Académique Documents
Professionnel Documents
Culture Documents
Lansium Domesticum: Skin and Leaf Extracts of This Fruit Tree Interrupt The Lifecycle of Plasmodium Falciparum, and Are Active Towards A Chloroquine-Resistant Strain of The Parasite (T9) in Vitro
Transféré par
Chikita Artia SariTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Lansium Domesticum: Skin and Leaf Extracts of This Fruit Tree Interrupt The Lifecycle of Plasmodium Falciparum, and Are Active Towards A Chloroquine-Resistant Strain of The Parasite (T9) in Vitro
Transféré par
Chikita Artia SariDroits d'auteur :
Formats disponibles
Journal of Ethnopharmacology 85 (2003) 145150
Lansium domesticum: skin and leaf extracts of this fruit tree interrupt the lifecycle of Plasmodium falciparum, and are active towards a chloroquine-resistant strain of the parasite (T9) in vitro
Donald T.T. Yapp , S.Y. Yap
The Institute of Health and Community Medicine, Universiti Malaysia Sarawak, 94300 Kota Samarahan, Sarawak, Malaysia Received 4 May 2002; received in revised form 27 November 2002; accepted 27 November 2002
Abstract Malaria remains a global problem in the light of chloroquine-resistant strains of Plasmodium falciparum. New compounds are needed for the development of novel antimalarial drugs. Seed, leaf, and fruit skin extracts of Lansium domesticum, a common fruit tree in South-East Asia, are used by indigenous tribes in Sabah, Malaysia for treating malaria. The skin and aqueous leaf extracts of the tree were found to reduce parasite populations of the drug sensitive strain (3D7) and the chloroquine-resistant strain (T9) of P. falciparum equally well. The skin extracts were also found to interrupt the lifecycle of the parasite. The data reported here indicate that extracts of L. domesticum are a potential source for compounds with activity towards chloroquine-resistant strains of P. falciparum. 2002 Elsevier Science Ireland Ltd. All rights reserved.
Keywords: Malaria; Plasmodium falciparum; T9; Lansium domesticum; Chloroquine resistance
1. Introduction Malaria is a global public health problem that primarily affects the people of the subtropical and tropical zones of the world. However, travelers to these endemic malaria zones are also at risk of contracting the disease. Global estimates of malaria deaths range from 0.7 to 2.7 million per year with over 75% of deaths occurring among children younger than 5 years in tropical Africa (Breman, 2001). In humans, the disease is caused by four species of the malaria parasite (Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, and Plasmodium ovale) of which P. falciparum is the most virulent and potentially deadly. Falciparum malaria is found throughout the tropics although infection in Africa tends to be endemic with high transmission rates while in South America, the Indian subcontinent, South-East Asia and China, transmission rates tend to be lower and seasonal in nature (Winstanley, 2000). The other three species are distributed unevenly throughout the tropical (and in the case of P. vivax, in some temperate) regions and do not generally cause severe disease although they are
Corresponding author. Present address: BCCRC, 601 West 10th Avenue, Vancouver, BC V5Z 1L3, Canada. Tel.: +1-608-267-1000; fax: +1-608-267-2275. E-mail address: dyapp@bccancer.bc.ca (D.T.T. Yapp).
