Académique Documents
Professionnel Documents
Culture Documents
MLE1101 - Tutorial 3 - Suggested Solutions
Transféré par
Yin HauCopyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
MLE1101 - Tutorial 3 - Suggested Solutions
Transféré par
Yin HauDroits d'auteur :
Formats disponibles
1
MLE1101 Tutorial 3 - Suggested Solutions
1. Calculate the number of atoms in a critically sized nucleus for the homogeneous nucleation
of pure iron. Assume AT (undercooling) = 0.2 T
m
. Use the data for pure iron as follows. Latent
heat of fusion = 2098 J/cm
3
, Surface energy = 20410
7
J/cm
2
, Melting temperature = 1808K
and lattice constant for its BCC unit cell = 0.28664 nm.
Solution:
( )
( )
( )
( )
( )( )
7 2
* 10
3
2 204 10 J/cm 1808K
2 2
9.72 10 m
0.2
2098J/cm 0.2 1808K
m m
f f m
T T
r
H T H T
= = = =
A A A
( )
3
3 10 27 3
4 4
vol. of critical-sized nucleus * 9.72 10 m 3.85 10 m
3 3
r x t t
= = =
( )
3
3 9 29 3
vol. of 1 unit cell of Fe 0.28664 10 m 2.355 10 m a
= = =
Since there are two atoms per BCC unit cell,
= =
29 3
29 3
2.355 10 m
Volume / atom 1.178 10 m
2
= =
27 3
29 3
vol. of nucleus 3.85 10 m
327 atoms
Volume/atom 1.178 10 m
2. Calculate the radius of the largest interstitial void in the BCC o iron lattice. The atomic radius
of the iron atoms in this lattice is 0.124 nm, and the largest interstitial voids occur at the
( ) ( ) ( ) ( )
3 3 1 1 1 1 1 1
4 2 2 4 4 2 2 4
, ,0 ; , ,0 ; , ,0 and , ,0 etc., type positions.
Solution:
For BCC structure,
( ) 4 0.124nm 4
0.286nm
3 3
R
a = = =
Let x = Fe atom radius + Interstitial void radius,
( )
2 2
2 2
2
2
5
4 2 16
5 5
0.286nm 0.160nm
16 16
a a
x a
x a
| | | |
= + =
| |
\ . \ .
= = =
void Fe
interstitial void radius, 0.160nm 0.124nm 0.036nm R x R = = =
2
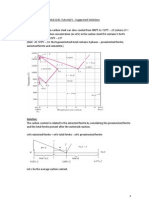
3. Describe and illustrate the edge- and screw-type dislocations. What types of strain fields
surround both types of dislocations?
Solution:
An edge dislocation is a line imperfection caused by an extra half plane of atoms between
two normal planes of atoms. Whereas a screw dislocation is a line imperfection created by
applying upward and downward shear stress to regions of a perfect crystal separated by a
common plane.
The strain fields associated with the edge and screw dislocations are shown below:
4. If there are 400 grains per square inch on a photomicrograph of a ceramic material at 200,
what is the ASTM grain-size number of the material?
Solution:
( )( )
1
1600 2
ln1600 1 ln2
10.64 1 11.64
n
N
n
n
= =
=
= + =
3
5. (a) Calculate the equilibrium concentration of vacancies per cubic meter in pure copper
at 850
o
C. Assume that the energy of formation of a vacancy in pure copper is 1.00
eV, constant C = 1.
(b) What is the vacancy fraction at 800
o
C?
(Atomic mass of copper = 63.54 g/mol; density of copper = 8.96 g/cm
3
; Avogadros
constant = 6.0210
23
atoms/mol; Boltzmanns constant = 8.6210
-5
eV/K)
Solution:
(a)
( )( )
( )
23 6 3
0 Cu
28 3
6.02 10 atoms /atomic mass 8.96 10 g /m
atomic mass of Cu 63.54g /atomic mass
8.49 10 atoms /m
N
N
= =
=
exp exp
v v v
v
n E E
C n NC
N kT kT
| | | |
= =
| |
\ . \ .
