Académique Documents
Professionnel Documents
Culture Documents
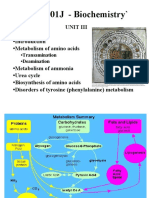
Mind Map: Amino Acid Metabolism
Transféré par
Abbey AyalaCopyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Mind Map: Amino Acid Metabolism
Transféré par
Abbey AyalaDroits d'auteur :
Formats disponibles
Tissue-specific metabolism
Liver
Main site of AA degradation
(deamination) & urea synthesis for
nitrogen excretion
During fasting, = main site of
gluconeogenesis, using carbon skeletons from
AAs
Major role in synthesis of
tripeptide glutathione
lntestine
Enterocytes take up glutamine &
metabolise it to alanine to produce
energy
Enterocytes = only cells to contain
glutamate reductase, the synthetic enzyme
for citrulline
Citrulline from gut metabolised to arginine in
liver, then to ornithine to increase capacity of
urea cycle during periods of increased protein
uptake
Skeletal muscle
Broken down during starvation
for gluconeogenesis in liver
Main AAs released = alanine &
glutamine
Alanine transported by blood to
liver for deamination &
gluconeogenesis
Glutamine taken up by
enterocytes for energy, & released
as alanine
Renal Cortex
Only tissue other than liver to perform
gluconeogenesis: capacity of up to 10& of total
glucose generation
Converts citrulline via arginine to creatine, used
by skM to store high-energy phosphate bonds
as creatine phosphate
Creatine phosphate
spontaneously forms creatine
Creatine excreted by kindeys;
blood level used to assess renal
function
Major site of carnitine
synthesis (with liver to lesser
extent) Carnitine used in FA B-oxidation
Urea Cycle
Under all conditions, body needs
to excrete amino groups (ie.
ammonia)
On Western diet, protein intake
generally >> need
Cannot store excess AAs
Use/store carbon skeletons as energy
source; excrete unwanted amino
groups
lf protein intake << need
Protein catabolism occurs to
free carbon skeletons for
energy
Excess amino groups need to
be excreted
Free ammonia toxic: blood levels
need to be kept low
(25-40microM)
lf ammonium levels rise, NH4+ reacts
with alpha-ketoglutarate to form
glutamate
At high levels in brain, this reduces
rate of ATP formation, starving cells of
energy
Healthy adults in 'nitrogen balance'
~80% of excess nitrogen excreted as
urea; remainder as free NH4+ &
creatine
Most urea synthesised by liver; rate
strictly controlled to prevent ammonia
build-up
Urea used in kidney as part of
urinary concentrating
mechanism
Urea cycle describes formation of urea from one
free ammonium ion & one donated from
aspartate
Occurs partly in mitochondrial
matrix, partly in cytoplasm
lnvolves 2 AAs not found in
proteins: ornithine & citrulline
Control
Acute Via Carbamoyl-phosphate synthetase
Regulated by [N-acetyl glutamate], formed by
N-acetyl glutamate synthase, stimulated by urea
cycle intermediate arginine
Chronic
Enzymes induced over 24-36hrs in
response to increased ammonia levels in
liver cells
Ammonia levels can vary 10-20-fold with diet &
under starvation conditions when muscle broken
down
Under prolonged starvation conditions, ability
for enzyme (protein) synthesis may be
compromised
Protein from diet (~70g/day)
broken down by sequential protein
digestion
Enzymes of protein degradation
= peptidases
From Stomach Pepsin
