Académique Documents
Professionnel Documents
Culture Documents
Nucleotide Degradation PDF
Transféré par
manoj_rkl_07Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Nucleotide Degradation PDF
Transféré par
manoj_rkl_07Droits d'auteur :
Formats disponibles
Nucleotide Degradation
H Anne Simmonds, Guys Hospital, London, UK Albert H van Gennip, Laboratory for Metabolic Diseases and Cancer, Amsterdam, The
Netherlands
Secondary article
Article Contents
. Introduction . Enzymes Catalysing Dephosphorylation and Deamination of Purine Nucleotides . Species Differences in the Enzymes of Nucleoside Degradation . Removal of Metabolic Waste from Nucleotide Degradation: Uric Acid is the End-product of Purine Catabolism Only in Humans . Purine Ribonucleotide Catabolism in Healthy Humans . Enzymes Catalysing Dephosphorylation and Deamination of Pyrimidine Nucleotides . What Can We Learn from Genetic Metabolic Defects of Purine and Pyrimidine Metabolism? . Summary
Nucleotide degradation is an integrated process in all human cells, being intimately linked with the pathways of nucleotide synthesis and salvage. The clinical conditions associated with defects of enzymes catalysing nucleotide degradation provide valuable insight into the importance of this network.
Introduction
Nucleotides have very early origins
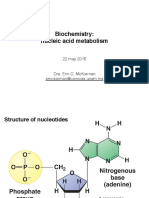
Purine and pyrimidine nucleotides were almost certainly the rst complex chemical structures to emerge from the primordial soup, the basic ring structure being synthesized rst through the action of ultraviolet light on mixtures of cyanide and methane, or urea. Addition of sugar and phosphate, also present at that time, could occur thereafter under abiotic conditions, resulting in the formation of purine and pyrimidine mononucleotides. More complex pathways that sustain these vital intracellular chemicals have developed subsequently. The diverse functions performed by nucleotides depends on an integrated network involving synthesis, degradation and salvage. This varies among species, and also within a given species, depending on the function of a particular cell or tissue. The multistep de novo pathways of nucleotide synthesis contrast with the less energetically expensive single-step salvage routes. This chapter focuses on the pathways of nucleotide degradation (Figure 1) that link these two and underlines how they dier in health and disease. Two types of nucleotides mononucleotides and polynucleotides are found in all nucleated cells and their routes of degradation vary, depending on the cell type and its function. Mononucleotides are composed of either purine or pyrimidine bases (Figures 1 and 2) with a pentose sugar attached at N9 for purines or N1 for pyrimidines (nucleosides), and additionally esteried with phosphoric acid (nucleotides). Most mononucleotides occur within the cell in the triphosphate form and these highly charged phosphorylated compounds do not normally cross cell membranes in the absence of carrier systems. Polynucleotides exist in two forms: deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). DNA is found principally in the nucleus, where the pentose is 2-deoxyribose (Figure 3a) and the bases are adenine (A), guanine (G), cytosine (C) and thymine (T). In RNA the pyrimidine uracil (U) replaces thymine and the pentose is ribose. Most of the RNA is produced in the nucleus on a DNA template, thereafter
being exported to the cytoplasm through pores in the nuclear membrane. In both DNA and RNA the pentose is linked to the C1 atom through a glycosidic linkage to the N9 atom of the purine group, or the N1 of the pyrimidine group (Figure 3a). DNA comprises two strands wound in the well-dened helical structure. Each nucleotide of one strand is associated through hydrogen bonding to a complimentary nucleotide on the other strand (AT and GC), with the deoxyribose and phosphate groups performing structural roles (Figure 3b,c). Genes, the hereditary material in the nucleus of human cells, are coded by long chains of double helical DNA packed into 23 chromosomes. The human genome is considered to contain between 70 000 and 100 000 genes. The four bases, the purines A and G and the pyrimidines T and C, linked through a sugarphosphate backbone, carry the genetic information of all prokaryotic and eukaryotic organisms, the innite variation in genetic programming being achieved by the particular sequence of these bases (Figure 3).
Why do cells need to degrade nucleotides?
The roles of adenosine triphosphate (ATP) (Figure 2) as the universal energy source, of guanosine triphosphate (GTP) in signal transduction and translation, and of uridine triphosphate (UTP) and cytidine triphosphate (CTP) as vital intermediates in the synthesis of the pyrimidine sugars and lipids involved in protein glycosylation and membrane biosynthesis, is well documented. During the wear and tear of daily life (muscle work, metabolism, wound healing, red cell senescence, etc.) a considerable amount of ATP is turned over daily. By contrast, DNA is relatively stable, except in cells with a high turnover rate, such as the skin, the lymphoid system (when required to mount an immune response), or the gut where there is a constant migration of cells from crypt to villus. In these circumstances DNA must be degraded also. Because de novo synthesis is energetically
1
ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Nature Publishing Group / www.els.net
Nucleotide Degradation
PYRIMIDINE O Uridine HN 3 4 5 CH monophosphate O C 2 1 6 CH N OH2C 5 O P O O (DNA) (RNA) Ribose 1 O O H H 4 H 2 3 H TMP TMP C OH OH HN CH C Thymidine Cytidine Uridine O N H H CH OH2C H H OH Nucleosides C Nucleotides
PURINE O HN 1 HC
2
C
6
5C
N
7 8 CH 9
Inosine 5monophosphate O P O O (RNA)
3 4C
N
1
H2C O 5 O
Ribose
2 3
H GMP
OH OH
Inosine
Guanosine
OH OH O HN O C C C CH3 O HN C O Bases C CH HN HC C C N CH H2N HN C C C C N CH N H Guanine O O
CH N H Thymine
CH N H Uracil
C N N Hypoxanthine H
O Metabolic end products O -Aminoisobutyric acid -Alanine HN C C C C N C O
N H Uric acid N
Figure 1 Metabolic end products of purine and pyrimidine degradation in humans. Routes of pyrimidine (green) and purine (red) mononucleotide degradation showing the structural formula and numbering of the atoms in the respective ring structures of UMP (uridine 5-monophosphate) and IMP (inosine 5-monophosphate), central intermediates in nucleotide degradation. CMP and UMP are degraded to uridine and largely recycled, since most human cells, except liver, lack uridine phosphorylase. A different battery of enzymes degrade TMP (thymidine 5-monophosphate) to thymine. The purine nucleotides IMP and GMP are degraded via the corresponding nucleosides to the constituent bases hypoxanthine and guanine respectively and recycled at this level. Further catabolism of nonrecycled degradation products in the liver leads to the formation of the metabolic end products b-alanine and baminoisobutyric acid for pyrimidines, uric acid for purines (blue).
