Académique Documents
Professionnel Documents
Culture Documents
# Week 5 Notes
Transféré par
timx123yDescription originale:
Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
# Week 5 Notes
Transféré par
timx123yDroits d'auteur :
Formats disponibles
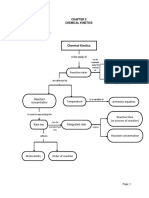
FACTORS THAT AFFECT REACTION RATES Although a balanced chemical equation describes the quantitative relationships between the
reactants and the products, it gives us no information about whether and how fast a given reaction will occur. This information is obtained by studying the chemical kinetics of a reaction, which depend on various factors: Higher concentration of reactants more frequent collisions between reactants higher reaction rate o Reaction rates usually decrease with time as reactants are used up (their concentration decreases) Higher temperature particles move faster more frequent collisions higher reaction rate o In systems where more than one reaction is possible, the same reactants can produce different products under different reaction conditions Homogeneous reactions, when all reactants are in the same phase (liquid or gas), have a higher reaction rate than heterogeneous reactions o The reaction rate of a heterogeneous reaction depends on the surface area of the more condensed phase Some reactions occur faster in some solvents. Also, the lower the viscosity of the solvent, the faster the reaction will happen Reactions normally do not occur in one step, but instead in a number of intermediate steps. The slowest intermediate reaction determines the overall reaction rate. Even though the overall products may be at a lower energy level than the reactants, the intermediaries may be at a higher level. In order for the reaction to occur, this barrier, called activation energy, must be overcome. For similar reactions under comparable conditions, the one with the lowest activation energy will occur most rapidly. Catalysts accelerate reactions. Sometimes catalysts lower the activation energy. Carefully selected catalysts will accelerate only one of possible reactions.
RATE LAWS Reaction rates are usually expressed as the change in concentration of any reactant consumed or the change in concentration of any product formed per unit time (moles per litre ( ) per second): [ ]
[ ] [ ]
[ ]
[ ] [ ]
ACTIVATION ENERGY
Activation energy can be calculated from the Arrhenius equation: ( ( [ ) ) ] [ ]
ZEROTH ORDER REACTIONS A zeroth order reaction is one whose rate is constant and independent of concentration: [ ] [ ] [ ] [ ]
FIRST ORDER REACTIONS
SECOND ORDER REACTIONS
[ ] [ ]
[ ][ ] [ ]
REACTION ORDER SUMMARY
In general, reaction order is the slope of the line obtained from plotting the log of reaction rate against the log of varying concentrations. [ ] [ ]
If there is more than one reactant, one must calculate the reaction order coefficients separately for each reactant to obtain the overall reaction order. Doubling the concentration of one reactant while keeping all others constant and observing the reaction rate, we can calculate the reaction order coefficient for the varying reactant. After doing the same for the other reactants, the overall reaction order is the sum of all coefficients. [ ] [ ] [ ]
Vous aimerez peut-être aussi
- 5.2 Introduction To Rate Law StudentDocument6 pages5.2 Introduction To Rate Law StudentSyed RazaPas encore d'évaluation
- 14 All-1B-CSMDocument486 pages14 All-1B-CSMShubham Chattopadhyay100% (2)
- English WB IgcseDocument23 pagesEnglish WB Igcsetoleen playzPas encore d'évaluation
- Chemical KineticsDocument71 pagesChemical KineticsLoraine Andrei DeLeonPas encore d'évaluation
