Académique Documents
Professionnel Documents
Culture Documents
Quality Control of Pesticide Products
Transféré par
premasarthyCopyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Quality Control of Pesticide Products
Transféré par
premasarthyDroits d'auteur :
Formats disponibles
Quality Control of Pesticide Products
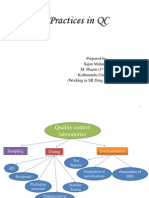
1. ORGANIZATION AND MANAGEMENT
1.1. Structure, responsibilities and authority of individuals in the laboratory - Awareness on national and international regulatory framework. - Comply with national pesticide standards (eg: FAO, WHO). - Up-to-date methods of analysis. - Key areas of responsibility presented clearly.
1.2. Quality assurance schemes - Management review of all systems and procedures once a year to identify improvements needed. - Laboratory quality manual consisting of quality planning, key procedures and record keeping for effective traceability.
1.3. Documents and data recording - Analytical methods, product specifications, standard operating procedures. - Consistent systems for maintenance of records (samples, COA from suppliers). - Quality records include Supplier lists Analytical test results Calibration records Customer complaints Training records and plans
1.4. Internal quality audits - By trained auditors. - Ensure corrective actions taken by follow-up audits.
2. STAFF QUALIFICATIONS AND TRAINING
- Trained personnel (technical aspects, personal development and management skills). - Key competencies Ability to see problems through Ability to think analytically and logically Ability to assess the impact of their work Ability to think creatively Ability to work to focus on detail - Training programmes, records and associated procedures regularly audited.
3. FACILITIES
3.1. Library and access to electronic database - FAO, WHO specifications - CIPAC methods - Pesticide manual - Information provided by pesticide manufacturers.
3.2. Laboratory facilities and safety provisions - Smooth, easily cleaned floors and other surfaces. - Location away from vibration. - Good electrical supply. - Air conditioning - Good drainage and waste disposal systems. - Piped supply of high quality gases for chromatography. - Adequate bench space for sample preparation. - Fume hoods to handle volatile, dusty and toxic materials. - Gas alarms, fire detector and personal protective equipment.
3.3. Areas for storage of data, sample, standards and reagents - Fire-proof cabinets for sensitive information. - Back-up copies - Storage of materials using first in first out basis.
2
4. EQUIPMENT
4.1. Range and type of equipment - Vary according to the tests performed. - eg: Gas chromatography High performance liquid chromatography Spectrophotometers Laboratory balances Volumetric glassware Ovens, refrigerators, desiccators
4.2. Procurement of equipment - Purchased from major suppliers supplying equipment which is fit for the purpose.
5. CHEMICALS
5.1. Quality and type - All chemicals and reagents sourced from laboratory chemical suppliers.
5.2. Analytical standards - Only certified reference materials should be used. - Stored according to instructions (refrigerator, moisture-free bottles).
5.3. Safe handling and storage - Proper personal protective equipment. - Storage of chemicals in secure, temperature controlled areas with good lighting and ventilation.
5.4. Waste disposal - Suitable containers to collect waste materials including solvents and organic matter. - Wastewater should pass to an appropriate water treatment system.
6. Operational procedures for quality control
6.1. Procurement of materials and services - Examples of materials Laboratory reagents Laboratory equipment and consumables Labels Software External maintenance and calibration Cleaning contractors
- Examples of services
6.2 Sample registration and traceability - Incoming samples must be clearly labeled with a sequential reference number and recorded. - Computerized laboratory information management systems with bar code samples.
6.3. Analytical and physical test method and validation - In-house procedures should be carefully validated. - Samples checked in replicates to ensure adequate accuracy and precision.
6.4. Product specifications - Products should be checked against the appropriate specification (by WHO, FAO or the manufacturer).