Secreted by chief cells of stomach as
pepsinogen, activated by pH<5 or by active
pepsin
Cleave internal peptide
bonds to release large
peptide fragments
Peptide fragments in duodenum stimulate
CCK production, stimulating pancreatic
secretion
Stomach acidity denatures proteins,
makes more susceptible to
hydrolysis
From Pancreas
Pancreatic secretion contains various
proteolytic enzymes active at neutral
pH:
Endopeptidases (serine proteases)
Trypsin
Chymotrypsin
Elastase
Exopeptidases
Carboxypeptidases A & B Remove last AA from peptide chain
Aminopeptidases Remove first AA from peptide chain
Produce mixture of AAs & small
peptides up to 6AAs long
(oligopeptides)
Neutral intestinal lumen achieved by secretion
of bicarbonate-rich pancreatic juice to
neutralise gastric acid
All proteases secreted as inactive precursors,
'zymogens': trypsinogen, chymotrypsinogen,
proelastase & procarboxypeptidase A & B
Trypsin activated by enteropeptidase from
enterocytes of small intestine, & by active
trypsin
Trypsin activates all other
proteolytic enzymes
Diseases eg. pancreatitis, Cystic Fibrosis, which
interfere with pancreatic secretions, prevent full
protein digestion --> malabsorption &
malnutrition
Overcome by supplying preparations of
exogenous pancreatic enzymes, or dietary
supplements of easily-digested proteins
Lumenal (Brush-border) membrane of
enterocytes contain enzymes that continue
digestion
Endopeptidases, aminopeptidases &
dipeptidases continue digestion to dipeptides,
tripeptides & free AAs
Mixture of di- & tri-peptides &
AAs taken up by enterocytes
Proton-couple transporter for peptides
Na-coupled transporter for AAs
lntracellularly, any
peptides broken down to
free AAs
AAs leave enterocytes via
basolateral membrane & enter
circulation
Some hydrolysis-resistant
peptides (& antibiotics) may
leave cell intact
Amino Acids
Essential AAs: Those the body
cannot make
Arginine, Histidine, lsoleucine, Leucine, Lysine,
Methionine, Phenylalanine, Threonine,
Tryptophan, Valine
'Private Tim Hill,' PVT TlM HLL'
Are His lsolated Looks Lies?
Merely Philosophies, Trying
Valiantly.
Arginine can be synthesised in
body, but not in large enough
quantities
Dietary lack of an essential AA -->
inability to synthesise proteins
containing
Non-essential: can be made by body
Alanine, aspartate, asparagine, cysteine,
glutamate, glutamine, glycine, proline, serine,
tyrosine
Variety of uses/fates:
Protein synthesis
Hormones, eg. adrenaline
Neurotransmitters, eg. 5-HT
Deaminated
Carbon skeleton oxidised in TCA cycle
Carbon skeleton converted to
glucose via gluconeogenesis Possible for 'glucogenic' AAs
Via pyruvate
Alanine, Glycine, Cysteine,
Serine, Threonine
Via TCA intermediates oxaloacetate,
fumarate, succinate & alpha-ketoglutarate
Asparagine & Aspartate -->
oxaloacetate
Carbon skeleton converted to
acetyl CoA or acetoacetyl CoA
for FA synthesis Possible for 'ketogenic' AAs Only leucine & lysine solely ketogenic
Breakdown Breakdown Breakdown Breakdown Oxidation
Excess AAs cannot be stored:
carbon skeletons used as energy
source
Transamination
Aminotransferases (transaminases) catalyse
transfer of NH3+ from AA to an alpha-ketoacid:
pyruvate, oxaloacetate, or - usually - alpha-ketoglutarate
New amino acid & new ketoacid formed
lf acceptor = a-ketoacetate, forms glutamate:
