Académique Documents
Professionnel Documents
Culture Documents
Chapter3 Properties
Transféré par
Shafiq HafizullahCopyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Chapter3 Properties
Transféré par
Shafiq HafizullahDroits d'auteur :
Formats disponibles
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
Chapter 3 Properties of Pure Substances
(Sifat Bahan Tulin)
oleh Lt Kol Prof Madya Ir Khalid bin Abd Jalil TUDM Jabatan Kejuruteraan Mekanikal Universiti Pertahanan Nasional Malsysia.
012-2094234
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
Objectives
Introduce the concept of pure substance. Discuss the physics of phase-change of processes. Illustrate the P-V-T property diagram. Determine the thermodynamics properties. Describe ideal gas and its behavior.
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
The Pure Substance
Definition
Pure Substance has a homogeneous and invariable chemical composition and may be exist in more than one phase with the same chemical composition. Water (solid, liquid and vapor phases) Mixture of liquid water and water vapor Carbon dioxide, CO2 Nitrogen, N2 Mixture of gases, such as air, as long as there is no change of phase.
Homogeneous Substance a substance that has uniform thermodynamic properties throughout is said to homogeneous. Simple Substance a substance whose changes in properties is dominated by the effect of a volume change as opposed to surface, magnetic, electrical changes.
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
Phases of Pure Substance
Substance exit in different phases and its depend on temperature and pressure. In principles, substance have 3 phases:- solid (pepejal) ~ molecules are relatively fixed positions in solid. - liquid (cecair) ~not much different from that of the solid phase, except the molecules are no longer at fixed positions relative to each other and they can rotate and translate freely. - vapor (wap) ~ molecules are far apart from each other and move about a random
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
Relationships between Pressure (P), Temperature (T) and Specific Volume (v)
P, T and v 3 important properties in the
thermodynamic and also a pure substances due to:v and T is a independent variable While P, is the function of both P = P(T,v) Relationships between P, T and v can be demonstrated in P v T Surface for a real substance diagram as shown next slide.
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
The P v T Surface for a Real Substance that expand upon freezing example; water
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
The P v T Surface for a Real Substance that contracts upon freezing. Example: Nitrogen, CO2
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
The P v T Surface for a Real Substance
From the Surface diagram, the 3 phases region where substance like water may exist as a solid, liquid or gas (vapor) can be easily defined. a. Single phase region (Satu fasa) area where P, T and v are independent properties, although one of the property can changed without changing of the other property. b. 2 phase region (Dua fasa) two properties are in equilibrium, where pressure and temperature are depends to one another without changing the other. Example : boiling water. c. 3 phase region (3 fasa) all properties, solid, liquid and vapor, were in equilibrium.
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
The P v T Surface for a Real Substancecont
d. Saturated condition (keadaan tepu) line for liquid and vapor meet at critical point and forming a dome. All compressed liquid are located in the region to the left of saturated liquid line, called the compressed liquid region. All superheated vapor states are located to the right of saturated vapor line, called superheated vapor region. e. Critical Point (titik genting) defined as the point at which the saturated liquid and saturated vapor states are identical and in equilibrium state. At this point, P =22.06 MPa and T = 373.95C .
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
Phase-Change Processes of Pure Substances
Consider piston-cylinder device containing water at 20C and 1 atm pressure. At this state, water exits in liquid phase and it is called a compressed liquid or a subcooled liquid. Process 1-2: T and v will increase from the compressed liquid or sub cooled liquid (cecair sub dingin) , state 1, to saturated liquid (cecair tepu) state 2. In the compressed liquid region (cecair termampat) , the properties of the liquid are approximately equal to the properties of saturated liquid state at the temperature. Process 2-3: At state 2, the liquid reached the boiling temperature, called the saturation temperature (suhu tepu) and is said to exist as a saturated liquid (cecair tepu). During the phase change, both temperature and pressure remain constant ( water boil at 100 C and P=1atm or 101.325 kPa)
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
Phase-Change Processes of Pure Substancesconts.