6.5. Special laboratory procedures - Required to cover Sample receipt, handling and storage Calibration programmes Procurement, storage and use of analytical standards Storage, use and disposal of reagents and test solutions Equipment cleaning protocols Selection of new equipment and suppliers Maintenance programmes and procedures Standard operating procedures for all laboratory equipment
Vous aimerez peut-être aussi
- GMP Audit Agenda FP Site 2023-24Document4 pagesGMP Audit Agenda FP Site 2023-24Vishweshwar L SangmulyPas encore d'évaluation
- Final Exame NumanDocument16 pagesFinal Exame NumanMayson BaliPas encore d'évaluation
- GMP QCDocument29 pagesGMP QCBhavishya KandulaPas encore d'évaluation
- DR - Baher: Analytical Control Laboratory (ACL)Document3 pagesDR - Baher: Analytical Control Laboratory (ACL)Mohamed TiemaPas encore d'évaluation
- Plant Master FileDocument3 pagesPlant Master Filea4623Pas encore d'évaluation
- Good Laboratory Practice: August 2013Document40 pagesGood Laboratory Practice: August 2013Somnath BanerjeePas encore d'évaluation
- Sops IndexDocument3 pagesSops IndexIbrahim IssaPas encore d'évaluation
- Quality AssuranceDocument3 pagesQuality AssuranceDevanshi JadaunPas encore d'évaluation
- BP606T Unit 4-6Document222 pagesBP606T Unit 4-6Gyampoh SolomonPas encore d'évaluation
- Unit IIIDocument46 pagesUnit IIIDIPALI TALELEPas encore d'évaluation
- Good Laboratory PracticesDocument21 pagesGood Laboratory PracticesSajanMaharjan100% (1)
- Good Practices For Quality Control Laboratories: Supplementary Training Modules On Good Manufacturing PracticeDocument16 pagesGood Practices For Quality Control Laboratories: Supplementary Training Modules On Good Manufacturing PracticelanikhilPas encore d'évaluation
- Microbiology Best Laboratory PracticesDocument47 pagesMicrobiology Best Laboratory PracticesQAV_CRS100% (1)
- Good Practices in Quality ControlDocument13 pagesGood Practices in Quality ControlTerrence Terry BhengoePas encore d'évaluation
- Wang - Microbiological Best Laboratory PracticesDocument47 pagesWang - Microbiological Best Laboratory PracticesGuna BabuPas encore d'évaluation
- HACCP, PRPS, SOPsDocument38 pagesHACCP, PRPS, SOPsakash kumarPas encore d'évaluation
- HACCP MaterialDocument38 pagesHACCP MaterialWahyu NugrahaPas encore d'évaluation
- Equipments and Raw Materials 1Document20 pagesEquipments and Raw Materials 1Vasu ManchandaPas encore d'évaluation
- Good Laboratory PracticesDocument39 pagesGood Laboratory PracticesGANESH KUMAR JELLA100% (2)
- Risk Based Validation and Requalification of Processes EquipmentDocument33 pagesRisk Based Validation and Requalification of Processes EquipmentfenixseravgonPas encore d'évaluation
- Tech RequirementsDocument26 pagesTech Requirementszubair ahmadPas encore d'évaluation
- CH 3. The Drug Manufacturing Process: The Highest Quality StandardsDocument55 pagesCH 3. The Drug Manufacturing Process: The Highest Quality StandardsShdefat Alaa AlaaPas encore d'évaluation
- Good Laboratory Practice (GLP)Document26 pagesGood Laboratory Practice (GLP)Ganesh V Gaonkar100% (2)
- Good Practices For Quality Control Laboratories: Part 1: Introduction, Management and InfrastructureDocument33 pagesGood Practices For Quality Control Laboratories: Part 1: Introduction, Management and InfrastructurelanikhilPas encore d'évaluation
- Schedule M - Good Manufacturing PracticesDocument6 pagesSchedule M - Good Manufacturing PracticesLankalapalli SrinivasPas encore d'évaluation
- SOP For Regulatory Annual Product Quality ReviewDocument9 pagesSOP For Regulatory Annual Product Quality ReviewisralmayoorPas encore d'évaluation
- KEL 1 Dewi Validasi KebersihanDocument24 pagesKEL 1 Dewi Validasi KebersihanMuhsin Mukhtar S. FarmPas encore d'évaluation
- Day 3 NABL Accreditation Control of Documents & RecordsDocument59 pagesDay 3 NABL Accreditation Control of Documents & RecordsChaudhari CnPas encore d'évaluation
- LABMANDocument7 pagesLABMANChellie GualbertoPas encore d'évaluation