provides pool of amino groups for making other
non-essential AAs, or for deamination
lf original amino acid = alanine,
glycine etc, ketoacid produced =
pyruvate
Each AA has own specific
aminotransferase
Reactions easily reversible,
require to energy input
Deamination
Glutamate deaminated by
glutamate dehydrogenase
Pooling of excess amino groups in
glutamate means only 1 deamination
pathway required
Deamination pathway regenerates alpha-ketoglutarate
& free ammonium (NH4), & an
NADH
Allosterically stimulated by increased ADP &
GDP: these signal that AAs required as energy
source. lnhibited by ATP/GTP
Occurs in mitochondria of liver cells
Major fate of NH4+ =
incorporation into urea by
liver as soluble, non-toxic
way of eliminating excess
ammonium
Renal cortex can also deaminate
glutamate
Ammonium used to assist with
acidifying urine
Mechanism conserves bicarbonate, which
would otherwise need to be used in urea
synthesis
Other sites of ammonium
production include:
Brain
Breakdown of GABA to succinate
& NH4+
Ammonium ion combined with alpha-ketoglutarate
to produce glutamate, which is
transported to liver for deamination & urea
production
Muscle
Natural protein turnover, muscle catabolism in
starvation & breakdown of excess ADP during
severe exercise
Ammonium ion + alpha-ketoglutarate
--> glutamate
Glutamate used to transaminate pyruvate,
forming alanine & regenerating alpha-ketoglutarate
Alanine released into
bloodstream & taken up by liver
Deamination of alanine releases pyruvate
for oxidation in TCA cycle or
gluconeogenesis
lntestinal Cells Glutamine used as energy source
Amino Acid Metabolism Amino Acid Metabolism Amino Acid Metabolism Amino Acid Metabolism
Vous aimerez peut-être aussi
- Health Benefits of Cascade Fermented Foods - "You'll Just Have To Live With It!" (No Thank You!)Document105 pagesHealth Benefits of Cascade Fermented Foods - "You'll Just Have To Live With It!" (No Thank You!)BiologicalMed99100% (4)
- Med Surg Test 4 Study GuideDocument29 pagesMed Surg Test 4 Study GuideJessPas encore d'évaluation
- Mercury Dental Amalgams: The Controversy Continues by Gary Null, PH.D., and Martin Feldman, M.D.Document55 pagesMercury Dental Amalgams: The Controversy Continues by Gary Null, PH.D., and Martin Feldman, M.D.Gary NullPas encore d'évaluation
- Metabolism of ProteinsDocument50 pagesMetabolism of ProteinsAbdur RehmanPas encore d'évaluation
- Amino Acid Catabolism Between OrgansDocument44 pagesAmino Acid Catabolism Between OrgansFarhati MardhiyahPas encore d'évaluation
- Lectures 1 3 Handout For PrintingDocument43 pagesLectures 1 3 Handout For Printingkriss Wong100% (2)
- Integration of Metabolism Integration of MetabolismDocument10 pagesIntegration of Metabolism Integration of MetabolismEdison LucianoPas encore d'évaluation
- AVS 172 Reproductive Physiology: Amin Ahamdzadeh Department of Animal and Veterinary Science University of IdahoDocument41 pagesAVS 172 Reproductive Physiology: Amin Ahamdzadeh Department of Animal and Veterinary Science University of IdahoCristina CarvalhoPas encore d'évaluation
- HW - Carbohydrate Metabolism II & Lipid MetabolismDocument2 pagesHW - Carbohydrate Metabolism II & Lipid MetabolismyanPas encore d'évaluation