often linked with anemia and low birth weights. In addition, P. vivax and P. ovale have liver forms which can cause relapses if left untreated, and P. malariae can persist in the blood for years if not eradicated completely (Gilles, 1993). Efforts to eradicate malaria began in the 1950s under the auspices of the WHO and were initially successful, but by the 1970s, the disease was resurgent again (Onori et al., 1993). Today, the eradication of malaria is one of the most pressing global public health issues (Winstanley, 2001). The reasons for the resurgence of malaria are complex and beyond the scope of this paper but one reason is the paucity of new antimalarial drugs that are both cheap and effective against drug-resistant parasite strains. 1.1. Drug resistance Many of the antimalarial drugs in widespread use today are losing their effectiveness in the face of multidrugresistant strains of Plasmodium that are becoming increasingly prevalent around the world (Hyde, 2002; Winstanley et al., 2002; Wongsrichanalai et al., 2002). Quinine, derived from the bark of the cinchona tree, was rst isolated in 1820 by Pelletier and Caventou (see Foley and Tilley, 1998 for historical review of the quinoline antimalarials) and is still used today for cases of severe chloroquine (CQ)-resistant malaria in Africa. The drug is not suitable for widespread
0378-8741/02/$ see front matter 2002 Elsevier Science Ireland Ltd. All rights reserved. doi:10.1016/S0378-8741(02)00375-6
146
D.T.T. Yapp, S.Y. Yap / Journal of Ethnopharmacology 85 (2003) 145150
use in third world countries due to its cost, dosing regime and toxicity, however, it remains useful as a second or third line drug in Africa but less so in South-East Asia due to widespread resistance in the region (Winstanley, 2001; Wongsrichanalai et al., 2002). CQ was rst introduced as an antimalarial drug in 1945 due to its potency, low cost, and ease of use but by 1957, resistance to CQ in Thailand was already being reported (Foley and Tilley, 1998). Today, there is widespread resistance to the drug in South-East Asia and in most parts of Africa although it is still widely used for economic reasons (Winstanley, 2001). The remaining cost-effective alternative to CQ is a combination of two drugssulfadoxine and pyrimethamine (PS), also known as Fansidar. Unfortunately, resistance to PS occurs very quickly, and resistance to PS is already well established in South-East Asia and in parts of Africa. Eight countries in Africa have switched to PS as the rst line drug against malaria and already failure rates of 2045% and 60% in rst and second clinical treatment, respectively, are already seen, and the drug is expected to become ineffective by 20062007 (Winstanley, 2001; Winstanley et al., 2002). The Chinese have used teas made from Artemisia annua L. (sweet or annual wormwood) to treat fevers and malaria for over 2000 years. The active component present in these teas is artemisinin, a highly potent compound that can reduce parasite loads within 48 h and has a short half life of 13 h when taken orally (van Agtmael et al., 1999). Artemisinins and its derivatives are also effective against drug-resistant malaria (Meshnick et al., 1996; Balint, 2001). The short half life of the artemisinins, however, leads to recrudescence rates of 4454% for 3-day dosing regimes, and this led to the suggestion that the artemisinins be used in combination with drugs with a long half life to obtain increased cure rates and to slow down the growth of drug resistance to the adjuvant drug (White and Olliaro, 1996; van Agtmael et al., 1999). The results of a long-term study on combination treatment with artesunate and meoquine have borne this out indicating that the artemisinins may perhaps best be used in combination with other drugs such as meoquine (Nosten et al., 2000). Unfortunately, meoquine is too expensive for widespread use and such combinations would therefore be of little practical use in third world countries. 1.2. Developing new antimalarial drugs The development of new, potent, and cheap antimalarials should be a global priority today if malaria is to be controlled in the near future. With the exception of the artemisinins and new combinations of existing drugs, there are few new antimalarial drugs being developed that are both cost-effective and have potent activity towards the drug-resistant variants of the parasite (Winstanley et al., 2002). The identication of natural product preparations used against malaria in traditional medicine is also likely to lead to compounds or classes of compounds that can be developed as new antiplasmodials
(Kirby, 1996, 1997). The chances of nding new drugs through investigation of natural products are good given the results of the pastabout 40% of all drugs approved between 1983 and 1994 were natural products or derived from natural products and on average, 70% of new antibacterial/anticancer drugs were derived solely from natural products (Cragg et al., 1997). The structural diversity afforded by natural products is also greater than that obtained from most combinatorial processes based on heterocyclic compounds which increases the chances of nding a compound with activity (Harvey, 1999). Lansium domesticum Corr., is a genus of small trees from the family Meliaceae found wild and cultivated in Malaysia and surrounding countries in the region (Thailand, Vietnam, Philippines, and Indonesia). The fruit is known locally as langsat, duku, dukulangsat, or dokong. The tree grows to a height of 4050 ft with long leaves that are pinnate, dark green, and glossy on the surface. The owers are found in clusters on the trunk and the older branches of the tree and tend to be small, eshy, white-yellow, and mostly bisexual. The fruit grows in clusters and is small and round with a diameter between 3 and 5 cm, with a yellow leathery skin that can be thick or thin depending on the variety. The esh of the fruit is juicy and translucent, and comes in ve or six segments with seeds contained in at least 13 segments (Morton, 1987). The fruit is produced commercially although it is not unusual for a household to have a langsat tree on their property. The fruit can be acidic or sweet depending on the variety and local growing conditions, and is generally eaten directly after peeling. The dried peel is also burnt as a mosquito repellent in parts of Java, and an arrow poison has reportedly been made from the fruit peel and bark of the tree (Morton, 1987). A survey carried out to determine the antimalarial measures taken by the indigenous lowland tribes of Sabah (North Borneo) indicated that the seeds, leaves, and bark of Lansium were used in the absence of standard antimalarial drugs (CoxSingh, unpublished data). As a result, we decided to examine the antimalarial activity of L. domesticum extracts in vitro to determine if these extracts had activity against the CQ-resistant strain of P. falciparum, T9. We report the results of this work here.
