Académique Documents
Professionnel Documents
Culture Documents
Incipient Wetness
Transféré par
Reny AR MalakianDescription originale:
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Incipient Wetness
Transféré par
Reny AR MalakianDroits d'auteur :
Formats disponibles
this was accomplished by impregnation of alumina to incipient wetness with a solution of nickel nitrate and urea.
careful heating avoided solvent evaporation and hydrolyzed the urea to provide the base needed to precipitate nickel hydroxide inside the pores. after drying and reduction NI/AL203 catalyst was produced having very small nickel cystallites uniformly distributed throughout the alumina. some difficulties have been observed in the preparation of supported ruthenium catalysts by incipient wetness using ruthenium chloride solutions with a silica support. the usual procedure of air calcination followed by reduction in hydrogen at 450-500 celcius gave a catalysts inferior to one which the dried material was reduced directly in hydrogen at 700 celcius. this direct reduction did not result in any metal sintering, even though the temperature was high. the high temperature was needed, though, to remove the chlorine impurities from the ruthenium surface. application of this same procedure to a ruthenium chloride supported on carbon resulted in considerable hydrogenation of the support. this could be minimized by first heating the supported salt in a stream og nitrogen followed by high temperature hydrogen reduction with short contact times. this modified procedure produced a particularly active Ru/C catalyst. a Ni/Si02 catalyst prepared by incipient wetness was shown to consist, intially, of the silica support filled with the nickel salt. on reduction the nickel particles coalesced to form large cystallites because of their weak interaction with the support. coprecipitated catalyst, however, were composed of layered silicate structures that were more difficult to reduce to metallic nickel. when reduction did take place, though, the reduced cystallites were fixed within the confines of the support so small metal particles with a narrow size range were produced. table 13.1 metal complexes commonly used to prepare supported catalyst by ion exchange.
cobalt nickel copper ruthenium rhodium palladium silver iridium platinum gold
ion exchange as discussed in chapter 9, the adsorption character af a support material is governed by the nature of its surface functionality. for oxides these are generally hydroxy groups and for carbon supports they are acidic functions such as phenols and carboxylic acids. depending on the acidity of these surface groups and the pH of the solution in which the support is suspended, the surface can be either positively or negatively charged as shown in Fig.9.4. the pH at which the net charge on the support is zero is referred to as the isoelectric point (IEP) of the material. when the surface is negative, cationic species are attracted to it and become adsorbed. a positive surface interacts with negative species. the common anionic and cationic complexes used in catalyst preparation are listed in table 13.1
for exchange on a support two conditions must be met : the pH of the impregnation solution must be either sufficiently high or low to provide the appropriate surface potential and the adsorbent must have the proper charge. the isoelectric points of a number of oxides are listed in table 9.1. along with the increase in the adsorption of negative ion results. incipient wetness
Vous aimerez peut-être aussi
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (121)
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (588)
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (400)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (266)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5794)
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (345)
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (895)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- 01 - 17 - 72 Equipment Form - UnlockedDocument40 pages01 - 17 - 72 Equipment Form - UnlockedMuthana JalladPas encore d'évaluation