- Lipid Profiles ExplainedDocument5 pagesLipid Profiles ExplainedFahd Abdullah Al-refaiPas encore d'évaluation
- EnzymesDocument21 pagesEnzymesCatherine Merilleno100% (1)
- Cholesterol SynthesisDocument16 pagesCholesterol Synthesispriya19866100% (1)
- Enzymes: Protein Catalysts That Increase The Rate of Reactions Without Being Changed in The Overall ProcessDocument49 pagesEnzymes: Protein Catalysts That Increase The Rate of Reactions Without Being Changed in The Overall ProcessGhafoor AzamPas encore d'évaluation
- Biochemistry of Digestive SystemDocument55 pagesBiochemistry of Digestive SystemSyam UnhasPas encore d'évaluation
- Glycolysis: Shekhar Chandra Yadav Lecturer Dept. of BiochemistryDocument25 pagesGlycolysis: Shekhar Chandra Yadav Lecturer Dept. of BiochemistryTULSI SHARMAPas encore d'évaluation
- Biochemistry Lecture 2 Cell and OrganellesDocument16 pagesBiochemistry Lecture 2 Cell and OrganellesProfessor Rakesh Sharma Biochemistry LecturesPas encore d'évaluation
- Enzymes and Their Importance in Plants and AnimalsDocument4 pagesEnzymes and Their Importance in Plants and Animalsanili50% (2)
- ChromosomesDocument9 pagesChromosomestayyabaPas encore d'évaluation
- 4 Nutrition and Metabolism Lipid-Updated (Compatibility Mode)Document45 pages4 Nutrition and Metabolism Lipid-Updated (Compatibility Mode)Anh Nguyen100% (1)
- Micronutrients in health and disease reviewDocument10 pagesMicronutrients in health and disease reviewLeonard LeonardPas encore d'évaluation
- Carbohydrates Slide-2 PHARM-D, SALUDocument85 pagesCarbohydrates Slide-2 PHARM-D, SALUShahid AhmedPas encore d'évaluation
- Avishkar 2019 PosterDocument1 pageAvishkar 2019 PosterVedant 23 GooglePas encore d'évaluation
- ProteinDocument39 pagesProteinNICHOLE MOJELLO100% (1)
- Endocrine Disruptors and Hormonal CancerDocument8 pagesEndocrine Disruptors and Hormonal CancerPol MaliaPas encore d'évaluation
- The Urea Cycle: A 40-Character SummaryDocument8 pagesThe Urea Cycle: A 40-Character SummaryManohar PattarPas encore d'évaluation
- CARBOHYDRATES Biochem Pre-LabDocument50 pagesCARBOHYDRATES Biochem Pre-LabGlen MangaliPas encore d'évaluation
- Classification of Genetic DiseasesDocument8 pagesClassification of Genetic DiseasesTyn TynPas encore d'évaluation
- Digestive System DisordersDocument11 pagesDigestive System DisordersDanie CabahugPas encore d'évaluation
- Mutations: Causes, Types and Genetic DisordersDocument18 pagesMutations: Causes, Types and Genetic DisordersSushanthPas encore d'évaluation
- Acid-Base Balance and DisodersDocument86 pagesAcid-Base Balance and DisodersPrincewill SeiyefaPas encore d'évaluation
- Reactions of MonosaccharidesDocument15 pagesReactions of Monosaccharidesvishnudurga88% (8)
- ACFrOgB4ugyviBu1XK4Lh1UX8Pt64wVQwa2 Exi6l8nAFhE Uu1QLN5OIKowfbqMbZ48dVUAL2yq7eDi4HBBlOHGoCWigzrxxjl305MoZfTvJxd54XHQjc6yi-YzD8c PDFDocument12 pagesACFrOgB4ugyviBu1XK4Lh1UX8Pt64wVQwa2 Exi6l8nAFhE Uu1QLN5OIKowfbqMbZ48dVUAL2yq7eDi4HBBlOHGoCWigzrxxjl305MoZfTvJxd54XHQjc6yi-YzD8c PDFLpPas encore d'évaluation
- Food HygieneDocument314 pagesFood Hygienefeyisa100% (2)
- Subject: Biochemistry Topic:Lipid Metabolism 2 Lecturer: Dr. Laygo DATE: NOV. 2010Document11 pagesSubject: Biochemistry Topic:Lipid Metabolism 2 Lecturer: Dr. Laygo DATE: NOV. 2010Std DlshsiPas encore d'évaluation