- Good Laboratory PracticesDocument52 pagesGood Laboratory Practicesankita pathania100% (1)
- SopDocument20 pagesSopanirban82inPas encore d'évaluation
- Quality Assurance Program For Feed Testing Laboratories PresentationDocument104 pagesQuality Assurance Program For Feed Testing Laboratories Presentationrsuerto100% (1)
- GLP ConceptDocument35 pagesGLP ConceptAbdelhamidgmPas encore d'évaluation
- Laboratory ManagementDocument33 pagesLaboratory ManagementsinglethienPas encore d'évaluation
- In-Process Quality Control, Stability, CosmeticDocument10 pagesIn-Process Quality Control, Stability, CosmeticWellanie Guillena TamalaPas encore d'évaluation
- Ich Q7Document35 pagesIch Q7bharat_sasiPas encore d'évaluation
- GLP FinalDocument7 pagesGLP Finalaakash sahaPas encore d'évaluation
- Seminar On GMP Requirements For Ophthalmic PreparationsDocument57 pagesSeminar On GMP Requirements For Ophthalmic Preparationsvkguptajss100% (1)
- Area of Microbiology Lab AuditDocument2 pagesArea of Microbiology Lab Auditsagor sagorPas encore d'évaluation
- Action PlanDocument2 pagesAction PlanЕвгения ЕвгенияPas encore d'évaluation
- Draft GMP For Ayurveda Ravindra PrakashDocument28 pagesDraft GMP For Ayurveda Ravindra Prakashreflectprakash3610Pas encore d'évaluation
- Validation of Pharmaceutical PackagingDocument28 pagesValidation of Pharmaceutical PackagingNaheed Malik100% (3)
- Akram El Sabrouty CV Dec-2019 PDFDocument7 pagesAkram El Sabrouty CV Dec-2019 PDFAkram El SabroutyPas encore d'évaluation
- EU - Quality ControlDocument3 pagesEU - Quality Controlapi-3859063Pas encore d'évaluation
- Materi 2Document23 pagesMateri 2Murdifin SulawesiPas encore d'évaluation
- Good Laboratory PracticesDocument48 pagesGood Laboratory PracticesRaja AbhilashPas encore d'évaluation
- Cleaning Validation: WHO Supplementary Training ModulesDocument43 pagesCleaning Validation: WHO Supplementary Training ModulesvkguptajssPas encore d'évaluation
- Akram El Sabrouty CV Dec-2019 (USA)Document5 pagesAkram El Sabrouty CV Dec-2019 (USA)Akram El SabroutyPas encore d'évaluation
- GLP Mahnoor Kazmi 7th SemDocument13 pagesGLP Mahnoor Kazmi 7th SemMusfira KhalidPas encore d'évaluation
- Good Laboratory Practices: Mr. Abhijeet A. AherDocument23 pagesGood Laboratory Practices: Mr. Abhijeet A. AherSUMIT SANJAY LAD Research StudentPas encore d'évaluation
- CGMP Training Suntara Cosmetics Pvt. LTD.: Prepared By: Pratham ConsultantsDocument76 pagesCGMP Training Suntara Cosmetics Pvt. LTD.: Prepared By: Pratham Consultantsmrugeshj100% (1)
- RAC Review Chapter 10Document35 pagesRAC Review Chapter 10Ayele BizunehPas encore d'évaluation
- Environmental Monitoring Considerations: Nancy Roscioli, Ph.D. Don Hill and Associates, IncDocument54 pagesEnvironmental Monitoring Considerations: Nancy Roscioli, Ph.D. Don Hill and Associates, IncKandhakatla PraveenkumarPas encore d'évaluation
- Inside the Pill Bottle: A Comprehensive Guide to the Pharmaceutical IndustryD'EverandInside the Pill Bottle: A Comprehensive Guide to the Pharmaceutical IndustryPas encore d'évaluation
- Molecular Microbial Diagnostic Methods: Pathways to Implementation for the Food and Water IndustriesD'EverandMolecular Microbial Diagnostic Methods: Pathways to Implementation for the Food and Water IndustriesPas encore d'évaluation
- Practical Approaches to Method Validation and Essential Instrument QualificationD'EverandPractical Approaches to Method Validation and Essential Instrument QualificationPas encore d'évaluation
- Laboratory Quality/Management: A Workbook with an Eye on AccreditationD'EverandLaboratory Quality/Management: A Workbook with an Eye on AccreditationÉvaluation : 5 sur 5 étoiles5/5 (1)
- Basic Life Science Methods: A Laboratory Manual for Students and ResearchersD'EverandBasic Life Science Methods: A Laboratory Manual for Students and ResearchersPas encore d'évaluation
- 63 NayanmaargalDocument8 pages63 NayanmaargalpremasarthyPas encore d'évaluation
- Good Laboratory Practice (GLP)Document10 pagesGood Laboratory Practice (GLP)premasarthyPas encore d'évaluation