- 2020 HL BrochureDocument12 pages2020 HL Brochurearkadi oikhman100% (1)
- The Structure, Function and Properties of The Acquired PellicleDocument36 pagesThe Structure, Function and Properties of The Acquired PellicleLaura RăschipPas encore d'évaluation
- Edited PPT On Carboxylic AcidDocument51 pagesEdited PPT On Carboxylic AcidRenante DavisPas encore d'évaluation
- Christopher James, The Book of Alternative Photographic Processes - Cap 6 - The-Cyanotype-ProcessDocument22 pagesChristopher James, The Book of Alternative Photographic Processes - Cap 6 - The-Cyanotype-ProcessFernanda Eda PazPas encore d'évaluation
- ASTM D6209-98 Determination of Gaseous and Particulate Policyclic Aromatic Hydrocarbons in Ambient AirDocument14 pagesASTM D6209-98 Determination of Gaseous and Particulate Policyclic Aromatic Hydrocarbons in Ambient AirFredi Cari CarreraPas encore d'évaluation
- Experiment 7 - Gravimetric Determination of Aluminum As OxinateDocument2 pagesExperiment 7 - Gravimetric Determination of Aluminum As OxinateSavita ChemistryPas encore d'évaluation
- A. 2003. Grimsky, G. R. Spectrophotometric Determinaion of Protein ConcentrationDocument9 pagesA. 2003. Grimsky, G. R. Spectrophotometric Determinaion of Protein ConcentrationJorge Luis RomeroPas encore d'évaluation
- Role of Chemical Engineering For Renewable EnergyDocument16 pagesRole of Chemical Engineering For Renewable EnergyAayush KumarPas encore d'évaluation
- Test Bank For Exercise Physiology 8th Edition Scott PowersDocument13 pagesTest Bank For Exercise Physiology 8th Edition Scott PowersAnthonyRiveraqion100% (32)
- US2789119Document3 pagesUS2789119Feride Elif ErtürkPas encore d'évaluation
- Chemical, Synonym, and Trade Name IndexDocument4 pagesChemical, Synonym, and Trade Name IndexLuis CastroPas encore d'évaluation
- FA - BiochemistryDocument62 pagesFA - BiochemistryMargaret GracePas encore d'évaluation
- Thermocouples and Resistance ThermometersDocument44 pagesThermocouples and Resistance ThermometersmihailiuhaszPas encore d'évaluation
- 7182 PDFDocument5 pages7182 PDFAslam SheriffPas encore d'évaluation
- Dry GranulationDocument17 pagesDry GranulationMd.Motaleb HossenPas encore d'évaluation
- 1.physical, Chemical and Biological Characteristics of WaterDocument203 pages1.physical, Chemical and Biological Characteristics of WaternikhilPas encore d'évaluation
- Sika Rep Fine MSDocument4 pagesSika Rep Fine MSmohghareib80Pas encore d'évaluation
- Practice Density Problems: Solve The Following Problems Showing All Your Work Including Equations and UnitsDocument2 pagesPractice Density Problems: Solve The Following Problems Showing All Your Work Including Equations and UnitsJudd ShPas encore d'évaluation
- Salivary Cortisol: Expanded Range High SensitivityDocument21 pagesSalivary Cortisol: Expanded Range High Sensitivityerik gunawanPas encore d'évaluation
- Mean Median Mode RangeDocument2 pagesMean Median Mode RangeShin WonHoPas encore d'évaluation
- Trafo Oil Testing TypesDocument10 pagesTrafo Oil Testing TypesShrikanth SolaPas encore d'évaluation
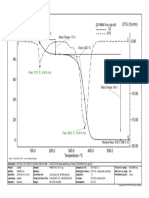
- TG /% DTG / (%/min) : Onset: 130.3 °C Mass Change: - 15 %Document1 pageTG /% DTG / (%/min) : Onset: 130.3 °C Mass Change: - 15 %Danay ManzoPas encore d'évaluation
- Photomer 6210 SDS USDocument11 pagesPhotomer 6210 SDS USPrabhakar RamachandranPas encore d'évaluation
- Actinobacillus Succinogenes NJ113Document6 pagesActinobacillus Succinogenes NJ113Мария КухароваPas encore d'évaluation
- JURNAL FixxDocument8 pagesJURNAL FixxNci Ncisri.mPas encore d'évaluation
- 05 Determining The Molar Volume of A GasDocument8 pages05 Determining The Molar Volume of A Gaslouise50% (4)
- Preliminary Evaluate Quiz: Ms. Khryss AyudaDocument2 pagesPreliminary Evaluate Quiz: Ms. Khryss Ayudaalex sshiPas encore d'évaluation
- Separations: A Brief Review of Chromatography in CroatiaDocument6 pagesSeparations: A Brief Review of Chromatography in CroatiaHarsha SharmaPas encore d'évaluation
- Solution Manual For Essentials of General Organic and Biochemistry 3rd Edition Denise GuinnDocument17 pagesSolution Manual For Essentials of General Organic and Biochemistry 3rd Edition Denise GuinnCameronHerreracapt100% (39)