2. Methodology 2.1. Extraction of L. domesticum The seeds, leaves, and fruit skin of L. domesticum were washed, air dried, and pulverized before extraction with solvents. Each plant part was extracted sequentially with diethylether (Mallinkrodt), reagent grade methanol (R&M), and UHQ water (Elga Water Systems). Plant material (300350 g) was immersed in ether and left for 48 h at room temperature, and the resulting solutions concentrated in vaccuo to obtain a dark yellow oil (typical yields were in
D.T.T. Yapp, S.Y. Yap / Journal of Ethnopharmacology 85 (2003) 145150
147
the range 12.5%, w/w). The ether extracted material was air dried before extraction with the next solvent. A Soxhlet extractor apparatus was used for the methanol extraction; in a typical extraction, 350 g of plant material was extracted continuously with methanol for 68 h. The resulting solutions were then concentrated to an oil in vaccuo. Typical yields were in the range 34 g (0.91.1%, w/w). Aqueous extracts of the seed, skin, and leaves were obtained by infusion. Approximately 300 g of plant material (previously extracted with ether and methanol) was added to water (800 ml), and the suspension stirred for 68 h with gentle heating (<80 C). The resulting dark yellow solutions were frozen and lyophilized (Iishin, FD5525, Korea) to yield brown, hygroscopic powders. Typical yields obtained were between 2 and 3 g (0.71%, w/w). Methanol and ether extracts were stored at 20 C while the water extracts were stored in a dry box until required. A clean white powder was precipitated from ether skin extracts with methanol, and this was used in assays instead of the original crude ether extract. Stock solutions (1 mg/ml) of the water and methanol extracts were made in media and sterile ltered prior to use. However, the ether extracts were only sparingly soluble in aqueous media, and solutions of the extracts in DMSO precipitated when diluted in media. Saturated solutions of the ether extracts in media (nominally 1 mg/ml) were therefore prepared, centrifuged, and ltered to remove insoluble particles before dilution. The concentrations of the ether extracts reported in this work are, therefore, not known exactly. 2.2. In vitro culture of P. falciparum P. falciparum was cultured in vitro according to methods established previously (Trager and Jensen, 1976). Briey, parasites were grown in erythrocytes suspended in RPMI 1640 medium (Gibco-BRL) supplemented with human serum (10%), HEPES (25 mmol), and gentamicin (40 g/ml; Gibco-BRL). The blood cultures were maintained at 37 C in candle jar conditions and 10% haematocrit levels. Thin blood smears were prepared, xed with methanol, stained with Giemsa (10% in PBS), and examined by microscopy daily to monitor parasite populations. Culture media was changed daily and fresh blood was added to the wells to dilute parasite populations as required. Excess parasite cultures were frozen in a glycerol/mannitol solution (28% glycerol, 3% mannitol, w/v in 0.9% NaCl solution) and stored at liquid nitrogen vapor temperatures for future use. 2.3. Parasite synchronization When parasite cultures were established, the parasites lifecycle was synchronized as described previously (Lambros and Vanderberg, 1979). Infected red blood cells were washed with RPMI 1640 media and suspended in an aqueous solution of d-sorbitol (Sigma, 5% w/v). Under