NH2
5C 2 4 HC 3 C
N1
C
6
N
9 8 CH
O O
5
O O P O O
O P O O
expensive, recycling of the respective base, or nucleoside/ deoxynucleoside to which these ribo- and deoxyribonucleotides are degraded predominates (Figure 1), resulting in little loss of these vital chemicals to the body.
N
1
H2C O H
Ribose 2 3
P O
OH OH Adenosine 5-triphosphate ATP
Figure 2 Structural formula of ATP. Structural formula of adenosine 5triphosphate (ATP), indicating the numbering of the atoms in the ribose, as well as the purine ring.
Nucleotide catabolism is tissue, cell and species specific
In humans the above recycling occurs at the base level for purines and the nucleoside level for pyrimidines (Figure 1), while prokaryotes recycle purines at the nucleoside level and pyrimidines at the base level. These dierences are important, for example, when devising analogue therapy
ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Nature Publishing Group / www.els.net
Nucleotide Degradation
NH2 N O
O 7 6 N 1 4 3 2 C CH
CH 8
5 4
C5
Adenine
DNA
P O
CH2 H
O H H
N
1
Deoxy ribose
O N C C C A NH C C Guanine H2N T C G G A A G T C A
3 2
H CH O H
O
O
P O
CH2 H H O
H H NH2 O HC 5 O CH2 H H O O H H H H3C C O C Thymine NH O G C
4 N 3 6 1 2 HC C
(b)
Cytosine A O C
P O
Part of a DNA chain
P O
HC C N CH2 O H H H H H
(a)
(c)
Figure 3 Part of a DNA chain. (a) Structural formulae of the four constituent bases, adenine and guanine (red), cytosine and thymine (green), showing that the deoxyribose has an H group at the 2 position on the pentose ring, instead of the OH group of ribose. These bases are linked via the 3-OH group of the deoxyribose-phosphate moiety to the 5-OH group of the next deoxyribose. (b) Schematic representation of the manner in which the above bases are linked on strands making infinite variation possible, depending on the order of these bases. (c) Schematic representation of the role of hydrogen bonding between bases on opposite strands in contributing to the stability of this double helical structure. The base pair guanine cytosine has three hydrogen bonds, that for adenine thymine two. Colour code for (b) and (c) as for (a).
toxic to invading organisms but not to human cells. Microorganisms in the gut catabolize exogenous nucleotides. In humans, purine nucleotide synthesis and degradation, unlike that for pyrimidines, is considered to be entirely endogenous. The gut mucosa contains a battery of enzymes capable of degrading dietary purine to uric acid (Figure 4), which thus adds to the endogenous uric acid pool, leading to the clinical syndrome of gout (Stone and Simmonds, 1991). By contrast, the gut mucosa lacks enzymes capable of degrading uridine, as evidenced by the successful treatment of hereditary oroticaciduria with oral uridine (Scriver et al., 1995). However, the gut ora may contain enzymes that degrade uridine to uracil (e.g. Escherichia coli). It is equally essential to realize that although multiple pathways for nucleotide degradation exist in all mammalian species, only certain of these may be functional in a particular cell. Furthermore, these pathways and controls on them can be very dierent in malignant cells.
Because standard texts rarely dene the organism or species from which the data were derived, there is potential confusion regarding routes of nucleotide degradation. This arises because much of the information has been obtained from studies in yeasts, E. coli, Drosophila or rats and mice, etc., as well as malignant cells. The dierences can be considerable and can lead to much wasted time and money in endeavours to develop analogues for the treatment of human disease. For example, such dierences can mean that potentially useful drugs for humans will fail their obligatory animal trials and, conversely, that drugs found to be safe in animals may prove to be toxic to humans.
Degradation of DNA, RNA and the mononucleotides ATP, GTP, UTP and CTP
In tissues with a high rate of cell turnover the skin, gut, lung, lymphoid and haematopoietic systems both
3
ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Nature Publishing Group / www.els.net
Nucleotide Degradation
CO2
CO2
Purines
Pyrimidines
XDH
XDH Uric acid Uridine
URP DHP etc
Uric acid
Amino acids ( CO2 + NH3)
Figure 4 Fate of oral purine and pyrimidine nucleotides in humans. (a) In the gut dietary purine is degraded first by gut bacteria to CO2 (up to 50%). Any purine not so degraded is broken down by a battery of enzymes present in the intestinal mucosa which include xanthine dehydrogenase (XDH), thereby ensuring their conversion to uric acid prior to absorption. (b) Dietary pyrimidines are partially degraded to CO2 in the gut by bacteria. Because uridine phosphorylase (URP) is not present in the intestinal mucosa in humans, only in the liver, dietary pyrimidines escaping bacterial degradation are absorbed in the form of uridine. Uridine can then be taken up by tissues lacking URP. Uridine reaching the liver is degraded to uracil by URP and catabolized further to amino acids, as shown in Figure 1.
polynucleotides and mononucleotides must be degraded, ve steps being involved. This contrasts with tissues where DNA is relatively stable and the principal nucleotide turned over will be ATP (Figure 2). The initial degradation of DNA and RNA which results in the release of the respective deoxyribo- and ribomononucleotides (Figure 1) is catalysed by enzymes collectively called nucleases. Subsequent degradation depends on the cell type and involves either direct dephosphorylation or dephosphorylation plus deamination to yield the corresponding riboor deoxyribonucleoside (Figure 1). Cleavage of the glycosidic bonds of the dierent nucleosides/deoxynucleosides then yields the bases hypoxanthine and guanine, or uracil and thymine, as described below.