Process 3: Once the boiling starts, the temperature will stop raising until the liquid is completely vaporized. At this state, liquid and vapor are in equilibrium. This phase called saturated liquid (cecair tepu) and saturated vapor (wap tepu) and also called saturated liquid vapor mixture (campuran cecair-wap tepu). Process 4: At state 4, a saturated vapor exists and vaporization is completed. Any heat loss from this vapor will cause some vapor to condense (phase change from vapor to liquid). The vapor that about to condense is called a saturated vapor (wap tepu). A substance at state between 2 and 4 is often referred to as a saturated liquid vapor mixture (campuran cecair-wap tepu).
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
Phase-Change Processes of Pure Substancesconts.
Process 4-5:
If the constant pressure heating is continued, the temperature will begin increase above the saturated temperature (suhu tepu) and the volume also increases. State 5, is called a superheated (wap panas lampau) state because T 5 is greater that saturation temperature (above 100C) for the pressure and the vapor is not about to condenser.
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
Phase-Change Processes of Pure Substances cont
Water boils at 100C at 1 atm pressure
T-v diagram for heating process of water at constant pressure.
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
Pc=22.09MPa Tc=374.14C
vc= 0.003155m/kg
T v diagram of constant pressure phase-change processes of a pure substance at various pressure
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
The T- v Diagram
The 2 lines (saturated liquid lines and saturated vapor lines) intersect at critical point and form is called the steam dome. The region between saturated liquid line and saturated vapor lines is called saturated liquid-vapor mixture region . At given pressure, temperature at which a pure change phase is called Saturation Temp., Tsat At given temperature, pressure at which a pure substance change phase is called saturation pressure Psat. Table A-1 page 910, Critical point properties.
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
Superheated vapor region Compressed liquid region
The region to the left of the saturated liquid line and below the critical temp is called the compressed liquid region. The region to the right of the saturated vapor line and above critical temp is called superheated region. At temp & pressure above the critical point, the phase transition from liquid to vapor is no longer discrete.
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
Relationship between solid, liquid and vapor
P=22.06 MPa
T=373.95 C
P=0.6113kPa T=0.01 C
Figure shows the P-T diagram, often called the phase diagram, for pure substances that contract and expand during freezing.
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
Property Table
For most substances, the relationships among thermodynamic properties are too complex to be expressed by simple equations. Therefore, properties are frequently presented in the form of tables. Table A-2 & A-3 (page 914/915) Properties of common liquids, solids & foods. Table A-4 (page 916) Saturated water Temperature table Table A-5 (page 918) saturated water Pressure table Table A-6 (page 920-923) superheated water Table A-7 (page 924) Compressed liquid water Table A-8 (page 925) saturated ice-vapor
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
FIGURE 2-40 A partial listing of Table A6.
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
Enthalpy is the thermodynamic property of fluid (combination property) and It can be
used to calculate the heat transfer during a quasistatic process taking place in a closed thermodynamic system under constant pressure. The enthalpy is a convenient grouping of the internal energy, pressure, and volume:-
h is useful to calculating the energy of mass streams flowing into and out of
control volumes. (kJ/kg)
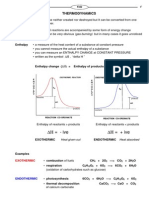
Note: Is called the enthaply of vaporization (or latent heat of vaporization). It decreases as temp. or px increase and becomes zero at the critical point.
hf specific enthaply of saturated liquid hg specific enthaply of saturated gas
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
Using saturated water Table A4 and A-5, find temperature/pressure, specific volume of saturated liquid, Vf , internal energy of saturated vapor, Ug and enthalpy of evaporation hfg , if the:a. pressure is 50kPa. b. Temperature 120C
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
Saturated Liquid-Vapor Mixture
(campuran Cecair-wap Tepu)
-During a vaporization process, a substance exists as part liquid and part vapor, also called a mixture of saturated liquid and saturated vapor. - at this point liquid and vapor are in equilibrium.
- To analyze this mixture properly, we need to know the proportions of the liquid and vapor phase in the mixture.
