLisandrea BrownPas encore d'évaluation
- WaterDocument24 pagesWaterAshley M NcubePas encore d'évaluation
- Lecture 2 - Lifes - Chemical - BasisDocument25 pagesLecture 2 - Lifes - Chemical - BasisPooja ChaudharyPas encore d'évaluation
- C9e Answers Active Reading 03Document6 pagesC9e Answers Active Reading 03Jaden Ventura0% (1)
- The Biochemistry of WaterDocument54 pagesThe Biochemistry of WaterIrene BungariaPas encore d'évaluation
- 5 Water LectureDocument37 pages5 Water Lecturevanessa biliyaPas encore d'évaluation
- Properties of Water: Hydrogen Bonds, Liquidity, ReactionsDocument2 pagesProperties of Water: Hydrogen Bonds, Liquidity, ReactionsasadPas encore d'évaluation
- AP BIO Campbell Reading Guide Answer KeyDocument3 pagesAP BIO Campbell Reading Guide Answer KeyRocio CastroPas encore d'évaluation
- Properties of Water LiquidsDocument23 pagesProperties of Water Liquidsmjlngpogi.walangibaPas encore d'évaluation
- Phase Diagram of WaterDocument29 pagesPhase Diagram of WaterSaif UllahPas encore d'évaluation
- Hydrogen: Syllabus: Unitix: Hydrogen 04 PeriodsDocument70 pagesHydrogen: Syllabus: Unitix: Hydrogen 04 Periodsanikesh JainPas encore d'évaluation
- Chapter 2Document12 pagesChapter 2Wany NurPas encore d'évaluation
- Water Definition: in The Gaseous PhaseDocument4 pagesWater Definition: in The Gaseous PhaseRica NorcioPas encore d'évaluation
- Unit 2: Biochemical MoleculesDocument105 pagesUnit 2: Biochemical MoleculesDaniel100% (1)
- Cell Biology and Genetics 16-08-17Document4 pagesCell Biology and Genetics 16-08-17Huda HalanePas encore d'évaluation
- Properties of Water GundaDocument15 pagesProperties of Water GundaGadzikaPas encore d'évaluation
- 1-1 Lecture , additionDocument10 pages1-1 Lecture , additionAnonymous guyPas encore d'évaluation
- Water NotesDocument5 pagesWater NotesYaron SaksPas encore d'évaluation
- 1.1 Bio 3Document4 pages1.1 Bio 3aelinsmy93Pas encore d'évaluation
- Water BME-211 (2120)Document50 pagesWater BME-211 (2120)Jack WengroskyPas encore d'évaluation
- Water Why Is Water PolarDocument3 pagesWater Why Is Water PolarDaneilla BanksPas encore d'évaluation
- Chemistry of Life Study Guide Answer KeyDocument3 pagesChemistry of Life Study Guide Answer Keyapi-293006069100% (1)
- The Structure and Function of WaterDocument23 pagesThe Structure and Function of WaterThomas JonesPas encore d'évaluation
- Solvent properties of water: How polarity enables life's chemistryDocument22 pagesSolvent properties of water: How polarity enables life's chemistryNana tseredianiPas encore d'évaluation
- CAPE 1 BIOLOGY - WaterDocument72 pagesCAPE 1 BIOLOGY - WaterTamicka BonnickPas encore d'évaluation
- Module 1 Lecture 3Document30 pagesModule 1 Lecture 3Amirs AmjadPas encore d'évaluation
- Water ChemDocument5 pagesWater ChemJohn harold De GuzmanPas encore d'évaluation
- Water 1Document9 pagesWater 1Michael NyaongoPas encore d'évaluation
- Biochemistry NotesDocument90 pagesBiochemistry Notespatialokkumar100% (2)
- Week 1 H2O Properties, Solutes Interactions & Types of H2ODocument56 pagesWeek 1 H2O Properties, Solutes Interactions & Types of H2Omunyee91100% (1)
- Water ChemistryDocument4 pagesWater Chemistryapi-296793567Pas encore d'évaluation
- StructureandpropertiesofwaterDocument57 pagesStructureandpropertiesofwaterDj Arts Tarpaulin PrintingPas encore d'évaluation
- Biochemistry 1.1 Introduction To Water and BuffersDocument7 pagesBiochemistry 1.1 Introduction To Water and Bufferslovelots1234Pas encore d'évaluation
- NEW WATERDocument55 pagesNEW WATERgostrider0093sPas encore d'évaluation
- The Properties of Water PresentationDocument21 pagesThe Properties of Water PresentationNurain Nasuha Tajul ArafatPas encore d'évaluation