Enzymes Catalysing Dephosphorylation and Deamination of Purine Nucleotides
Dephosphorylation of nucleoside di- and triphosphates to monophosphates is performed by enzymes with dierent specicities from those degrading monophosphates. The former include nonspecic pyrophosphatases as well as
4
phosphatases specic for the dierent nucleoside diphosphates. Degradation of monophosphates to nucleosides may be catalysed also by nonspecic acid or alkaline phosphatases, regardless of the base or the phosphate position (2, 3 or 5). AMP is generally rst deaminated at the nucleotide level by AMP deaminase (AMPDA; EC 3.5.4.6) prior to dephosphorylation (Figure 5). AMPDA has at least three tissue-specic isoforms (Gross, 1997; McKusick, 1998). Isozymes of GMP reductase have not been identied. Xanthosine monophosphate (XMP) is not a metabolic intermediate in human cells. The reductive deamination of GMP to inosine monophosphate (IMP) requires NADPH (reduced nicotinamideadenine dinucleotide phosphate), while the conversion of AMP to IMP requires no coenzyme. In some tissues, AMP dephosphorylation may occur rst, with subsequent deamination at the nucleoside level catalysed by adenosine deaminase (ADA; EC 3.5.4.4). Specic nucleotidases dephosphorylating AMP, IMP or GMP have been the subject of extensive research, particularly by those studying the pharmacological actions of adenosine (Stone and Simmonds, 1991). In general, these nucleotidases appear to be tissue dened and relate specically to the function of that tissue. 5-Nucleotidases may be ecto- (on the outer membrane), or endo-nucleotidases, depending on the particular tissue, their activity being controlled by factors which carefully regulate their expression. The role of ecto-5-nucleotidase (5-NT, also known as CD73), which is expressed by a variety of cell types, has been studied in depth. The highly variable distribution of 5-NT suggests a tissue-specic regulation of expression. In some tissues, 5-NT and ADA appear to be expressed in a reciprocal manner, suggesting that they share genetic elements which coordinate adenine nucleotide degradation. Deoxy-AMP is not a substrate for AMPDA in humans and must be degraded rst to deoxyadenosine and thereafter by ADA. By contrast there is no comparable enzyme capable of deaminating guanosine/deoxyguanosine to inosine/deoxyinosine, deamination occurring at the nucleotide level from GMP to IMP, which can then be dephosphorylated under appropriate conditions. Xanthosine is not found in human cells. Purine nucleoside phosphorylase (PNP; EC 2.4.2.1) catalyses the only route of degradation of purine nucleosides and deoxynucleosides in mammals. Adenosine and deoxyadenosine are not substrates for PNP. However, guanosine, inosine or their deoxyanalogues derived from either ATP, dATP, IMP, GTP or dGTP all feed into this pathway to be degraded to hypoxanthine or guanine respectively, making PNP possibly the most important enzyme of purine nucleotide catabolism in mammals. Although the reaction catalysed by PNP is essentially reversible, in vivo studies conrm that PNP in humans functions primarily in the direction of nucleoside breakdown, the concentration of inorganic phosphate being considerably greater than that of either
ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Nature Publishing Group / www.els.net
Nucleotide Degradation
Purines ATP DNA d-AMP AMP RNA AMPDA1 RNA IMP GMP GTP DNA d-GMP CTP RNA (d)-CMP UMPH-1 (d)-Adenosine ADA (d)-Inosine PNP Hypoxanthine XDH (d)-Guanosine Cytidine UTP
Pyrimidines TTP RNA (d)-UMP DNA TMP Nucleoside (d)-Uridine Thymidine Nucleotide
Polyamines
Adenine
Guanine
Uracil DPD Dihydrouracil
Thymine
Base
Dihydrothymine DPA -Ureodoisobutyric acid UP -Aminoisobutyric acid BAIBPAT Methylmalonic semialdehyde Metabolic waste
8-Hydroxyadenine
Xanthine XDH -Ureodopropionic acid -Alanine BAKAT Malonic semialdehyde
2,8-Dihydroxyadenine
Uric acid
Figure 5 Enzymes defective in inborn errors of nucleotide degradation. Metabolic pathways of purine (red) and pyrimidine (green) nucleotide degradation, via the nucleoside and base to the respective metabolic end products (blue), indicating the enzymes (pink) deficient in genetic disorders affecting these pathways. The extra-purine origin of adenine (end product of the polyamine pathway, cyan) and its degradation to 2,8-dihydroxyadenine by xanthine dehydrogenase (XDH), when the salvage enzyme adenine phosphoribosyltransferase is defective, is indicated in the inset on the left. Abbreviations: ADA, adenosine deaminase; AMPDA, myoadenylate deaminase; BAPAT, b-alanine pyruvate aminotransferase; BAKAT, b-alanine ketoglutarate aminotransferase; BAIBPAT, b-amino isobutyrate pyruvate aminotransferase; DHP, dihydropyrimidinase; DPD, dihydropyrimidine dehydrogenase; PNP, purine nucleoside phosphorylase; UMPH-1, UMP-hydrolase (or Py5-N, pyrimidine 5-nucleotidase); UP, ureidopropionase; XDH, xanthine dehydrogenase.
(2-deoxy) ribose-1-phosphate, or hypoxanthine and guanine (Figure 1). In the interests of whole body economy, most of the hypoxanthine and guanine formed is largely recycled, only a small fraction being lost to the body as metabolic waste. Thus purine nucleotide catabolism essentially stops at this point.
Species Differences in the Enzymes of Nucleoside Degradation
The obligatory prior deamination of adenine nucleotides to IMP, or adenine nucleosides to inosine or deoxyinosine, relates to the fact that adenosine phosphorylase is not detectable in mammalian cells (Figure 5). By contrast, adenosine phosphorylase is abundant in certain prokaryotes, being used as a reliable indicator of mycoplasma infection. An adenosine cycle is reportedly present in trypanosomes which, unlike mammals, lack ADA. Another striking dierence in prokaryotes is that adenine formed in this way is further catabolized to hypoxanthine by adenine deaminase, also not present in human cells. Adenine in humans is derived exclusively as a byproduct of the polyamine pathway (Stone and Simmonds, 1991). Adenine accumulating when the salvage enzyme adenine
phosphoribosyltransferase (APRT; EC 2.4.2.7) is defective is degraded by xanthine dehydrogenase (XDH) to 2,8dihydroxyadenine (Figure 5). Considerable species dierences also exist in the activity of PNP, which is ubiquitously distributed in human cells, activity being extremely high in the erythrocyte. The release of inosine from ischaemic human but not rat heart illustrates the very low PNP activity in human heart, indicating considerable tissue-specic variation in expression for this enzyme too.
Removal of Metabolic Waste from Nucleotide Degradation: Uric Acid is the End-product of Purine Catabolism Only in Humans
The nal steps of nucleotide degradation leading to the irretrievable loss of the purine bases guanine and hypoxanthine are catalysed by guanine aminohydrolase (guanase; EC 3.5.4.3) and xanthine dehydrogenase (XDH; EC 1.2.1.37) respectively. Xanthine formed by either route is a metabolic end-product and does not normally accumulate, being further oxidized by XDH to uric acid
5
ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Nature Publishing Group / www.els.net
Nucleotide Degradation
(Figure 4). Expression of XDH is also tissue-specic and subject to considerable species variation, being conned exclusively to the liver and intestinal mucosa in humans and pigs only (Scriver et al., 1995). By contrast, it is ubiquitously distributed in rodents, dogs, cats and bovine species, while camels appear to lack XDH activity. Uric acid is the end-product of purine nucleotide catabolism only in humans, birds and some reptiles. Most mammalian species possess the enzyme uricase, which converts the insoluble uric acid to the more soluble allantoin. Although humans possess the gene for uricase the enzyme is not expressed (Scriver et al., 1995).