these conditions, red blood cells containing mature trophozoites and schizonts were lysed, effectively killing the parasite within. Only erythrocytes infected with ring forms or young trophozoites survived the treatment. The parasite cultures were synchronized 23 times over several lifecycles. Thin blood smears were obtained daily, and the parasite forms examined for complete synchronization (>90% of one particular life cycle stage). Synchronized parasite cultures were used for all assays described in this work. 2.4. In vitro assays The antiparasitical effects of the extracts were determined using a modication of the in vitro microtechnique described previously (Rieckmann et al., 1978; Ang et al., 1995). Briey, infected erythrocytes (50 l, 0.2% parasitemia) suspended in media supplemented with gentamicin and human serum (20%) were added to test solutions (50 l) in a 96 well microplate; parasitemia (%P) and haematocrit in nal solutions were 0.1 and 10%, respectively. Test solutions consisted of extract dissolved in RPMI 1640 media and sterilized by ltration. Final extract concentrations in the well were 400 g/ml with the exception of extract SKE where the concentration was unknown (see Section 2.1). Control solutions containing no extract or CQ (20 nmol) were also included as part of the assay. The parasites were then incubated in test solutions at 37 C under candle jar conditions for 48 h. Thin blood smears were made and stained with Giemsa. The degree of parasitemia was determined by examining a minimum of 2000 erythrocytes for infection by parasites using microscopy. The parasitemia was determined from the number of parasites in 2000 erythrocytes, normalized to control cultures. 2.5. Life cycle effects In order to determine the effect of the extracts on the parasites life cycle, the ratio of rings, trophozoites and schizonts in T9 cultures exposed to extracts were determined, and the results compared with those from control cultures containing media or CQ (30 nmol). Extract concentrations of 1 mg/ml were used except in the case of ether extracts where a saturated solution was used. The experiments were carried out in 12 well plates with higher %P (4%) and larger solution volumes (1 ml) than in the in vitro assays but otherwise the procedure remained the same. Thin blood smears were taken, xed, and stained with Giemsa every 24 h for 3 days. Lifecycle stages in parasite cultures were examined, and their ratios calculated daily based on examination of >100 infected erythrocytes per slide. The ratio of rings to trophozoites and schizonts in the presence of media, CQ (30 nmol), SKW, and SKE, are shown in Fig. 2ad, respectively. The %P is shown as a line above the bars. The purpose of this experiment was to examine changes in the life cycle stages so the initial parasitemia was higher than that used to determine extract activity (4 vs. 0.1%P, respectively). Thus
148
D.T.T. Yapp, S.Y. Yap / Journal of Ethnopharmacology 85 (2003) 145150
a higher concentration of CQ was used (30 nmol) to elicit a dose response, and the reported changes in %P are not necessarily indicative of the extracts potency. After 72 h, the parasites were collected and washed with media to remove test solutions. Fresh erythrocytes were added to reduce the %P (to 2%), the cultures resuspended in fresh media without extract, and returned to candle jar conditions. The %P of the various cultures were measured daily for another 3 days to determine if the parasites were still viable following exposure to the skin extracts.
3. Results and discussion 3.1. Extract activity The fruits of L. domesticum were purchased from local markets, and the leaves obtained from a tree belonging to a local land owner. Dried samples of the fruit skin, seed, and leaves have been deposited in the Institute of Health and Community Medicine. Nine extracts were obtained from the seeds, leaves, and fruit skins of L. domesticum and tested for antiplasmodial activity (data not shown). This preliminary assay identied four extracts (leafmethanol, leafwater, skinwater and skinether; LEM, LEW, SKW, and SKE, respectively) that reduced parasite populations in vitro (3D7, drug sensitive) by more than 50%. The four extracts were therefore studied in greater detail, and the results shown in Fig. 1.