- Hexose Monophosphate Shunt CHEM3119"TITLE"Biometabolism Lecture on HMP Shunt and G6PD DeficiencyDocument18 pagesHexose Monophosphate Shunt CHEM3119"TITLE"Biometabolism Lecture on HMP Shunt and G6PD DeficiencyAbdul Jabbar Abdul JabbarPas encore d'évaluation
- Dna and Rna Powerpoint 2Document46 pagesDna and Rna Powerpoint 2api-267309851Pas encore d'évaluation
- CarbohydratesDocument156 pagesCarbohydrateseiddnewPas encore d'évaluation
- Color Reactions of ProteinsDocument1 pageColor Reactions of ProteinsZarah Pauline JimenezPas encore d'évaluation
- 6.1 Types of NutritionDocument11 pages6.1 Types of NutritionNoor Hidayah SambliPas encore d'évaluation
- Beta OxidationDocument19 pagesBeta Oxidationindra100% (1)
- Clinical Biochemistry - Clinical Pathology and Procedures - VetDocument9 pagesClinical Biochemistry - Clinical Pathology and Procedures - VetHimmet AslanPas encore d'évaluation
- Animal Diseases CollectionDocument94 pagesAnimal Diseases CollectionNabin Kumar YadavPas encore d'évaluation
- Chapter 18 Endocrine SystemDocument40 pagesChapter 18 Endocrine SystemlolasparklePas encore d'évaluation
- Primary Structure of ProteinDocument21 pagesPrimary Structure of ProteinNico RobinPas encore d'évaluation
- 10 Steps Glycolysis ExplainedDocument8 pages10 Steps Glycolysis Explaineddani2703Pas encore d'évaluation
- Protein TherapeuticsDocument14 pagesProtein TherapeuticsSumanth Kumar ReddyPas encore d'évaluation
- Metabolic Pathway of Carbohydrate and GlycolysisDocument22 pagesMetabolic Pathway of Carbohydrate and GlycolysisDarshansinh MahidaPas encore d'évaluation
- Nutrition BiochemDocument45 pagesNutrition BiochemKesha Marie TalloPas encore d'évaluation
- Handouts - CarbohydratesDocument9 pagesHandouts - CarbohydratesJerrold CruzPas encore d'évaluation
- The Adrenal GlandDocument41 pagesThe Adrenal GlandRujha Haniena Ahmad RidzuanPas encore d'évaluation
- Regulation of Gluconeogenesis and Glycogen MetabolismDocument35 pagesRegulation of Gluconeogenesis and Glycogen MetabolismdanielachynaPas encore d'évaluation
- Carbohydrates SummaryDocument9 pagesCarbohydrates SummaryHarold NagunaPas encore d'évaluation
- Endocrine System GuideDocument6 pagesEndocrine System GuideChechan AmbaPas encore d'évaluation
- Biochemistry of Kidneys and UrineDocument18 pagesBiochemistry of Kidneys and UrineAndrias PutriPas encore d'évaluation
- Carbs: The sugars that feed usDocument4 pagesCarbs: The sugars that feed usMaria Sophia Faith100% (1)
- CHEM 140 Unit 8 Lec 1 LipidsDocument93 pagesCHEM 140 Unit 8 Lec 1 LipidsMevil Jane MabrasPas encore d'évaluation
- 1.protein Digestion, Urea Cy, MbbsDocument81 pages1.protein Digestion, Urea Cy, MbbsDebarghya MukherjeePas encore d'évaluation
- Biochemistry Unit 03 PDFDocument66 pagesBiochemistry Unit 03 PDFDhanush kannanPas encore d'évaluation
- 15BT103 Biochem-UNIT 3Document53 pages15BT103 Biochem-UNIT 3Adityanair RA1711009010128Pas encore d'évaluation
- Amino Acid Metabolism NotesDocument152 pagesAmino Acid Metabolism NotesAkhilesh TiwariPas encore d'évaluation
- Amino Acid Metabolism NotesDocument8 pagesAmino Acid Metabolism Notessean100% (2)
- Amino Acid Metabolism All LecturesDocument18 pagesAmino Acid Metabolism All Lecturesmizare29gPas encore d'évaluation
- Abdominal X RayDocument64 pagesAbdominal X RayabhishekbmcPas encore d'évaluation