Purine Ribonucleotide Catabolism in Healthy Humans
Degradation of the adenine nucleotides varies in different tissues
ATP represents the largest single ribonucleotide pool in the human body, the ATP content of skeletal muscle (4 5 mmol kg 2 1) being twice that in brain and three times that in human erythrocytes (Stone and Simmonds, 1991). Intense muscular exercise can deplete the total adenine nucleotide pool (ATP/ADP/AMP) by about 15% with a corresponding increase in urinary hypoxanthine, but this ATP pool is restored rapidly, generally within 30 minutes. However, catabolism in muscle rarely proceeds beyond IMP, the prime function of the adenine nucleotide cycle, which essentially involves the interconversion of AMP and IMP, being the maintenance of ATP pools. The tissue specicity of purine degradation in skeletal muscle relates to the high activity and low Km of the muscle-specic isozyme of AMPDA, myoadenylate deaminase (AMPD1; EC 3.5.4.6), catalysing the conversion of AMP to IMP. This directs AMP catabolism to the nondiusible IMP, where a low IMP 5-nucleotidase activity prevents dephosphorylation to the diusible inosine and ensures recycling to AMP. In heart there is also a constant need for an uninterrupted supply of ATP, but the controls on the nucleotide cycle are quite dierent. The rst part of the cycle is essentially inoperative due to the low activity of AMPDA resulting from a high Km for AMP and subtle controls by phospholipids. This regulation by phospholipids appears to be lost in the necrotic heart, mimicking infarct, but is regained during the recovery period. The low activity of AMPDA, coupled with a 3-fold higher activity of the ectoand endonucleotidases specic for AMP particularly the endothelial cell ecto-5-nucleotidase ensures the formation of the diusible and vasoactive adenosine necessary to relax vascular tone. Such adenosine is then recycled to AMP, depending on the energy state of the cell, only a minor portion being degraded by ADA to inosine and
6
thence hypoxanthine. Thus, the cycle in the human heart involves the interconversion of AMP and adenosine and is geared to providing a rapid bolus of adenosine in times of hypoxia. Studies of ATP catabolism in human brain are scanty, but the brain is exquisitely sensitive to changes in ATP as well as oxygen supply. Brain has unusually high ATP requirements for both protein and nucleoprotein synthesis. This would involve a considerable turnover of both ATP and GTP. As in heart the Km for AMPDA is high. Changes in membrane lipid composition, induced by ageing or pathology, might thus inuence cellular metabolism by controlling the ow of adenine nucleotide metabolites toward adenosine production. By contrast, the kidney appears to combine elements of ATP degradation present in both skeletal and heart muscle, but the adenosine released when controls on AMPDA favour degradation via 5-nucleotidase acts as a vasoconstrictor, not a vasodilator (Stone and Simmonds, 1991). In liver, the nucleotide prole is much more complex than in muscle, undoubtedly due to the diverse synthetic and detoxifying functions. Knowledge of purine degradative pathways derives principally from fructose-loading studies, where rapid utilization of inorganic phosphate (Pi), results in GTP and Pi depletion, both potent inhibitors of liver AMPDA. ATP concentrations suer a 10-fold reduction, degradation occurring via AMPDA, not ADA. The fact that liver does not contain signicant amounts of fast mobilizable energy in the form of creatine phosphate, coupled with the high XDH activity, can result in the irretrievable loss of ATP as uric acid under appropriate conditions. Since most other human tissues lack XDH, there is normally very little loss of purines to the body as uric acid, as already discussed.
Inherited defects of purine nucleotide catabolism
The metabolites accumulating, coupled with the clinical consequences of inherited enzyme defects of nucleotide catabolism, have provided valuable insights into the importance of purine catabolic pathways in humans. Myoadenylate deaminase deciency is the most common enzyme defect in humans (Figure 5). Although the frequencies of the mutant allele are high, the disorder has a low incidence and is relatively benign, the principal symptom being exercise-related cramps and myalgias (Gross, 1997). Deciency of the other deaminating enzyme, ADA, presents as a potentially lethal immunodeciency, with severe lymphopenia involving both T and B cells. The lymphospecic toxicity in this defect relates to the fact that ADA represents the only route of deoxyadenosine nucleotide catabolism in human cells. dAMP is not a substrate for AMPDA. The accumulating deoxyadenosine is converted to dATP which inhibits a variety of
ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Nature Publishing Group / www.els.net
Nucleotide Degradation
enzymes essential to lymphocyte proliferation and apoptosis ensues (Stone and Simmonds, 1991). The companion T lymphocyte disorder, PNP deciency, has underlined the importance of PNP, as well as ADA, in degrading toxic DNA waste (Figure 5). PNP deciency generally presents rst with neurological abnormalities, similar to those in severe hypoxanthineguanine phosphoribosyltransferase (HPRT) deciency (Scriver et al., 1995). This nding highlights the importance of HPRT to the human brain, for although HPRT is not defective it cannot function without the substrates (hypoxanthine, guanine) normally provided by PNP. Interestingly, successful bone marrow transplantation has corrected the immunological problems in PNP deciency, but not the neurological abnormalities (Carpenter et al., 1996). Thus correction of the latter in both PNP and HPRT deciency will require strategies to deliver the enzyme directly to the brain. Deciency of XDH was the rst defect reported in humans (Scriver et al., 1995). Clinical problems (predominantly renal) relate solely to the insolubility of this metabolic waste. Classical xanthinuria results either from an isolated deciency of XDH type 1, or type 11. The latter is due to a deciency of both XDH and aldehyde oxidase (AOX; EC 1.2.3.1). AOX is considered to have arisen from XDH by gene duplication. These two enzymes, as well as sulphite oxidase (SO; EC 1.8.2.1) are non-functional in molybdenum cofactor deciency, when the clinical features of SO deciency overshadow those of XDH deciency. Presentation with neonatal tting, ocular lens dislocation and death in the rst year is common (Scriver et al., 1995). However, for all the above disorders there is considerable heterogeneity in clinical expression, both within and between families, and late presentation is not infrequent.
basis for XDH2 is undened. The MOCS1 gene (molybdenum cofactor synthesis, step 1) is unusual in that alternative open reading frames direct the synthesis of two dierent proteins from the same mRNA. Mutations aecting the function of either protein result in molybdenum cofactor deciency.
Enzymes Catalysing Dephosphorylation and Deamination of Pyrimidine Nucleotides
Analogously to the purine nucleotides, dephosphorylation of the pyrimidine nucleoside, di- and triphosphates is performed by nonspecic (pyro)phosphatases. Monophosphates are also dephosphorylated to nucleosides by nonspecic phosphatases or specic nucleotidases. dCMP is generally rst deaminated to dUMP at the nucleotide level by dCMP deaminase (EC 3.5.4.12) prior to dephosphorylation (Figure 5). Dephosphorylation of UMP, CMP and dTMP to the corresponding nucleosides uridine, cytidine and thymidine is carried out by UMP hydrolase (pyrimidine-5-nucleotidase, Py5-N, UMPH: EC 3.1.3.5). Two specic pyrimidine-5-nucleotidases, UMPH-1 and 2, have been identied in human erythrocytes which have dierent anities towards the various substrates (Paglia et al., 1984). UMPH-1 does not dephosphorylate purine nucleotides or the 2-pyrimidine-, 3-pyrimidine-, or cyclic pyrimidine nucleotides. Activity with UMP and CMP is about twice that with dTMP, while UMPH-2 is specic for the deoxynucleotides dUMP and dTMP. There is also a nucleotidase specic for human erythrocyte OMP (orotidine 5-monophosphate).