- 1.1 Water & PHDocument105 pages1.1 Water & PHfardeensattar785Pas encore d'évaluation
- Biophysical Chemistry Lecture 1 CHE 212Document55 pagesBiophysical Chemistry Lecture 1 CHE 212Solomon MbewePas encore d'évaluation
- Water Chemistry PropertiesDocument19 pagesWater Chemistry Propertiesyuvimessi100% (2)
- Chapter 2 Water ChemistryDocument15 pagesChapter 2 Water ChemistryKathy Del CastilloPas encore d'évaluation
- Chapter 03Document4 pagesChapter 03Edward LeePas encore d'évaluation
- Physical and Chemical Properties of WaterDocument4 pagesPhysical and Chemical Properties of WaterSifatPas encore d'évaluation
- Biomolecules and Cells:: Mr. Derrick Banda MSC, BSCDocument42 pagesBiomolecules and Cells:: Mr. Derrick Banda MSC, BSCAmon SangulubePas encore d'évaluation
- 16 Water ChemistryDocument21 pages16 Water ChemistryMohit KambojPas encore d'évaluation
- Lesson 3.1. Acid-Base ChemistryDocument6 pagesLesson 3.1. Acid-Base ChemistryGemma CabañasPas encore d'évaluation
- Lecture Notes First Semester Yr 2 BPham BMLS BDSDocument57 pagesLecture Notes First Semester Yr 2 BPham BMLS BDSsriPas encore d'évaluation
- Water: The Solvent of Life Where There Is Water, There Is LifeDocument18 pagesWater: The Solvent of Life Where There Is Water, There Is LifeIsaiah Fidelis MajiPas encore d'évaluation
- Life, The Universe, and EverythingDocument12 pagesLife, The Universe, and EverythingBatisane Kantsu MathumoPas encore d'évaluation
- Acosta, Niel Task 5Document3 pagesAcosta, Niel Task 5Algrin AcostaPas encore d'évaluation
- Basic Water PropertiesDocument12 pagesBasic Water PropertiesBryan GraczykPas encore d'évaluation
- Properties of Water STUDENT'sDocument36 pagesProperties of Water STUDENT'sKim TangoPas encore d'évaluation
- Water: 1. Water Is Distributed On Earth As A Solid, Liquid and GasDocument10 pagesWater: 1. Water Is Distributed On Earth As A Solid, Liquid and GasIra Katriel NunagPas encore d'évaluation
- Biochem- Organic Compound and WaterDocument3 pagesBiochem- Organic Compound and WaterRaine TaclaPas encore d'évaluation
- 1c8eaproperties of Water, Acids Bases and BuffersDocument27 pages1c8eaproperties of Water, Acids Bases and BuffersAnita NegiPas encore d'évaluation
- Physical Science - CH 11Document5 pagesPhysical Science - CH 11suhughes100% (5)
- Lesson 2 Matter in The Liquid PhaseDocument27 pagesLesson 2 Matter in The Liquid PhaseDarren Daniel InfantePas encore d'évaluation
- Combining Chemicals - Fun Chemistry Book for 4th Graders | Children's Chemistry BooksD'EverandCombining Chemicals - Fun Chemistry Book for 4th Graders | Children's Chemistry BooksPas encore d'évaluation
- Water on Earth: Physicochemical and Biological PropertiesD'EverandWater on Earth: Physicochemical and Biological PropertiesPas encore d'évaluation
- Air Arabia Maroc HR Case StudyDocument23 pagesAir Arabia Maroc HR Case Studysara9990Pas encore d'évaluation
- Final Project OperatorsDocument19 pagesFinal Project Operatorssara9990Pas encore d'évaluation
- Blanks SolutionDocument1 pageBlanks Solutionsara9990Pas encore d'évaluation
- Concept of God in Major World Religions - Dr. Zakir NaikDocument29 pagesConcept of God in Major World Religions - Dr. Zakir NaikArshad Farooqui100% (5)
- Concept of God in Major World Religions - Dr. Zakir NaikDocument29 pagesConcept of God in Major World Religions - Dr. Zakir NaikArshad Farooqui100% (5)
- The Scope and Challenge of International MarketingDocument14 pagesThe Scope and Challenge of International Marketingsara9990Pas encore d'évaluation
- Global Aspects of Entrepreneurship: 2011pearson Education, Inc. Publishing As Prentice HallDocument31 pagesGlobal Aspects of Entrepreneurship: 2011pearson Education, Inc. Publishing As Prentice Hallsara9990Pas encore d'évaluation