( )
( )
( )
28 3
5
24 3
1eV
8.49 10 atoms /m exp
8.62 10 eV/K 850K 273K
2.77 10 vacancies /m
v
n
(
(
=
( +
=
(b) vacancy fraction at 1073K is,
( )
( )
5
5
1eV
exp exp
8.62 10 eV/K 800K 273K
2.02 10 vacancies /atom
v v
n E
N kT
(
| |
(
= =
|
( \ . +
=
6. Write the equation for Ficks first law of diffusion, and define each term in SI units.
Solution:
2
2 3
atoms m atoms 1
or in SI unit form
s m m s m
dC
J D
dx
| |
| |
= =
` ` | |
) \ .
\ .
)
where J = flux or net flow of atoms; D = proportionality constant called the diffusitivity
(atomic conductivity) or diffusion coefficient;
dC
dx
= concentration gradient.
4
7. Consider the gas carburizing of a gear of 1018 steel (0.18wt%) at 927
o
C (1700
o
C). Calculate
the time necessary to increase the carbon content to 0.35wt% at 0.40 mm below the surface
of the gear. Assume the carbon content at the surface to be 1.15wt% and that the nominal
carbon content of the steel gear before carburizing is 0.18wt%.
D (C in iron) at 927
o
C = 1.2810
-11
m
2
/s.
Solution:
( )
( )
3
11 2
0
1.15 0.35 0.4 10 m
1.15 0.18 2
2 1.28 10 m /s
S X
S
C C x
erf erf
C C Dt
t
| |
| |
|
= =
|
|
\ .
|
\ .
55.9017
0.8247 erf
t
| |
=
|
\ .
1 0.8427 0.8247
1 0.95 0.8427 0.8209
0.9587
z
z
=
=
55.9017
0.9587
3400s 56.67mins
t
t
=
= =
8. The diffusivity of copper atoms in the aluminium lattice is 7.5010
-13
m
2
/s at 600
o
C and
2.5010
-15
m
2
/s at 400
o
C. Calculate the activation energy for this case in this temperature
range. Given R = 8.314 J/molK.
Solution:
1
0
2 1 2
1 1
exp exp
D Q Q
D D
RT D R T T
(
| |
| |
= =
( | |
\ .
( \ .
( ) ( )
13 2
15 2
7.5 10 m /s 1 1
exp
8.314J/molK 600K 273K 400K 273K 2.5 10 m /s
Q
(
| |
= ( |
|
+ +
(
\ .
139.3kJ/mol Q=
z erf(z)
0.95 0.8209
z 0.8247
1 0.8427
Vous aimerez peut-être aussi
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (119)
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (265)
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (399)
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (587)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2219)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5794)
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (344)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (890)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (73)
- CBSE Class 10 Science NCERT Exemplar Solutions CHAPTER 1 Chemical Reactions and Equations - Chapter 1Document25 pagesCBSE Class 10 Science NCERT Exemplar Solutions CHAPTER 1 Chemical Reactions and Equations - Chapter 1Govu GovardhanPas encore d'évaluation
- Physical Science Quarter 1 Module 4Document32 pagesPhysical Science Quarter 1 Module 4Luanne Jali-JaliPas encore d'évaluation
- EC1301 Mid-Term Exam Questions (09102009 - Make-Up Exam)Document11 pagesEC1301 Mid-Term Exam Questions (09102009 - Make-Up Exam)Yin Hau100% (1)
- 2 - Solubility of Organic CompoundsDocument4 pages2 - Solubility of Organic CompoundsJade AsparinPas encore d'évaluation
- ME2142E Feedback Control Systems-CheatsheetDocument2 pagesME2142E Feedback Control Systems-CheatsheetPhyo Wai Aung67% (9)