Genetics and molecular biology of purine degradation defects
AMPD1 and AMPD2 are closely linked on chromosome 1, have a similar sequence and gene structure and are likely to have arisen by gene duplication (McKusick, 1998). Two mutations, a nonsense mutation in exon 2 and a missense mutation in exon 3 of AMPD1 occur at a high frequency in some population groups. In the homozygous state these mutations are associated with AMPD1 deciency. Alternative splicing of exon 2 may ameliorate the severity of the phenotype by eliminating the nonsense mutation in a proportion of mRNA transcripts, producing some functional enzyme. The DNA sequences and chromosomal locations of ADA (20q1220q11), PNP (14q11.2) and XDH1 (2p22.3 2p22.20) have been established (McKusick, 1998). Mutations at these loci are heterogeneous. All three show an autosomal recessive pattern of inheritance. The genetic
Enzymes catalysing degradation of nucleosides
As mentioned above, salvage of the pyrimidine ribonucleosides/deoxyribonucleosides occurs at the nucleoside level in most human tissues, there being little degradation to the constituent base (Figure 1). As for purines, there is also no direct degradation of cytidine/deoxycytidine to the constituent base, both must rst be deaminated by specic deaminases (EC 3.5.1.4.5/EC 3.5.4.14) to uridine/deoxyuridine. Here too, there is considerable species as well as tissue-specic variation in enzyme expression, activity being high in malignant cells, with little activity in most normal human cells, while the enzyme is widely distributed in rodent cells. Uridine/deoxyuridine deriving from degradation, or deamination and not recycled, are degraded to uracil by uridine phosphorylase (EC 2.4.2.3), present only in liver in humans. Species dierences also apply for this enzyme. Although this reaction is essentially
7
ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Nature Publishing Group / www.els.net
Nucleotide Degradation
reversible also, the in vivo concentration of (2-deoxy)ribose 1-phosphate is too low for it to proceed (Figure 1). Thymidine can be degraded to thymine by a specic thymidine phosphorylase (EC 2.4.2.4) which in humans only seems to be present in platelets and macrophages. Such cell-specic dierence can be used advantageously (e.g. to treat viral infections such as human immunodeciency virus (HIV) with pyrimidine analogues).
Enzymes catalysing the degradation of the pyrimidine bases
The rst three steps in the degradation of uracil and thymine are catalysed by the same three enzymes which convert these bases into b-alanine and b-aminoisobutyric acid, respectively (Figure 5): dihydropyrimidine dehydrogenase (DPD; EC 1.3.1.2), dihydropyrimidinase (DHP; EC 3.5.2.2) and ureidopropionase (UP; EC 3.5.1.6). Subsequent degradation is performed by three separate aminotransferases. b-Alanine is converted into malonic semialdehyde by b-alaninepyruvate aminotransferase (BAPAT; EC 2.6.1.18) and b-alanineketoglutarate aminotransferase (BAKAT; EC 2.6.1.19), while b-aminoisobutyric acid is converted into methylmalonic semialdehyde by the action of b-aminoisobutyratepyruvate aminotransferase (BAIBPAT; EC 2.6.1.40). DPD, BAIBPAT, BAPAT and BAKAT are expressed in various tissues although at dierent levels (Van Gennip et al., 1997). By contrast, DHP and UP are expressed only in liver (Figure 4). The two semialdehydes are subsequently metabolized in the citric acid cycle.
(Scriver et al., 1999). Defects of the rst three enzymes (DPD, DHP and UP), shared by uracil and thymine, present with a variable clinical picture comprising seizures, psychomotor retardation, microcephaly, dysmorphic features, growth retardation and ocular abnormalities (Van Gennip et al., 1997). DPD, DHP and UP deciency all lead to reduced concentrations of the neurotransmitter balanine. The one reported case with a complete deciency of BAKAT suered from hypotonia, hyporeexia, generalized tonic clonic seizures, intermittent lethargy and died in infancy. A deciency of this enzyme leads to accumulation of b-alanine. Partial deciency of BAKAT (50% of normal) leading to hyper-b-alaninaemia is associated with Cohen syndrome. A deciency of BAIBPAT in liver has been proposed as the cause of permanent hyper-b-aminoisobutyric aciduria. This genetically determined phenomenon is thought to be a benign polymorphism. Deciency of BAPAT has not yet been discovered in humans (Van Gennip et al., 1997).
Genetics and molecular biology of pyrimidine degradation defects
The various defects of pyrimidine degradation are inherited as autosomal recessive traits. At present two separate structural genes are assumed for Py5-N-1 (UMPH-1) and Py5-N-2 (UMPH-2) respectively. However, since the genes have not yet been isolated, mutations are unknown. The entire human DPD gene consists of 23 exons, is at least 950 kb in length with 3 kb of coding sequence and a minimal average intron size of about 43 kb. It has been mapped to chromosome 1p22 and is present as a single copy gene. Seven dierent mutations have been reported so far, including one splice-site mutation, two deletions and four missense mutations (Van Kuilenberg et al., 1999). The human DHP gene spans over 80 kb and contains 10 exons. All introns contain the conserved 5-GT splice donor and 3-AG splice acceptor sites. The gene has been assigned to chromosome 8q22 and also exists as a single copy. One frameshift mutation and ve missense mutations have been established (Hamajima et al., 1998). At present, the human genes for the other enzymes of the pyrimidine degradation pathways or mutations causing disease have not yet been reported.
Inherited defects of pyrimidine nucleotide catabolism
As for purines, these defects have provided valuable insights into normal catabolic routes in humans. Py5-N (UMPH-1) deciency (Figure 5) is the only defect directly aecting pyrimidine nucleotide degradation, the eects being conned to blood cells (Paglia et al., 1984). UMPH-1 hydrolyses nucleotides to the corresponding nucleosides in the developing erythrocyte, which can be transported to other tissues for recycling. The defect results in substantial accumulation of cytidine and uridine nucleotides can be demonstrated in lymphocytes, granulocytes (De Korte et al., 1989) and erythrocytes. However, the defect is seemingly only harmful to erythrocytes, resulting in nonspherocytic haemolytic anaemia with prominent basophilic stippling. In contrast to the purines, defects of uridine and thymidine phosphorylase have not yet been reported in humans. All other defects of pyrimidine catabolism occur within the four-step pathway (Figure 5) which degrades the bases uracil and thymine to their corresponding amino acids b-alanine and b-aminoisobutyric acid respectively
8
What Can We Learn from Genetic Metabolic Defects of Purine and Pyrimidine Metabolism?