The difference in response between the two strains of P. falciparum, 3D7 and T9 to CQ is evident. 3D7 parasite populations are lowered by approximately 50% more than T9 parasite populations, reecting the intrinsic resistance of T9 parasites to CQ. The data for CQ was included to show the difference in response between 3D7 and T9 to CQ. Unfortunately no comparison can be drawn between this potent antimalarial and the extracts reported since the former is a pure compound while the latter are crude mixtures. At concentrations of 400 g/ml (see Fig. 1), extract SKW was most effective against both strains of P. falciparum (3D7 and T9). Parasite response to LEW was similar but the extract was slightly less effective than SKW. Antiplasmodial effects were also observed with extract LEM although the T9 strain was less sensitive to the extract than 3D7. The concentration of SKE is unknown (see Section 2.1) but is considerably less than 400 g/ml. However, its activity towards both parasite strains appears to be just as effective as the other extracts. 3.2. Effect of the skin extracts on the parasites life cycle The effect of SKE on the parasites life cycle was rst observed during the in vitro assays; parasite cultures that had been exposed to the skin extracts appeared to have a high number of tight rings compared to control cultures and cultures exposed to leaf and seed extracts. Therefore an attempt to quantify this observation was made using T9 cultures as described in Section 2.5. The results of this experiment are shown in Fig. 2, and are quite startling for extract SKE, less so for SKW. Parasites were synchronized and experiments were started when >90% of the parasites were in the young trophozoite form. The parasite populations cultured in media alone and CQ solutions have similar distributions of blood stage life forms reecting the normal development of the parasite over time. The slight dip in parasite population after 48 h and subsequent recovery in the CQ solutions reects the resistance of the T9 strain to CQ, and is not unexpected. The population proles of cultures exposed to both skin extracts are however quite different. The parasite population falls steadily while the percentage of rings increases with time. In the presence of SKW, the change is gradual, taking up to 72 h before the ratio of rings reaches 80%; however, in the presence of SKE the change is much more dramaticalmost 90% of the parasite life forms are present as rings after 24 h exposure to SKE. These tight rings were not viable despite replacement of the extract solution with fresh media and dilution with fresh erythrocytes. The parasite population in control and CQ solutions treated this way rebound whereas the parasites exposed to skin extracts remained mordant. At present, there is no explanation for why the parasites become such tight rings following exposure to the extracts. Presumably this is one result of the extracts active component(s) but until the active ingredient is isolated and studied, no conclusions concerning its mode of
Fig. 1. The effect of L. domesticum extracts LEM (leafmethanol), LEW (leafwater), SKW (fruit skinwater) and SKE (fruit skinether) on the WHO drug-sensitive and CQ-resistant strains of P. falciparum, 3D7 () and T9 (), respectively. Extract concentrations are 400 g/ml except for those SKE, which is unknown. Control consists of media alone without extract. () CQ concentration is 20 nmol. All data points are the average of three experiments and error bars indicate the standard deviation.
D.T.T. Yapp, S.Y. Yap / Journal of Ethnopharmacology 85 (2003) 145150
149
Fig. 2. Changes in the lifecycle of the parasite (T9) with time. The bars indicate the percentage of rings, trophozoites, and schizonts (black, blank, and striped bars, respectively) found in each culture with time. The change in %P (normalized to control wells) with time is indicated in the line above the bars. Control cultures contain media without extract and 30 nmol CQ (a and b, respectively). Test solutions contain SKW (1 mg/ml) and SKE (unknown concentrations; c and d, respectively).
action can be made. The results, however general, are encouraging as they show compounds present in the fruit skin of L. domesticum are as active towards CQ-resistant strains of P. falciparum as to drug sensitive strains. Although the data presented here are preliminary, it is clear that the leaves and fruit skin of L. domesticum contain compounds that, either singly or in combination, have antimalarial properties against the CQ-resistant strain of P. falciparum, T9. The current work in our laboratories is focused on fractionation of the extracts and isolating groups of compounds/pure compounds to determine the active component responsible for the extracts antiplasmodial activity. Recently, we reported the isolation of a cyclic amino acid (n-methyl-4-hydroxyproline from methanolic extracts of the fruit skin (Yapp et al., 2002) although the compound itself only exhibited antiplasmodial activity at concentrations greater than 1 mg/ml. There are few other reports on compounds isolated from L. domesticum and none on its use for the treatment of malaria. Nishizawa et al. (1988, 1989) have reported the isolation of three tetranotriterpenoids from the seed (1988), and new cycloartanoid triterpene compounds from the leaves of the plant (1989). The latter compounds also showed inhibitory effects on skin tumor promoters in vitro but none have been tested for antiplasmodial activity in vitro.