- The ratio of the mass of vapor to the total mass of the mixture is called the quality, x
X = m vapor m vapor + m liquid
mg mg + m f
(1)
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
Saturated Liquid-Vapor Mixturecont
(campuran Cecair-wap Tepu)
-The quality is zero (0) for the saturated liquid and one (1) for the saturated vapor, (0 < x < 1). - the bigger x values, showed the total of saturated vapor are increased, and we can write as follow:-
( 1- x) =
mf m g+mf
(2)
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
Saturated Liquid-Vapor Mixturecont
(campuran Cecair-wap Tepu)
- Consider a tank that contains a saturated liquid-vapor mixture. The volume occupied by saturated liquid is Vf and the volume occupied by saturated vapor Vg,. The total volume V is sum of the two:
V = Vf + Vg
If, V = mv
(3)
mvav = mfvf + mgvg
(mg+ m f ) v = m g v g +
mfvf
Specific Volume
v=
mf . vf + mg . vg m g+ m f m g+ m f
x v g = vf + x (v g - v f )
Replace (1) and (2)
By replacing
v = (1 - x ) v f +
v fg = v g - v f
v = vf +
x v gf
m/kg
(4)
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
Saturated Liquid-Vapor Mixturecont
(campuran Cecair-wap Tepu)
- The analysis given can be repeated for internal energy, u and enthalpy, h with the following results: Specific enthalpy
h = (1 - x ) h f + x h g = hf + x (h g - h f ) = h fg + x h g f
(kJ/kg) (5)
In thermodynamics and molecular chemistry, the enthalpy or heat content (denoted as H, h, or rarely as ) is a quotient or description of thermodynamic potential of a system, which can be used to calculate the "useful" work obtainable from a closed thermodynamic system under constant pressure and entropy.
Specific internal energy
u = (1 - x ) u f + x u g = uf + x (u g - u f ) = u fg + x u g f
(kJ/kg) (6)
Note: Persamaan (4), (5) dan (6) digunakan untuk mendapatkan sifat-sifat termodinamik bahan pada fasa cecair dan wap tepu sahaja
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
Compressed Liquid (Cecair termampat)
It happen when the temperature is below from saturated temperature under certain pressure. Different between saturated temperature and compressed liquid temperature under same pressure is called sub cooled degree (darjah sub dingin)
Tsub=Ts T
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
Compressed Liquid (Cecair Termampat)
A substance is said to be compressed liquid when the pressure is greater than the saturation pressure for the given temperature. Compressed Liquid properties depends on temperature much more than pressure. For analysis, the value for saturated liquid properties is used as a reference at the given temperature, such as:
V = Vf at given temperature or V= Vf (T) U= Uf at given temperature or U = Uf (T) h = h f at given temperature or h = h f (T) In general, the compressed liquid is characterized by: Higher Pressure (P>Ps at given T) lower Temperature (T<Ts at given P) Lower specific Volume (V < V f at given P or T) Lower specific internal energy (U < U f at given P or T) Lower enthalpies (h< h f at given P or T)
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
Superheat Vapor
(Wap panas lampau)
In the region to the right of the saturated vapor line and temperature above the critical point, a substance exists as superheated vapor. Since the superheated region is a single-phase region, temperature and pressure are no longer dependent properties and there can conveniently be used in as the two properties in the tables.
Tpl = T Ts
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
Superheat Vapor
(Wap panas lampau)
A substance is said to be superheated if the given temperature is greater than the saturation temperature for the given pressure. In the region to the right of the saturated vapor line and temperature above the critical point, a substance exists as superheated vapor. Since the superheated region is a single-phase region, temperature and pressure are no longer dependent properties and there can conveniently be used in as the two properties in the tables. Superheated vapor can be characterized by : Low Pressure (P<Ps at given T) Higher Temperature (T>Ts at given P) Higher specific Volume (V > V g at given P and T) Higher specific internal energies (U > Ug at given P or T) Lower Specific enthalpies (h > h g at given P or T)
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
Interpolation Method
(kaedah tentu dalaman)
if required data is in between 2 given data. Using linear function y=f(x) Properties 1 Example: x1
(yt y1)
Properties 2
y1 yt
(y2 y1)
(xt x1)
(x2 x1) (xt x1)
xt
x2
y2
yt
(x2 x1)
(y2 y1)
+ y1
Link to excel file to calculate
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
Property Table (Jadual Sifat)
For most substance, the relationships among the thermodynamic properties are too complex to be express by simple equation, therefore, properties value are frequently presented in the form of table.