Defects of enzymes catalysing purine and pyrimidine nucleotides provide a valuable insight into the
ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Nature Publishing Group / www.els.net
Nucleotide Degradation
tissue-specic function of a particular enzyme. The broad spectrum of presentation, involving the immunological, haematological, neurological or renal systems also highlights the importance of particular steps to the integrated functioning of this network in humans. The altered concentrations of b-alanine resulting from the pyrimidine degradation defects may be of relevance with regard to understanding the cerebral dysfunction often seen in patients with these defects. The neurotransmitter b-alanine is a structural analogue of glycine and GABA (gaminobutyric acid), the major inhibitory neurotransmitters of the central nervous system (Van Kuilenberg et al., 1999). Thus altered levels might have a profound eect on the activation of glycine and GABA receptors as well as glial transport into glial cells, as has been demonstrated in chick spinal cord neurons and mouse brain. Both purine and pyrimidine enzyme defects can also be the cause of catastrophic responses to antimetabolite therapy (Scriver et al., 1995, 2000). Examples are deciencies in thiopurine methyltransferase (TPMT; EC 2.1.1.67) and XDH which provide alternative degradative routes for thiopurine analogues. Polymorphism for TPMT deciency occurs in 11% of caucasians. Deciency will thus increase the eective therapeutic dose, leading to lifethreatening bone marrow depression, even in TPMT carriers. Severe neurotoxicity due to 5-uorouracil (5FU), which may result from exposure of the nervous system to relatively high concentrations of 5-FU, leading to increased incorporation into cellular RNA, can also occur in individuals with less than 30% of normal DPD activity. Depletion of b-alanine by 5-FU acting as competitive substrate in partial DPD deciency may also contribute to this condition (Scriver et al., 1999). Similarly, although not yet reported, increased sensitivity to 5-FU may be expected in DHP deciency.
Furthermore, these pathways, and controls on them, can be very dierent in malignant cells, as well as varying greatly between humans and other mammals. Inborn errors of purine and pyrimidine metabolism have provided valuable insight into the relative importance of these pathways and their diering controls.
References
Carpenter PA, Ziegler JB and Vowels MR (1996) Late diagnosis of purine nucleoside phosphorylase deciency with allogeneic bone marrow transplantation. Bone Marrow Transplantation 17: 121124. De Korte D, Van Doorn CCH, Sijstermans JMJ, Van Gennip AH and Roos D (1989) Deciency of pyrimidine 5-nucleotidase in human leukocytes. Journal of Inherited Metabolic Disorders 12: 267272. Gross M (1997) Clinical heterogeneity and molecular mechanisms in inborn muscle AMP deaminase deciency. Journal of Inherited Metabolic Disorders 20: 186192. Hamajima N, Kouwaki M, Vreken P et al. (1998) Dihydropyrimidinase deciency: structural organisation, chromosomal localization and mutation analysis of the human dihydropyrimidinase gene. American Journal of Human Genetics 63: 717726. McKusick VA (1998) Mendelian Inheritance in Man. Catalogs of Human Genes and Genetic Disorders, 12th edn. Baltimore: Johns Hopkins University Press. Paglia DE, Valentine WN and Brockway RA (1984) Identication of thymidine nucleotidase and deoxyribonucleotidase activities among normal isoenzymes of 5-nucleotidase in human erythrocytes. Proceedings of the National Academy of Sciences of the USA 81: 588592. Scriver CR, Beaudet AL, Sly WS and Valle D (eds) (1995) The Metabolic and Molecular Basis of Inherited Disease, 7th edn, vol. II, chaps 4955, Purines and pyrimidines, pp. 16551940. New York: McGraw-Hill. Scriver CR, Beaudet AL, Sly WS et al. (eds) (2000) The Metabolic and Molecular Basis of Inherited Disease, 8th edn, Purines and pyrimidines. New York: McGraw-Hill. (In press). Stone TW and Simmonds HA (1991) Purines: Basic and Clinical Aspects. London: Kluwer. Van Gennip AH, Abeling NGGM, Vreken P and Van Kuilenburg ABP (1997) Inborn errors of pyrimidine degradation: clinical biochemical and molecular aspects. Journal of Inherited Metabolic Diseases 20: 203213. Van Kuilenburg ABP, Vreken P, Abeling NGGM et al. (1999) Genotype and phenotype in patients with dihydropyrimidine dehydrogenase deciency. Human Genetics 104: 19.
Summary
Nucleotide degradation is part of an integrated network which ensures that the component base or nucleoside is recycled, thereby sparing nucleotide synthesis via the de novo route. A considerable amount of ATP and to a lesser extent GTP, DNA and RNA is turned over daily, but only a small fraction of this is lost to the body as uric acid or amino acids in humans. Although multiple pathways for nucleotide degradation exist in all species, only some of these may be functional in a particular cell type.
Further Reading
Henderson JF and Paterson ARP (1973) Nucleotide Metabolism: An Introduction. New York: Academic Press. Simmonds HA (1994) Purine and pyrimidine disorders. In: Holton JB (ed.) The Inherited Metabolic Diseases, chap. 6, pp. 297330. Edinburgh: Churchill Livingstone.
ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Nature Publishing Group / www.els.net
Vous aimerez peut-être aussi
- Revised BSC Nursing SyllabusDocument218 pagesRevised BSC Nursing SyllabusManjunatha HR86% (7)
- Step 1 BiochemistryDocument12 pagesStep 1 Biochemistrylotusnelum7100% (2)
- (Bernhard Ø. Palsson) Systems Biology SimulationDocument332 pages(Bernhard Ø. Palsson) Systems Biology SimulationTecuitlatl100% (2)
- From My Students Most of AllDocument147 pagesFrom My Students Most of AllRamin HamzaPas encore d'évaluation
- Nucleic AcidDocument88 pagesNucleic AcidJoyce Jimenez100% (1)
- Pyrimidine SynthesisDocument35 pagesPyrimidine Synthesisdr.yogita.rajPas encore d'évaluation
- BIO 1412: Molecular Biology and Genetics LectureDocument119 pagesBIO 1412: Molecular Biology and Genetics LectureJOHN MVULA IIPas encore d'évaluation
- Alkaloids Induce Programmed Cell Death in Bloodstream Forms of Trypanosomes (Trypanosoma B. Brucei)Document12 pagesAlkaloids Induce Programmed Cell Death in Bloodstream Forms of Trypanosomes (Trypanosoma B. Brucei)Kayo PaivaPas encore d'évaluation
- Cells: Yeast To Study Human Purine Metabolism DiseasesDocument15 pagesCells: Yeast To Study Human Purine Metabolism DiseasesKanaPas encore d'évaluation
- Chapter 14 Biomolecules Worksheet Answers Set 4Document9 pagesChapter 14 Biomolecules Worksheet Answers Set 4kyomuhendobright4Pas encore d'évaluation
- A Stereoselective Approach To Nucleosides andDocument4 pagesA Stereoselective Approach To Nucleosides andcarloscampanherPas encore d'évaluation
- Genetika ManusiaDocument65 pagesGenetika Manusiadies100% (1)
- Tutorial 3 - Biology 101 Answer MemoDocument18 pagesTutorial 3 - Biology 101 Answer MemoKaizer NdoloPas encore d'évaluation
- Nucleic Acid Structure and FunctionDocument30 pagesNucleic Acid Structure and FunctionZayan HaiderPas encore d'évaluation
- DNA Structure and Function ExplainedDocument10 pagesDNA Structure and Function ExplainedEllu SeethaPas encore d'évaluation
- Synthesis and Degra NuclotidesDocument58 pagesSynthesis and Degra Nuclotidesur.yared21Pas encore d'évaluation
- Zamow Art PDF - PHTMLDocument17 pagesZamow Art PDF - PHTMLمحمد نعيمPas encore d'évaluation
- The Replication of DNA: NiveditaDocument53 pagesThe Replication of DNA: NiveditaLalruatdiki CPas encore d'évaluation
- Nucleotide Metabolism PPDocument65 pagesNucleotide Metabolism PPCLEMENTPas encore d'évaluation
- Nucleobase: Nucleobases, Also Known As Nitrogenous BasesDocument5 pagesNucleobase: Nucleobases, Also Known As Nitrogenous BasesLuis Felipe Mera GrandasPas encore d'évaluation
- First Professional Mbbs Fourth Semester EXAMINATION SESSION-2007-2008 Subject - BiochemistryDocument9 pagesFirst Professional Mbbs Fourth Semester EXAMINATION SESSION-2007-2008 Subject - BiochemistryIsrasami NaparPas encore d'évaluation
- Chapter 1. Molecular Basis of Genetics: DNA R NA ProteinDocument54 pagesChapter 1. Molecular Basis of Genetics: DNA R NA Protein뷸한연Pas encore d'évaluation
- Nucleic Acids BC21C 2018 StudentDocument120 pagesNucleic Acids BC21C 2018 StudentPheona BlagrovePas encore d'évaluation
- Heartfelt Enlightenment: News and ViewsDocument3 pagesHeartfelt Enlightenment: News and Viewsbenefits35Pas encore d'évaluation
- Ôn Thi SHPTDocument7 pagesÔn Thi SHPTHạ Thi LêPas encore d'évaluation
- Nucleic Acid (Organic Chemistry) : By: Septiana Saputri (1800023180)Document7 pagesNucleic Acid (Organic Chemistry) : By: Septiana Saputri (1800023180)septianasptrPas encore d'évaluation
- DNA Damage and RepairDocument7 pagesDNA Damage and RepairAparna AbiPas encore d'évaluation
- Material Review of Nucleic AcidDocument9 pagesMaterial Review of Nucleic AcidIrvandar NurviandyPas encore d'évaluation
- Notes On Nucleic AcidsDocument12 pagesNotes On Nucleic AcidsChristian Josh EspedillonPas encore d'évaluation
- Some Answer of Problemset - 7 - KEYDocument3 pagesSome Answer of Problemset - 7 - KEYNihir PatelPas encore d'évaluation
- The Warburg Effect and Its Cancer Therapeutic Implications: Mini ReviewDocument8 pagesThe Warburg Effect and Its Cancer Therapeutic Implications: Mini ReviewAndrea RangelPas encore d'évaluation
- Carcinogen Metabolism: BC DeckerDocument3 pagesCarcinogen Metabolism: BC DeckerTanu WijayaPas encore d'évaluation
- DNA Damage and RepairDocument47 pagesDNA Damage and RepairdewiulfaPas encore d'évaluation
- Molecular Biology 2022Document52 pagesMolecular Biology 2022Ming MingPas encore d'évaluation
- Nucleic acid study notes - Fall 2018Document10 pagesNucleic acid study notes - Fall 2018ReggiePas encore d'évaluation
- DNA Structure and ReplicationDocument30 pagesDNA Structure and ReplicationasaadsarfrazPas encore d'évaluation
- Biochemistry Book Additional PlatesDocument8 pagesBiochemistry Book Additional PlatesKiana TehraniPas encore d'évaluation
- Bacterial Mutation Types, Mechanisms and Mutant Detection Methods: A ReviewDocument11 pagesBacterial Mutation Types, Mechanisms and Mutant Detection Methods: A ReviewDenisse PxndithxPas encore d'évaluation
- Nucleic Acids and The Code of LifeDocument42 pagesNucleic Acids and The Code of LifePau LopezPas encore d'évaluation
- Nucleic Acids. Dna. Rna. Nucleic AcidsDocument7 pagesNucleic Acids. Dna. Rna. Nucleic Acidsm_tankovaPas encore d'évaluation
- How protein folding determines diseases like Alzheimer's and Parkinson'sDocument2 pagesHow protein folding determines diseases like Alzheimer's and Parkinson'sDarren ZimmermanPas encore d'évaluation
- Cisplatin As An Anti Cancer DrugDocument10 pagesCisplatin As An Anti Cancer DrugMahima KamraPas encore d'évaluation
- Must Review ThisDocument19 pagesMust Review Thispmp123456Pas encore d'évaluation