4. Conclusion This paper is, to the best of our knowledge, the rst to verify that L. domesticum has antiplasmodial activity, and was initiated by reports on the use of L. domesticum to combat episodic fevers and malaria by indigenous peoples of Sabah, Malaysia. The work presented here is a good example of how indigenous knowledge of plants with medicinal qualities can lead to potentially useful compounds. Our data show that the extracts of the leaves and skin exhibit activity towards the P. falciparum, and of particular interest, are active towards a CQ-resistant strain of the parasite, T9. Acknowledgements This work was funded in part by the Malaysian Ministry of Science (IRPA), The International Foundation for Science (IFS), and Universiti Malaysia Sarawak. References
Ang, H.C., Chan, K.L., Mak, J.W., 1995. Quantitative assessment of antimalarial activities from Malaysian Plasmodium falciparum isolates by modied in vitro microtechnique. Antimicrobial Agents and Chemotherapy 39, 626628.
150
D.T.T. Yapp, S.Y. Yap / Journal of Ethnopharmacology 85 (2003) 145150 leaves of Lansium domesticum: novel skin-tumor promotion inhibitors. Tetrahedron Letters 30, 56165618. Nosten, F., van Vugt, M., Price, R., Luxemburger, C., Thway, K.L., Brockman, A., McGready, R., ter Kuile, F., 2000. Effects of artesunatemeoquine combination on incidence of Plasmodium falciparum malaria and meoquine resistance in Western Thailand: a prospective study. Lancet 356, 297302. Onori, E., Beales, P.F., Gilles, H.M., 1993. From malaria eradication to malaria control: the past, the present and the future. In: Gilles, H.M., Warrell, D.A. (Eds.), Bruce-Chwatts Essential Malariology, 3rd ed. Edward Arnold, Great Britain, pp. 267282. Rieckmann, K.H., Campbell, G.H., Sax, L.J., Mrema, J.E., 1978. Drug sensitivity of Plasmodium falciparum: an in vitro microtechnique. The Lancet January 7, 2223. Trager, W., Jensen, J.B., 1976. Human malaria parasites in continuous culture. Science 193, 673675. van Agtmael, M.A., Eggelte, T.A., Boxtel, C.J., 1999. Artemesinin drugs in the treatment of malaria: from medicinal herb to registered medication. Trends in Pharmacological Sciences 20, 199205. White, N.J., Olliaro, P.L., 1996. Strategies for the prevention of antimalarial drug resistance: rationale for combination chemotherapy for malaria. Parasitology Today 12, 399401. Winstanley, P.A., 2000. Chemotherapy for falciparum malaria: the armoury, the problem and the prospects. Parasitology Today 16, 146 153. Winstanley, P.A., 2001. Modern chemotherapeutic options for malaria. The Lancet Infectious Diseases 1, 242250. Winstanley, P.A., Ward, S.A., Snow, R.W., 2002. Clinical status and implications of antimalarial drug resistance. Microbes and Infection 4, 157164. Wongsrichanalai, C., Pickard, A.L., Wernsdorfer, W.H., Meshnick, S.R., 2002. Epidemiology of drug resistant malaria. Lancet Infectious Diseases 2, 209218. Yapp, D.T.T., Steed, J., Yap, S.Y., Houghton, P.J., 2002. The isolation and structural characterization of n-methyl-4-hydroxyproline from the fruit skin of Lansium spp. Crystallographic Acta E 58, 988989.