- Chemical Kinetics: By:-Divyam Verma Ankur Kumar Deepak KumarDocument36 pagesChemical Kinetics: By:-Divyam Verma Ankur Kumar Deepak KumarAnindya BhattacharyaPas encore d'évaluation
- Chemical Kinetics 2Document39 pagesChemical Kinetics 2Md. Hasanur RahmanPas encore d'évaluation
- Chemical Kinetics - LectureDocument37 pagesChemical Kinetics - LectureEsmira Melić ŠutkovićPas encore d'évaluation
- FactorDocument2 pagesFactorJitendra KumarPas encore d'évaluation
- 1-Chemical Kinetics First LectureDocument27 pages1-Chemical Kinetics First LecturealakaolamuhammadPas encore d'évaluation
- Rates OF ReactionDocument6 pagesRates OF ReactionAdam WilliamsPas encore d'évaluation
- Chemical Kinetics in Under 40Document18 pagesChemical Kinetics in Under 40JeromePas encore d'évaluation
- Rates of ReactionDocument6 pagesRates of ReactionesotericgraphicapparelPas encore d'évaluation
- Chemistry Notes For Class 12 Chapter 4 Chemical KineticsDocument11 pagesChemistry Notes For Class 12 Chapter 4 Chemical KineticsAyush singh PrincePas encore d'évaluation
- Fair Use NoticeDocument15 pagesFair Use NoticeImran UnarPas encore d'évaluation
- Rates of Reaction NotesDocument13 pagesRates of Reaction NotesNia RamPas encore d'évaluation
- Chemical Kinetics 1234 FinalDocument22 pagesChemical Kinetics 1234 FinalJayesh SavaliyaPas encore d'évaluation
- Rangkuman TRK (Deva Punya)Document4 pagesRangkuman TRK (Deva Punya)gamalielPas encore d'évaluation
- Peter J. NassiffDocument11 pagesPeter J. Nassifflary77Pas encore d'évaluation
- KineticsDocument55 pagesKineticsSaptanshu SamalPas encore d'évaluation
- Chapter-04 Chemical KineticsDocument11 pagesChapter-04 Chemical Kineticsshrey4602Pas encore d'évaluation
- Understanding Chemical KineticsDocument96 pagesUnderstanding Chemical Kineticssalma khanPas encore d'évaluation
- Approval SheetDocument25 pagesApproval SheetSelni Sandabunga'Pas encore d'évaluation
- Rate of Reactions 18 April 2024(1)Document46 pagesRate of Reactions 18 April 2024(1)Amahle KudaPas encore d'évaluation
- Rates of Reaction Explained: How Variables Like Temperature and Concentration Affect Chemical KineticsDocument33 pagesRates of Reaction Explained: How Variables Like Temperature and Concentration Affect Chemical KineticsMd. Mujahid HasanPas encore d'évaluation
- SS 2 Week 3Document71 pagesSS 2 Week 3Denzel MusaPas encore d'évaluation
- Chemical KineticsDocument3 pagesChemical KineticsSunny RohidaPas encore d'évaluation
- Week 5 Chemical KineticsDocument60 pagesWeek 5 Chemical KineticsLuke BelmarPas encore d'évaluation
- Chemistry Unit 4 PDFDocument60 pagesChemistry Unit 4 PDFsammam mahdi samiPas encore d'évaluation
- Chemical KineticsDocument53 pagesChemical KineticsEuann MagtibayPas encore d'évaluation
- CBSE Class 12 Chemistry Notes: Chemical Kinetics: HomepageDocument14 pagesCBSE Class 12 Chemistry Notes: Chemical Kinetics: HomepageBHAVYA BPas encore d'évaluation
- Dr. Pedro Julio VillegasDocument57 pagesDr. Pedro Julio VillegasSheikh Samir HassanPas encore d'évaluation
- Rates and Rate Laws: SpectrosDocument6 pagesRates and Rate Laws: Spectrosdharul khairPas encore d'évaluation
- Pharmaceutical Kinetics and Stability-Lec-2Document27 pagesPharmaceutical Kinetics and Stability-Lec-2husseinkamilhamid123456789Pas encore d'évaluation
- CATALYTIC REACTIONS ACCELERATE CHEMICAL PROCESSESDocument41 pagesCATALYTIC REACTIONS ACCELERATE CHEMICAL PROCESSESKhuza EmahPas encore d'évaluation
- Unit 2 Chemical Kinetics: Adnan Chowdhury Chemistry TeacherDocument9 pagesUnit 2 Chemical Kinetics: Adnan Chowdhury Chemistry TeacherZulfikarPas encore d'évaluation