- Astm d6423 - PheDocument3 pagesAstm d6423 - PheNinit Miyu100% (1)
- MNO1001X Cheat SheetDocument8 pagesMNO1001X Cheat SheetYin HauPas encore d'évaluation
- VII - Chemistry KAT Worksheet - I PDFDocument3 pagesVII - Chemistry KAT Worksheet - I PDFSahithi100% (2)
- Michaelis-Menten model accounts for enzyme kineticsDocument11 pagesMichaelis-Menten model accounts for enzyme kineticsPhenyo Mmereki100% (2)
- Thermodynamics and Its Application in RefrigerationDocument8 pagesThermodynamics and Its Application in RefrigerationYin HauPas encore d'évaluation
- Laplace Transform TableDocument3 pagesLaplace Transform TableYin HauPas encore d'évaluation
- Entropy 15 01221Document11 pagesEntropy 15 01221Yin HauPas encore d'évaluation
- Gek 1540 Tut 2 QBDocument2 pagesGek 1540 Tut 2 QBYin HauPas encore d'évaluation
- Gek1540 Chapter 6Document4 pagesGek1540 Chapter 6Yin HauPas encore d'évaluation
- Tutorial 7 Suggested AnswersDocument4 pagesTutorial 7 Suggested AnswersYin HauPas encore d'évaluation
- Mechanical Properties and Testing of Materials Chapter 2Document7 pagesMechanical Properties and Testing of Materials Chapter 2Yin HauPas encore d'évaluation
- LSM1301Document7 pagesLSM1301Yin HauPas encore d'évaluation
- Engineers & EnvironmentDocument8 pagesEngineers & EnvironmentYin HauPas encore d'évaluation
- Heat Transfer Radiation Problem SetDocument1 pageHeat Transfer Radiation Problem SetLakshmi BalasubramaniamPas encore d'évaluation
- CA Lab Manual ScopeDocument10 pagesCA Lab Manual Scopea2367916100% (1)
- Tutorial 7 Suggested AnswersDocument4 pagesTutorial 7 Suggested AnswersYin HauPas encore d'évaluation
- ME3112-PART 2 Tutorial 2 & 3: ShahrokhDocument14 pagesME3112-PART 2 Tutorial 2 & 3: ShahrokhYin HauPas encore d'évaluation
- TimetableDocument2 pagesTimetableYin HauPas encore d'évaluation
- ME3162 Questions PDFDocument2 pagesME3162 Questions PDFYin HauPas encore d'évaluation
- 12 TransientDocument39 pages12 TransientYin HauPas encore d'évaluation
- Heat Transfer Radiation Problem SetDocument1 pageHeat Transfer Radiation Problem SetLakshmi BalasubramaniamPas encore d'évaluation
- MLE1101 - Tutorial 5 - Suggested SolutionsDocument5 pagesMLE1101 - Tutorial 5 - Suggested SolutionsYin HauPas encore d'évaluation
- Chapter 15Document2 pagesChapter 15Yin HauPas encore d'évaluation
- LSM1301Document7 pagesLSM1301Yin HauPas encore d'évaluation
- L2 - LeadershipDocument2 pagesL2 - LeadershipYin HauPas encore d'évaluation
- MLE1101 - Tutorial 6 - Suggested SolutionsDocument5 pagesMLE1101 - Tutorial 6 - Suggested SolutionsYin HauPas encore d'évaluation
- MLE1101 Tutorial 4 - Suggested Solutions AnalysisDocument7 pagesMLE1101 Tutorial 4 - Suggested Solutions AnalysisYin HauPas encore d'évaluation
- MLE1101 - Tutorial 1 - Suggested SolutionsDocument5 pagesMLE1101 - Tutorial 1 - Suggested SolutionsYin HauPas encore d'évaluation
- MLE1101 Tutorial 2 - Suggested Solutions for BCC Crystal Structure, Lattice Constant and Element IdentificationDocument8 pagesMLE1101 Tutorial 2 - Suggested Solutions for BCC Crystal Structure, Lattice Constant and Element IdentificationYin HauPas encore d'évaluation