- A Tour of The CellDocument4 pagesA Tour of The Cellsara9990Pas encore d'évaluation
- Polymer Hydrolysis KineticsDocument9 pagesPolymer Hydrolysis Kineticsdavid proxgamerPas encore d'évaluation
- Chemical and Ionic Equilibrium - Short Notes - Yakeen NEET 2024Document4 pagesChemical and Ionic Equilibrium - Short Notes - Yakeen NEET 2024Habibi AmjidPas encore d'évaluation
- 4 Determination of The Equilibrium Constant For Bromocresol GreenDocument12 pages4 Determination of The Equilibrium Constant For Bromocresol GreenKarlos Lds NvPas encore d'évaluation
- Using "Solver" To Solve Equilibrium Calculations: ExceletDocument8 pagesUsing "Solver" To Solve Equilibrium Calculations: Exceletdr_m_azharPas encore d'évaluation
- Lecture PDF IIT BombayDocument593 pagesLecture PDF IIT BombayKhan SalimPas encore d'évaluation
- Lesson Plan: A. Learning ObjectivesDocument4 pagesLesson Plan: A. Learning ObjectivesNazla Qonita PonotPas encore d'évaluation
- Chemical Equilibrium: Experiment No. 3Document12 pagesChemical Equilibrium: Experiment No. 3JV MandigmaPas encore d'évaluation
- The Langmuir IsothermDocument12 pagesThe Langmuir IsothermAlejandro MartinPas encore d'évaluation
- ReportDocument14 pagesReportAnh Lương QuỳnhPas encore d'évaluation
- PSRK Group Contribution Equation of State: Revision and Extension IIIDocument14 pagesPSRK Group Contribution Equation of State: Revision and Extension IIIAndrés F. CáceresPas encore d'évaluation
- Chem HW SolutionsDocument22 pagesChem HW Solutionsabdulrehman786100% (1)
- ASSIGNMENT3Document9 pagesASSIGNMENT3Eternal MiraclePas encore d'évaluation
- Lower 6 - Tutorial 17Document2 pagesLower 6 - Tutorial 17ronese augustusPas encore d'évaluation
- B.Sc. Biochemistry Syllabus at University of CalcuttaDocument32 pagesB.Sc. Biochemistry Syllabus at University of Calcuttanic315Pas encore d'évaluation
- Vogel, Arthur - Qualitative INORGANIC Analysis - (5th Ed - 1979) PDFDocument617 pagesVogel, Arthur - Qualitative INORGANIC Analysis - (5th Ed - 1979) PDFmanix_23100% (4)
- Chemistry 9701 Paper 1 - EquilibriaDocument27 pagesChemistry 9701 Paper 1 - EquilibriaSahana KumarPas encore d'évaluation
- FT Syllabus Upto 4th Year 14.03.14 PDFDocument28 pagesFT Syllabus Upto 4th Year 14.03.14 PDFhmce kalyaniPas encore d'évaluation
- International Journal of Mineral Processing: Senol Cetinkaya, Serafettin ErogluDocument3 pagesInternational Journal of Mineral Processing: Senol Cetinkaya, Serafettin ErogluMIzan NursiadiPas encore d'évaluation
- Chapter 4Document10 pagesChapter 4Lucy BrownPas encore d'évaluation
- PourbaixDocument648 pagesPourbaixKeyur GajjarPas encore d'évaluation
- SolubilidadDocument30 pagesSolubilidadWingsDavidPas encore d'évaluation
- Grand Viva QuestionsDocument9 pagesGrand Viva Questionsbaniya is herePas encore d'évaluation
- Thermochemistry Lab Report AnalysisDocument18 pagesThermochemistry Lab Report AnalysisAfina AnuariPas encore d'évaluation
- KDV EquationDocument4 pagesKDV Equationdr_s_m_afzali8662Pas encore d'évaluation
- Thermo DPP QuestionDocument35 pagesThermo DPP QuestionArush GuptaPas encore d'évaluation
- Larrabee JChem Educ 1990,67,267Document3 pagesLarrabee JChem Educ 1990,67,267κ.μ.α «— Brakat»Pas encore d'évaluation
- The Problem Set of The Four Rounds: ProblemsDocument29 pagesThe Problem Set of The Four Rounds: ProblemsMinh TieuPas encore d'évaluation
- REFFIPLANT Training CourseDocument76 pagesREFFIPLANT Training CourseKESAVARAPU UMA SAI MAHESHPas encore d'évaluation
- Boudouard ReactionDocument5 pagesBoudouard ReactionHailey17100% (1)
- Unit Kimia Kolej Matrikulasi Kedah: SK 015, Chemistry Unit, KMK Pra PSPM Set 1Document7 pagesUnit Kimia Kolej Matrikulasi Kedah: SK 015, Chemistry Unit, KMK Pra PSPM Set 1aPas encore d'évaluation