3 types of Table
(Compress liquid table A-7 ) (Saturated liquid table A-4 and A-5) (Superheated table A-6)
(Saturated water table)
(Saturated liquid table A-4) (Saturated liquid table A-5)
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
Table selection If U < U f - compressed liquid U f < U < Ufg - saturated mixture U > Ug superherated If P>Ps - compressed liquid P = Ps saturated mixture P<Ps superheated If T<Ts compressed liquid T=Ts - saturated mixture T>Ts super heated If V < V f - compressed liquid Vf <V < Vfg - saturated mixture V> Vg superheated.
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
(kJ)
entropy is a measure of the unavailability of a systems energy to do work. that energy which cannot be used for external work
Thermodynamics I (EMM2503) Chapter 3 Properties of Pure Substances
Duty, Honor and Integrity
Example : 1. Determine saturated temperature Ts of water if the pressure is 5.3 bar.
2. Determine specific volume and specific enthalpy of water at pressure 14bar and temperature 420C.
3. Determine the enthalpy of 1.5 kg of water contained in volume of 1.2 m at pressure 200kPa. 4. 1kg of water vapor at 200kPa fills the 1.1989m left chamber of a partitioned system shown in figure below. The right chamber has twice the volume of the left and is initially evacuated. Determine the final state of volume and pressure of the water after the partition has been removed and enough heat has been transferred so that the temp. of the water is 3 C.
Vous aimerez peut-être aussi
- Walkin App Lnform My S Dec 17Document2 pagesWalkin App Lnform My S Dec 17Shafiq HafizullahPas encore d'évaluation
- Eel1166 CK1Document11 pagesEel1166 CK1Shafiq HafizullahPas encore d'évaluation
- Assimen Tronic 2Document23 pagesAssimen Tronic 2Shafiq HafizullahPas encore d'évaluation
- Alternating Current CircuitsDocument8 pagesAlternating Current CircuitsShafiq HafizullahPas encore d'évaluation
- Chapter 2Document44 pagesChapter 2Shafiq HafizullahPas encore d'évaluation
- Chapter 5Document16 pagesChapter 5Shafiq HafizullahPas encore d'évaluation
- When Worlds Collide - The Rise of Social Media For Professional and Personal UseDocument25 pagesWhen Worlds Collide - The Rise of Social Media For Professional and Personal UseShafiq HafizullahPas encore d'évaluation
- Energy, Work and Heat: (Tenaga, Kerja & Haba)Document63 pagesEnergy, Work and Heat: (Tenaga, Kerja & Haba)Shafiq HafizullahPas encore d'évaluation
- Chapter3 - Ideal GasDocument19 pagesChapter3 - Ideal GasShafiq HafizullahPas encore d'évaluation
- Chapter 5 Practice Problems SolutionsDocument8 pagesChapter 5 Practice Problems SolutionsShafiq HafizullahPas encore d'évaluation
- Ch03 - The Structure of Crystalline SolidsDocument40 pagesCh03 - The Structure of Crystalline SolidsShafiq HafizullahPas encore d'évaluation
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5794)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (895)
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (588)
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (400)
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2259)
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (74)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (345)
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (121)
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)
- Introduction To Unit 3 Lesson 10Document8 pagesIntroduction To Unit 3 Lesson 10DJ GalonPas encore d'évaluation
- Slope Stabilization in Residual Soils of PeruDocument6 pagesSlope Stabilization in Residual Soils of PerurpazbPas encore d'évaluation