Balint, G.A., 2001. Artemisinin and its derivatives: an important new class of antimalarial agents. Pharmacology and Therapeutics 90, 261265. Breman, J.G., 2001. The ears of the hippopotamus: manifestations, determinants, and estimates of the malaria burden. American Journal of Tropical Medicine and Hygiene 64, 111. Cragg, G.M., Newman, D.J., Snader, K.M., 1997. Natural products in drug discovery and development. The Journal of Natural Products 60, 5260. Foley, M., Tilley, L., 1998. Quinoline antimalarials: mechanisms of action and resistance and prospects for new agents. Pharmacology and Therapeutics 1, 5587. Gilles, H.M., 1993. The malaria parasites. In: Gilles, H.M., Warrell, D.A. (Eds.), Bruce-Chwatts Essential Malariology, 3rd ed. Edward Arnold, Great Britain, pp. 1234. Harvey, A.L., 1999. Medicines from nature: are natural products still relevant to drug discovery? Trends in Pharmacological Sciences 20, 196198. Hyde, J.E., 2002. Mechanisms of resistance of Plasmodium falciparum to antimalarial drugs. Microbes and Infection 4, 165174. Kirby, G.C., 1996. Medicinal plants and the control of parasites. Transactions of the Royal Society of Tropical Medicine and Hygiene 90, 60609. Kirby, G.C., 1997. Plants as a source of antimalarial drugs. Tropical Doctor 27, 711. Lambros, C., Vanderberg, J.P., 1979. Synchronization of Plasmodium falciparum erythrocyte stages in culture. Journal of Parasitology 65, 418420. Meshnick, S.R., Taylor, T.E., Kamchonwongpaisan, S., 1996. Artemesinin and the antimalarial endoperoxides: from herbal remedy to targeted chemotherapy. Microbiology Reviews 60, 301315. Morton, J., 1987. Fruits of Warm Climates. J.F.M., Miami, p. 201. Nishizawa, M., Nademoto, Y., Sastrapradja, S., Shiro, M., Hayahi, Y., 1988. Dukunolide D, E and F: new tetranortriterpenoids from the seeds of Lansium domesticum. Phytochemistry 27, 237239. Nishizawa, M., Emura, M., Yamada, H., Shiro, M., Chairul, , Yuji, H., Tokuda, H., 1989. Isolation of a new cycloartanoid triterpene from
Vous aimerez peut-être aussi
- Retinopathy of PrematurityDocument20 pagesRetinopathy of PrematurityChikita Artia SariPas encore d'évaluation
- Retinal DetachmentDocument14 pagesRetinal DetachmentChikita Artia Sari100% (1)
- Hypertensive RetinopathyDocument27 pagesHypertensive RetinopathyChikita Artia SariPas encore d'évaluation
- Optic NeuritisDocument25 pagesOptic NeuritisChikita Artia SariPas encore d'évaluation
- Cover 2Document1 pageCover 2Chikita Artia SariPas encore d'évaluation
- EPISCLERITIS & ScleritisDocument14 pagesEPISCLERITIS & ScleritisChikita Artia SariPas encore d'évaluation
- Case Report Mature Cataract: Created By: Chikita Artia Sari I 11109014Document1 pageCase Report Mature Cataract: Created By: Chikita Artia Sari I 11109014Chikita Artia SariPas encore d'évaluation
- ROP Paeds PresentDocument16 pagesROP Paeds PresentChikita Artia SariPas encore d'évaluation
- Photophobia in Viral Conjunctivitis: Homework Ophtalmology DepartementDocument1 pagePhotophobia in Viral Conjunctivitis: Homework Ophtalmology DepartementChikita Artia SariPas encore d'évaluation
- Metoprolol Succinate Therapy Associated With Erythema MultiformeDocument2 pagesMetoprolol Succinate Therapy Associated With Erythema MultiformeChikita Artia SariPas encore d'évaluation
- Determination of Statement of Principles Concerning PHOTOCONTACT DERMATITISDocument3 pagesDetermination of Statement of Principles Concerning PHOTOCONTACT DERMATITISChikita Artia SariPas encore d'évaluation
- Varicella Zooster VirusDocument9 pagesVaricella Zooster VirusChikita Artia SariPas encore d'évaluation
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (588)
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5795)
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (895)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (345)