- EC1301 - Tutorial 4 (14-18 September 2009) - AnswersDocument9 pagesEC1301 - Tutorial 4 (14-18 September 2009) - AnswersYin HauPas encore d'évaluation
- Corrosivity of SoilsDocument5 pagesCorrosivity of SoilsMorched TounsiPas encore d'évaluation
- Liquid SolutionDocument11 pagesLiquid SolutionBikashPas encore d'évaluation
- Camera Tubes NDocument30 pagesCamera Tubes NRamakrishna VadlamudiPas encore d'évaluation
- ECE 3223 Separation Processes I: B. Eng (Hons.) Chemical EngineeringDocument19 pagesECE 3223 Separation Processes I: B. Eng (Hons.) Chemical EngineeringJosh VatomPas encore d'évaluation
- Enhanced Efficiency and Stability of N-I-P Perovskite Solar Cells by Incorporation of Fluorinated Graphene in The Spiro-OMeTAD Hole Transport LayerDocument11 pagesEnhanced Efficiency and Stability of N-I-P Perovskite Solar Cells by Incorporation of Fluorinated Graphene in The Spiro-OMeTAD Hole Transport Layer北科大-洪珮倫Pas encore d'évaluation
- D0597189 CHEM12 C1700 SWBT Mig PDFDocument16 pagesD0597189 CHEM12 C1700 SWBT Mig PDFMr: Mohamed BesharaPas encore d'évaluation
- Enzymes-Biology PresentationDocument52 pagesEnzymes-Biology PresentationAdeenPas encore d'évaluation
- Chemistry Criterion B and C Lab ReportDocument7 pagesChemistry Criterion B and C Lab ReportBRIGHTON ONYANGOPas encore d'évaluation
- Buffer Systems Maintain pHDocument10 pagesBuffer Systems Maintain pHEzat JrPas encore d'évaluation
- Pauli Paramagnetism of Cubic V3Al, CrVTiAl, and Related 18-Electron Heusler CompoundsDocument10 pagesPauli Paramagnetism of Cubic V3Al, CrVTiAl, and Related 18-Electron Heusler CompoundsRSF IITBPas encore d'évaluation
- 10.1007@978 3 030 43009 2Document378 pages10.1007@978 3 030 43009 2ali ghalibPas encore d'évaluation
- D664 PDFDocument7 pagesD664 PDFROHITPas encore d'évaluation
- 9D17101 Advanced ThermodynamicsDocument1 page9D17101 Advanced ThermodynamicssubbuPas encore d'évaluation
- En Tds A-80Document3 pagesEn Tds A-80cnotebookPas encore d'évaluation
- Declaration of Performance K-Roc UKDocument2 pagesDeclaration of Performance K-Roc UKDavid midlandPas encore d'évaluation
- Humidity Derate ChartDocument1 pageHumidity Derate ChartMd Sh100% (1)
- SMJC 2701 Exp2Document14 pagesSMJC 2701 Exp2norsiahPas encore d'évaluation
- TUTORIAL 3 Thermodynamics PDFDocument5 pagesTUTORIAL 3 Thermodynamics PDFNelson0% (1)
- 1st Summative Test in Science 10 Quarter 4Document2 pages1st Summative Test in Science 10 Quarter 4Aj De CastroPas encore d'évaluation
- Van Der WaalsDocument2 pagesVan Der WaalsDwi Esti KusumandariPas encore d'évaluation
- EP-300 Operating Manual E1Document343 pagesEP-300 Operating Manual E1leman quliyevaPas encore d'évaluation
- EurokinDocument14 pagesEurokinapitbhuPas encore d'évaluation
- Coal and Petroleum ProcessingDocument32 pagesCoal and Petroleum ProcessingVarshi RaagaPas encore d'évaluation
- Bio-Soft N-Series PDFDocument9 pagesBio-Soft N-Series PDFGina AriasPas encore d'évaluation