- Plasma Physiology (1-2020) by DR Khaled A AbulfadleDocument9 pagesPlasma Physiology (1-2020) by DR Khaled A AbulfadleUzama Binu AliPas encore d'évaluation
- Digestive System Breakdown and AbsorptionDocument9 pagesDigestive System Breakdown and AbsorptionTana OquendoPas encore d'évaluation
- Tests for Liver Function: Serum BilirubinDocument4 pagesTests for Liver Function: Serum BilirubinHiba EmadPas encore d'évaluation
- Fetal Pig Dissection Lab: Project WeblinkDocument6 pagesFetal Pig Dissection Lab: Project WeblinkJill KoehlerPas encore d'évaluation
- CHRONIC HEPATITIS Prof DR Tarek ShetaDocument38 pagesCHRONIC HEPATITIS Prof DR Tarek ShetaSheren GamaleldenPas encore d'évaluation
- Pathophysiology of Hypovolemic ShockDocument8 pagesPathophysiology of Hypovolemic ShockKAYCEEPas encore d'évaluation
- Cholelytiasis 2013 2015Document37 pagesCholelytiasis 2013 2015Nurul RamadhantyPas encore d'évaluation
- Anatomy of The Digestive Tube of Toco ToucanDocument11 pagesAnatomy of The Digestive Tube of Toco ToucanYinneth SakuritaPas encore d'évaluation
- HepC Handbook Web2008Document151 pagesHepC Handbook Web2008susilorini100% (1)
- Practical 4 Cardivascular and Hepatobiliary SystemDocument9 pagesPractical 4 Cardivascular and Hepatobiliary SystemchinPas encore d'évaluation
- ANEURSYMDocument24 pagesANEURSYMMYLENE GRACE ELARCOSAPas encore d'évaluation
- Mind Map GitDocument7 pagesMind Map Gitronron2008Pas encore d'évaluation
- Liver Alterations in Cats - A Literature ReviewDocument39 pagesLiver Alterations in Cats - A Literature ReviewJoanna ChrysanthouPas encore d'évaluation
- Assessment of Normal DopplerDocument4 pagesAssessment of Normal Dopplerharrinson89Pas encore d'évaluation
- Circulatory System TurtleDocument32 pagesCirculatory System TurtleVillamorchardPas encore d'évaluation
- The Longmire I, II, and III Operations: L. William Traverso, M.D.Document8 pagesThe Longmire I, II, and III Operations: L. William Traverso, M.D.Abu ZidanePas encore d'évaluation
- Innovations and Prospects in Modern Science 13 15.02.23Document481 pagesInnovations and Prospects in Modern Science 13 15.02.23Miroslav Ekman-KremenetskyPas encore d'évaluation
- Pathology Liver Jars FixedDocument7 pagesPathology Liver Jars Fixedapi-3730040Pas encore d'évaluation
- 007-Hepatobiliary & PancreaticDocument59 pages007-Hepatobiliary & PancreaticAhmed ZaghwPas encore d'évaluation
- ABDOMENDocument16 pagesABDOMENHussein Al SaediPas encore d'évaluation
- Cancer: Causes and Risks FactorsDocument88 pagesCancer: Causes and Risks FactorsClancy Anne Garcia NavalPas encore d'évaluation
- AD285230700512 LO230700610: Patient ReportDocument1 pageAD285230700512 LO230700610: Patient ReportReal Gamer 6Pas encore d'évaluation
- Anatomy and Physiology of Biliary TreeDocument48 pagesAnatomy and Physiology of Biliary TreeKamalakanta Das100% (1)
- 2018 Ultrasound A Core Review PDFDocument617 pages2018 Ultrasound A Core Review PDFsun sealPas encore d'évaluation
- Ascites PDFDocument8 pagesAscites PDFJanina Patricia BuddlePas encore d'évaluation
- The Liver in Systemic Disease A Clinician's Guide To Abnormal LiverDocument285 pagesThe Liver in Systemic Disease A Clinician's Guide To Abnormal Liveroleksandra.bilotkachPas encore d'évaluation