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (400)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (74)
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (266)
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (121)
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)
- Ashkenazi Jewish ScreeningDocument10 pagesAshkenazi Jewish ScreeningJorge SHPas encore d'évaluation
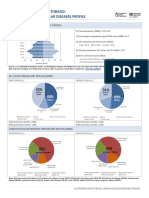
- Trinidad and Tobago: Cardiovascular Diseases ProfileDocument2 pagesTrinidad and Tobago: Cardiovascular Diseases ProfileKay BristolPas encore d'évaluation
- Meditation Its EffectDocument11 pagesMeditation Its Effectmanisami7036Pas encore d'évaluation
- Tracheostomy Tube Brochure - 086034Document4 pagesTracheostomy Tube Brochure - 086034adelin litanPas encore d'évaluation
- Raasn October NewsletterDocument6 pagesRaasn October Newsletterapi-231055558Pas encore d'évaluation
- End-Stage Heart DiseaseDocument44 pagesEnd-Stage Heart DiseaseCyrille AgnesPas encore d'évaluation
- Clinical Guideline For Management of Gallstones Pathology in AdultsDocument11 pagesClinical Guideline For Management of Gallstones Pathology in AdultsrintyosoPas encore d'évaluation
- Transitional CareDocument8 pagesTransitional CareJayvee FerandezPas encore d'évaluation
- Ace ArsenalDocument11 pagesAce ArsenalJustus WeißePas encore d'évaluation
- HIV and PregnancyDocument9 pagesHIV and PregnancyUm HamoOdPas encore d'évaluation
- Hybrid Hues 2008Document196 pagesHybrid Hues 2008Kulsharma100% (1)
- Basics of Pharmacology: Speaker - DR - SantoshDocument76 pagesBasics of Pharmacology: Speaker - DR - SantoshRamya ChallaPas encore d'évaluation
- Bell's Palsy Treatments & Medications - SingleCareDocument11 pagesBell's Palsy Treatments & Medications - SingleCareRoxan PacsayPas encore d'évaluation
- My First Authored PublicationDocument16 pagesMy First Authored PublicationSamuel UzonduPas encore d'évaluation
- Felicias Resume 1Document1 pageFelicias Resume 1api-357153848Pas encore d'évaluation
- Proposal For Defense Group 3Document77 pagesProposal For Defense Group 3Kristiene Kyle AquinoPas encore d'évaluation
- Palpation and Assessment SkillsDocument305 pagesPalpation and Assessment SkillsElin Taopan97% (34)
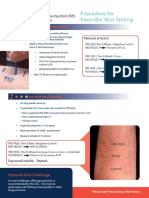
- Procedure For Penicillin Skin TestingDocument4 pagesProcedure For Penicillin Skin TestingHarshan Isuru KumaraPas encore d'évaluation
- HR PWDDocument13 pagesHR PWDDiane UyPas encore d'évaluation
- E B I W: Safemedicate Rounding Rules GuidelinesDocument9 pagesE B I W: Safemedicate Rounding Rules Guidelineslesky17Pas encore d'évaluation
- The PRISMAFLEX System: Making Possible PersonalDocument12 pagesThe PRISMAFLEX System: Making Possible PersonalluisfulaPas encore d'évaluation
- Induction Update2Document30 pagesInduction Update2yusepPas encore d'évaluation
- Hepar Sulph - The Homeopathic AntibioticDocument54 pagesHepar Sulph - The Homeopathic AntibioticGer DersenPas encore d'évaluation
- By - Derina FixDocument1 pageBy - Derina FixSellyta HasugianPas encore d'évaluation
- RetailerPriceList OHDocument1 pageRetailerPriceList OHMuhammad Samiur RahmanPas encore d'évaluation
- MCQ Thoracic SurgeryDocument5 pagesMCQ Thoracic SurgeryKhamis Belal100% (6)
- Nursing Group Research PaperDocument16 pagesNursing Group Research Paperapi-368267454Pas encore d'évaluation
- OT HoppysAppReviewDocument1 pageOT HoppysAppReviewIris De La CalzadaPas encore d'évaluation
- PRICE - LIST - Price List 2023 - Price List Bbraun-DNR Reguler - Basic - Care - 2023Document2 pagesPRICE - LIST - Price List 2023 - Price List Bbraun-DNR Reguler - Basic - Care - 2023yu2nwahyuni.aptPas encore d'évaluation
- Lipoxome Business PresentationDocument20 pagesLipoxome Business PresentationCaerwyn AshPas encore d'évaluation