- Tamil ExerciseDocument2 pagesTamil ExercisepremasarthyPas encore d'évaluation
- Science Test (STD 5)Document5 pagesScience Test (STD 5)premasarthyPas encore d'évaluation
- Science Chapter 5 - The Air Around UsDocument3 pagesScience Chapter 5 - The Air Around UspremasarthyPas encore d'évaluation
- 70-410 R2 Lesson 03 - Configuring Local StorageDocument23 pages70-410 R2 Lesson 03 - Configuring Local StorageCésar MarínPas encore d'évaluation
- It SopDocument42 pagesIt SopMoe Kaungkin100% (1)
- Mitsubishi GT2000 Catalogue - Contact ASC PH 03 9720 0211Document108 pagesMitsubishi GT2000 Catalogue - Contact ASC PH 03 9720 0211John Forbes NashPas encore d'évaluation
- Material Handling: By: Josh ConleyDocument20 pagesMaterial Handling: By: Josh ConleyDocMoodyPas encore d'évaluation
- Define and Explain The Internet of Things.: 1) Physical ObjectDocument32 pagesDefine and Explain The Internet of Things.: 1) Physical Objectfaiyaz pardiwalaPas encore d'évaluation
- XGT+Panel+HW+Manual eXP Eng V1.1 Oix08d5qDocument115 pagesXGT+Panel+HW+Manual eXP Eng V1.1 Oix08d5qcucuPas encore d'évaluation
- Data Structure in MainframeDocument10 pagesData Structure in MainframeKunalPas encore d'évaluation
- IT-Answers JELODocument4 pagesIT-Answers JELORaymond PascualPas encore d'évaluation
- Computer HardwareDocument2 pagesComputer Hardwarereymilyn zuluetaPas encore d'évaluation
- Problem Set 2Document4 pagesProblem Set 2bijan shresthaPas encore d'évaluation
- Computer Architecture I: Digital DesignDocument89 pagesComputer Architecture I: Digital DesignJitendra PatelPas encore d'évaluation
- Dell PowerProtect DD Concepts and Features - Participant GuideDocument76 pagesDell PowerProtect DD Concepts and Features - Participant GuideMWANAHAWA BAKARIPas encore d'évaluation
- CS609 CURRENT MIDTERM SOLVED SUBJECTIVE by JUNAID-1Document9 pagesCS609 CURRENT MIDTERM SOLVED SUBJECTIVE by JUNAID-1Hamza KhanPas encore d'évaluation
- s71200 Easy Book en-US en-USDocument364 pagess71200 Easy Book en-US en-USReynanBorliniPas encore d'évaluation
- SQL 2014 - Move Database FilesDocument50 pagesSQL 2014 - Move Database FilesPedro OliveiraPas encore d'évaluation
- USB Bus Interface Chip CH375Document18 pagesUSB Bus Interface Chip CH375Mario Esteban100% (1)
- GT Designer 3 Manual (Unlockplc - Com)Document1 316 pagesGT Designer 3 Manual (Unlockplc - Com)Unlock PLC100% (1)
- (M2S2-PDF) Debug PDFDocument26 pages(M2S2-PDF) Debug PDFKimCanillasVincerePas encore d'évaluation
- Flash Memory Programming SpecificationDocument22 pagesFlash Memory Programming SpecificationYonatan José Roche AndradePas encore d'évaluation
- Manoj Oswal V State of Maharashtra and Another (P. 38)Document18 pagesManoj Oswal V State of Maharashtra and Another (P. 38)nigamaishwaryaPas encore d'évaluation
- Component of A Computer SystemDocument34 pagesComponent of A Computer SystemmasulaPas encore d'évaluation
- Senior DBA Interview QuestionsDocument8 pagesSenior DBA Interview QuestionsKadambari RanjanPas encore d'évaluation
- X4450 - 3713255 - LSI 106x - RAID - User - GuideDocument156 pagesX4450 - 3713255 - LSI 106x - RAID - User - Guidec154333Pas encore d'évaluation
- Introduction To Operating Systems, System StructuresDocument35 pagesIntroduction To Operating Systems, System StructuresShri DeerPas encore d'évaluation
- Module 1 Test PDFDocument21 pagesModule 1 Test PDFMuhamad Hassan100% (1)
- Computer Science: 1. Introduction To ComputersDocument2 pagesComputer Science: 1. Introduction To ComputersMumtaz H. TurkPas encore d'évaluation
- ExtechDocument333 pagesExtechWilliam ProveauxPas encore d'évaluation
- Computer Arithmetic Practice Exercises ProgrammingDocument213 pagesComputer Arithmetic Practice Exercises ProgrammingjflksdjfPas encore d'évaluation
- UBI - Unsorted Block ImagesDocument19 pagesUBI - Unsorted Block ImagesDablyo FigueiredoPas encore d'évaluation
- Sherlog: Energy Network MonitorsDocument6 pagesSherlog: Energy Network Monitorskessir taouilPas encore d'évaluation