- Chemical Kinetics Rate Laws and Reaction OrdersDocument30 pagesChemical Kinetics Rate Laws and Reaction OrdersBichitra GautamPas encore d'évaluation
- Understanding reaction orders and kineticsDocument5 pagesUnderstanding reaction orders and kineticsdineshPas encore d'évaluation
- KineticsDocument73 pagesKineticsshireen O. IsmaelPas encore d'évaluation
- IB Chemistry - Unit 6 - Chemical Kinetics Study GuideDocument6 pagesIB Chemistry - Unit 6 - Chemical Kinetics Study GuideHamzah JoharPas encore d'évaluation
- Module Group 1 & 2Document27 pagesModule Group 1 & 2Jhosh bllstrsPas encore d'évaluation
- Chemical Kinetics NotesDocument11 pagesChemical Kinetics Notespala100% (1)
- Rate of ReactionDocument6 pagesRate of ReactionDavid ThompsonPas encore d'évaluation
- Kinetics NotesDocument4 pagesKinetics NotesDharaneesh S.k.Pas encore d'évaluation
- Short Note Chemistry Form 5-Chapter 1 Rate of ReactionDocument4 pagesShort Note Chemistry Form 5-Chapter 1 Rate of Reactionsalamah_sabri100% (1)
- Chemical Kinetics: Quick RevisionDocument20 pagesChemical Kinetics: Quick Revisionfakeheartattack8kPas encore d'évaluation
- Chapter 17 - Reaction RatesDocument3 pagesChapter 17 - Reaction RatescaffeinewriterPas encore d'évaluation
- Reactions and ReactorsssDocument28 pagesReactions and ReactorsssPrince HenryPas encore d'évaluation
- ChemistryDocument2 pagesChemistryKuldeep SinghPas encore d'évaluation
- For Exer 3Document16 pagesFor Exer 3Louiegi AlvarezPas encore d'évaluation
- Chemical Kinetics PDFDocument8 pagesChemical Kinetics PDFAlexandra minPas encore d'évaluation
- Measuring Rates of Reaction: Things To MeasureDocument3 pagesMeasuring Rates of Reaction: Things To MeasureMuhammad AfnanPas encore d'évaluation
- Physical Change: Chemical ReactionsDocument9 pagesPhysical Change: Chemical ReactionsAishi GuptaPas encore d'évaluation
- ChemistryDocument49 pagesChemistryAnam FPas encore d'évaluation
- Chemical KineticsDocument8 pagesChemical KineticsHosam Hasan Abd ElhadyPas encore d'évaluation
- Kinetic Methods of Analysis: Chapter 15 - 1Document9 pagesKinetic Methods of Analysis: Chapter 15 - 1natsdorfPas encore d'évaluation
- Rate of ReactionDocument23 pagesRate of ReactionVirly vcPas encore d'évaluation
- Chemical Kinetics: Factors Affecting Reaction RateDocument4 pagesChemical Kinetics: Factors Affecting Reaction RateMontassar DridiPas encore d'évaluation
- Group 4 - Chemical KineticsDocument54 pagesGroup 4 - Chemical KineticsMark Harold GonzalesPas encore d'évaluation
- Practice Makes Perfect in Chemistry: Kinetics and EquilibriumD'EverandPractice Makes Perfect in Chemistry: Kinetics and EquilibriumPas encore d'évaluation
- Reaction Kinetics: Reactions in SolutionD'EverandReaction Kinetics: Reactions in SolutionÉvaluation : 3.5 sur 5 étoiles3.5/5 (4)
- # Week 7 NotesDocument10 pages# Week 7 Notestimx123yPas encore d'évaluation
- # Week 3 NotesDocument13 pages# Week 3 Notestimx123yPas encore d'évaluation
- # Week 4 NotesDocument17 pages# Week 4 Notestimx123yPas encore d'évaluation
- # Week 9 NotesDocument5 pages# Week 9 Notestimx123yPas encore d'évaluation
- # Week 1 NotesDocument9 pages# Week 1 Notestimx123yPas encore d'évaluation
- # Week 8 NotesDocument14 pages# Week 8 Notestimx123yPas encore d'évaluation
- # Week 6 NotesDocument12 pages# Week 6 Notestimx123yPas encore d'évaluation
- # Week 2 NotesDocument21 pages# Week 2 Notestimx123yPas encore d'évaluation
- 3.091x - Spring 2013 - Logbook: Week 6Document19 pages3.091x - Spring 2013 - Logbook: Week 6timx123yPas encore d'évaluation
- 3.091x - Table of Physical Constants PDFDocument2 pages3.091x - Table of Physical Constants PDFtimx123yPas encore d'évaluation