- ArticleDocument72 pagesArticleVictor OmotoriogunPas encore d'évaluation
- Chapter 6 - Fluid Mechanics - UpdatedDocument43 pagesChapter 6 - Fluid Mechanics - UpdatedMuhammad Aminnur Hasmin B. HasminPas encore d'évaluation
- EE-LEC-6 - Air PollutionDocument52 pagesEE-LEC-6 - Air PollutionVijendraPas encore d'évaluation
- Learning Objectives: Describe What Friction Is. (S6Fe-Iiia-C-1) Q3W1D1Document21 pagesLearning Objectives: Describe What Friction Is. (S6Fe-Iiia-C-1) Q3W1D1Gelyn Torrejos Gawaran100% (2)
- Ebook PDF Chemistry A Molecular Approach Second Canadian Edition PDFDocument41 pagesEbook PDF Chemistry A Molecular Approach Second Canadian Edition PDFkathleen.williams876100% (37)
- 氢燃料电池技术发展现状及未来展望 刘应都Document10 pages氢燃料电池技术发展现状及未来展望 刘应都1 1Pas encore d'évaluation
- Equipment Budget JustificationDocument4 pagesEquipment Budget Justificationapi-307945760Pas encore d'évaluation
- Slope Stability: Civil Engineering DeptDocument79 pagesSlope Stability: Civil Engineering DeptSEDIMPas encore d'évaluation
- IEEE 1125-1993 - SF6 Equipment - Dew PointDocument21 pagesIEEE 1125-1993 - SF6 Equipment - Dew Pointangela_pp100% (1)
- G4 Geology AssignmentDocument5 pagesG4 Geology AssignmentAmanuel AlemayehuPas encore d'évaluation
- Thermo - Anlytical Study of Linagliptin and EmpagliflozinDocument14 pagesThermo - Anlytical Study of Linagliptin and EmpagliflozinHeba Yousry100% (1)
- Science Week 2Document10 pagesScience Week 2Bon Grace TañalaPas encore d'évaluation
- Edited Light - Particle or A Wave ArticleDocument5 pagesEdited Light - Particle or A Wave Articleapi-253993915Pas encore d'évaluation
- X Ray CircuitsDocument9 pagesX Ray CircuitsNguyen Duc DungPas encore d'évaluation
- Theoretical Assessment For The Mixing Properties of Almg Liquid Alloys at 1073KDocument9 pagesTheoretical Assessment For The Mixing Properties of Almg Liquid Alloys at 1073KShayista AhmadPas encore d'évaluation
- Q3 LAW Science 9 WEEKS 3 4 1Document9 pagesQ3 LAW Science 9 WEEKS 3 4 1reynaldo antonioPas encore d'évaluation
- NA14631A - CristopiaDocument3 pagesNA14631A - CristopiaVietHienPas encore d'évaluation
- Solar Oriented Architecture: Guided By: Vinay JainDocument39 pagesSolar Oriented Architecture: Guided By: Vinay JainpallaviPas encore d'évaluation
- Topic 1: Major Bulk Organic Major Bulk Organic Chemicals From MethaneDocument24 pagesTopic 1: Major Bulk Organic Major Bulk Organic Chemicals From MethaneYong LiPas encore d'évaluation
- Ive + Ive: ThermodynamicsDocument10 pagesIve + Ive: ThermodynamicsAbbas HaiderPas encore d'évaluation
- HP Simplify Your Thermal Efficiency Calculation PDFDocument9 pagesHP Simplify Your Thermal Efficiency Calculation PDFGovind RaoPas encore d'évaluation
- 1PH0 1H MSC 20210211Document30 pages1PH0 1H MSC 20210211RandomPas encore d'évaluation
- Case Study of Forest Ecosystem: by Bunty SinghDocument7 pagesCase Study of Forest Ecosystem: by Bunty SinghBunty SinghPas encore d'évaluation
- PH1 G7 Humanities W2 Slide2 Ch1 Sec2 PDFDocument9 pagesPH1 G7 Humanities W2 Slide2 Ch1 Sec2 PDFhussienmadyanPas encore d'évaluation
- The Crust, Mantle, and CoreDocument30 pagesThe Crust, Mantle, and CoreKianPas encore d'évaluation
- Rotational MotionDocument39 pagesRotational MotionKhushal BhavsarPas encore d'évaluation
- Preparation of Diesel From Plastic WasteDocument13 pagesPreparation of Diesel From Plastic WasteAYUSH SINGHPas encore d'évaluation
- One-Dimensional Flow of Water Through Soils: ImportanceDocument26 pagesOne-Dimensional Flow of Water Through Soils: ImportanceAngiePas encore d'évaluation