- Philips DVD-Recorder User Manual PDFDocument77 pagesPhilips DVD-Recorder User Manual PDFtimx123yPas encore d'évaluation
- Aqa 1 5Document19 pagesAqa 1 5leonidas.wujieweiPas encore d'évaluation
- Determination of Rate EquationDocument9 pagesDetermination of Rate EquationIsabella ThomasPas encore d'évaluation
- Chap 52 Activation EnergyDocument7 pagesChap 52 Activation Energybreakfast noPas encore d'évaluation
- AP Chemistry - Kinetics of A Reaction LabDocument8 pagesAP Chemistry - Kinetics of A Reaction LabJonathan Chen50% (2)
- Chemical Kinetics SlidesDocument87 pagesChemical Kinetics SlidesFarith AfifiPas encore d'évaluation
- SCH 421 Reactor Design WEEK 1 & 2 Notes-1Document15 pagesSCH 421 Reactor Design WEEK 1 & 2 Notes-1vivaline AchiengPas encore d'évaluation
- Enzyme Part 1-5 Vikneswaran 260110132004Document7 pagesEnzyme Part 1-5 Vikneswaran 260110132004Vikneswaran VîçkýPas encore d'évaluation
- Kinetics Lecture 26Document30 pagesKinetics Lecture 26sus023Pas encore d'évaluation
- Mekelle University Ethiopian Institute of Technology-Mekelle Department of Chemical Engineering Process EngineeringDocument77 pagesMekelle University Ethiopian Institute of Technology-Mekelle Department of Chemical Engineering Process EngineeringetayhailuPas encore d'évaluation
- Understanding Chemical KineticsDocument50 pagesUnderstanding Chemical Kineticsomer faruqePas encore d'évaluation
- CHE3164 Problem Set 5 Solution (1809)Document9 pagesCHE3164 Problem Set 5 Solution (1809)Divya KariaPas encore d'évaluation
- Block 2BBCCT 107Document44 pagesBlock 2BBCCT 107robinPas encore d'évaluation
- Chapter 1 - Chemical Kinetics Part 1Document46 pagesChapter 1 - Chemical Kinetics Part 1NUR DINI MAISARAH BINTI HEZAL / UPMPas encore d'évaluation
- Chem27 Chap15 Lecture 2021Document72 pagesChem27 Chap15 Lecture 2021Babeejay2Pas encore d'évaluation
- Determining Reaction MechanismsDocument34 pagesDetermining Reaction MechanismsMahmoud AbdAllahPas encore d'évaluation
- Advancedchemistry-Lecture Slides-Kinetics Lessons Student VersionDocument26 pagesAdvancedchemistry-Lecture Slides-Kinetics Lessons Student VersionJavier Blanco AlvarezPas encore d'évaluation
- EnzymesDocument17 pagesEnzymesakshaymoga0% (1)
- 6.2hl Rate Expression and Reaction MechanismDocument44 pages6.2hl Rate Expression and Reaction MechanismJESUS EDUARDO CARBONO NIEBLESPas encore d'évaluation
- Enzymes: Biological Catalysts ExplainedDocument41 pagesEnzymes: Biological Catalysts ExplainedANWESHA BALPas encore d'évaluation
- Chemical Kinetics Rate LawDocument28 pagesChemical Kinetics Rate LawReginal MoralesPas encore d'évaluation
- 2021 ATNAA Competitve Product InhibitionDocument14 pages2021 ATNAA Competitve Product InhibitionvinhPas encore d'évaluation
- Kinetics of the hydrolysis of ethyl acetateDocument6 pagesKinetics of the hydrolysis of ethyl acetateOnkar BhoslePas encore d'évaluation
- Lecture 9Document4 pagesLecture 9Asif AliPas encore d'évaluation
- Lecture 2 - Mole BalancesDocument20 pagesLecture 2 - Mole Balances88l8Pas encore d'évaluation
- Kinetics of Esterification of Acetic Acid With Methanol in The Presence of Ion Exchange Resin CatalystsDocument6 pagesKinetics of Esterification of Acetic Acid With Methanol in The Presence of Ion Exchange Resin CatalystsEduardo Jacobo SillerPas encore d'évaluation
- Enzyme KineticsDocument22 pagesEnzyme Kineticsmercy koPas encore d'évaluation
- Industrial Chemistry MCQDocument69 pagesIndustrial Chemistry MCQSatvik BeheraPas encore d'évaluation
- CHEM 103 CHEMICAL KINETICS TUTORIAL SHEETSDocument2 pagesCHEM 103 CHEMICAL KINETICS TUTORIAL SHEETSSwapnil TripathiPas encore d'évaluation