- Magni2004 Article EnzymologyOfNADHomeostasisInMaDocument16 pagesMagni2004 Article EnzymologyOfNADHomeostasisInMaLeidy Constanza Villalobos GonzalezPas encore d'évaluation
- As Trial Jan 14 p1 QDocument15 pagesAs Trial Jan 14 p1 QArvin DiNozzoPas encore d'évaluation
- Diseases of MetabolismDocument35 pagesDiseases of MetabolismBushra YaqubPas encore d'évaluation
- Mol Bio Part 1 2014Document95 pagesMol Bio Part 1 2014Manish SoniPas encore d'évaluation
- 2005 Prevention of Obesity in MiceDocument9 pages2005 Prevention of Obesity in MiceFerroBemPas encore d'évaluation
- Seminar of Cell Culture TechniquesDocument55 pagesSeminar of Cell Culture TechniquesAhmed J AlhindawePas encore d'évaluation
- MDSC1104 Tutorial on Hereditary Material and Gene ExpressionDocument2 pagesMDSC1104 Tutorial on Hereditary Material and Gene ExpressionJordan ConstantinePas encore d'évaluation
- Nucleotides and Nucleic AcidDocument56 pagesNucleotides and Nucleic AcidMrs RehanPas encore d'évaluation
- Lac TranspDocument13 pagesLac Transpfarinha2009Pas encore d'évaluation
- The Spectrum of Mitochondrial Disease Ep-3-10Document8 pagesThe Spectrum of Mitochondrial Disease Ep-3-10F4AR100% (1)
- Biochem Metabolismo Ácidos NucléicosDocument27 pagesBiochem Metabolismo Ácidos NucléicosLidia Escutia GuadarramaPas encore d'évaluation
- CH. 19 Replication, Repair and Recombination-2Document69 pagesCH. 19 Replication, Repair and Recombination-2IffatnazPas encore d'évaluation
- Mitochondrial DnaDocument10 pagesMitochondrial DnaGerardo GonzalezPas encore d'évaluation
- Nucleic Acids From WileyDocument38 pagesNucleic Acids From Wileyahmad jamalPas encore d'évaluation
- Biochimie Volume 81 Issue 6-Supp-S1 1999 (Doi 10.1016/s0300-9084 (99) 80112-6) - The Sir Hans Krebs LectureDocument390 pagesBiochimie Volume 81 Issue 6-Supp-S1 1999 (Doi 10.1016/s0300-9084 (99) 80112-6) - The Sir Hans Krebs LectureAllcrissPas encore d'évaluation
- PKD BiochemistryDocument7 pagesPKD BiochemistryHyun Jae WonPas encore d'évaluation
- Paper - IV Molecular BiologyDocument236 pagesPaper - IV Molecular BiologyNaruto UzumakiPas encore d'évaluation
- Benzing-2021-Insights Into Glomerular FiltrationDocument10 pagesBenzing-2021-Insights Into Glomerular FiltrationNguyen LequyenPas encore d'évaluation
- Electroporation: Jac A NickoloffDocument3 pagesElectroporation: Jac A Nickoloffmanoj_rkl_07Pas encore d'évaluation
- Ca Binding Proteins A0001347-001-000 PDFDocument8 pagesCa Binding Proteins A0001347-001-000 PDFmanoj_rkl_07Pas encore d'évaluation
- Evolution of Genome Organizn1699-001-000 PDFDocument5 pagesEvolution of Genome Organizn1699-001-000 PDFmanoj_rkl_07Pas encore d'évaluation
- 2,4-Thiazolidinedione As Antimicrobial and Cytotoxic AgentsDocument10 pages2,4-Thiazolidinedione As Antimicrobial and Cytotoxic Agentsmanoj_rkl_07Pas encore d'évaluation
- Evolution of Ecosystems - Terrestrial PDFDocument4 pagesEvolution of Ecosystems - Terrestrial PDFmanoj_rkl_07Pas encore d'évaluation
- Electron Carriers PDFDocument8 pagesElectron Carriers PDFmanoj_rkl_07100% (1)
- BMP Antags &nural Inducna0000805-001-000 PDFDocument5 pagesBMP Antags &nural Inducna0000805-001-000 PDFmanoj_rkl_07Pas encore d'évaluation
- Evolution of Development A0001661-001-000 PDFDocument4 pagesEvolution of Development A0001661-001-000 PDFmanoj_rkl_07Pas encore d'évaluation
- Ephrins: Ru Diger KleinDocument6 pagesEphrins: Ru Diger Kleinmanoj_rkl_07Pas encore d'évaluation
- FGF1Document9 pagesFGF1manoj_rkl_07Pas encore d'évaluation
- Plant Macro-And Micronutrient MineralsDocument5 pagesPlant Macro-And Micronutrient Mineralsmanoj_rkl_07Pas encore d'évaluation
- Cyanogenesis in Higher Plant and InsectsDocument3 pagesCyanogenesis in Higher Plant and Insectsmanoj_rkl_07Pas encore d'évaluation
- DNA Repair by Reversal of Damage PDFDocument8 pagesDNA Repair by Reversal of Damage PDFmanoj_rkl_07100% (1)
- DNA Damage: Paul W DoetschDocument7 pagesDNA Damage: Paul W Doetschmanoj_rkl_07Pas encore d'évaluation
- Forward-Cristopher Reeve PDFDocument1 pageForward-Cristopher Reeve PDFmanoj_rkl_07Pas encore d'évaluation
- Calibrating a UV Transilluminator for DNA FixationDocument2 pagesCalibrating a UV Transilluminator for DNA Fixationmanoj_rkl_07Pas encore d'évaluation
- Flocyt Anal Nstem Cel in DevDocument11 pagesFlocyt Anal Nstem Cel in Devmanoj_rkl_07Pas encore d'évaluation
- Heavy Metal Adaptation PDFDocument4 pagesHeavy Metal Adaptation PDFmanoj_rkl_07Pas encore d'évaluation
- Phyllosphere PDFDocument8 pagesPhyllosphere PDFmanoj_rkl_07Pas encore d'évaluation
- Heavy Metal Adaptation PDFDocument4 pagesHeavy Metal Adaptation PDFmanoj_rkl_07Pas encore d'évaluation
- Immunologival Tolerance Therpeutic Induction PDFDocument6 pagesImmunologival Tolerance Therpeutic Induction PDFmanoj_rkl_07Pas encore d'évaluation
- Genetic Code Introduction PDFDocument10 pagesGenetic Code Introduction PDFmanoj_rkl_07Pas encore d'évaluation
- Capillary Blotting of RNA and DNA Gels PDFDocument3 pagesCapillary Blotting of RNA and DNA Gels PDFmanoj_rkl_07Pas encore d'évaluation
- Dideoxy Sequencing of DNA PDFDocument16 pagesDideoxy Sequencing of DNA PDFmanoj_rkl_07Pas encore d'évaluation
- Calibrating a UV Transilluminator for DNA FixationDocument2 pagesCalibrating a UV Transilluminator for DNA Fixationmanoj_rkl_07Pas encore d'évaluation
- Gertrude Belle Elion PDFDocument1 pageGertrude Belle Elion PDFmanoj_rkl_07Pas encore d'évaluation
- Closteroviridae: Historical PerspectiveDocument6 pagesClosteroviridae: Historical Perspectivemanoj_rkl_07Pas encore d'évaluation
- Dideoxy Sequencing of DNA PDFDocument16 pagesDideoxy Sequencing of DNA PDFmanoj_rkl_07Pas encore d'évaluation
- Terpenoids Lower PDFDocument7 pagesTerpenoids Lower PDFmanoj_rkl_07Pas encore d'évaluation
- Root Nodules (Rhizobium Legumes) PDFDocument2 pagesRoot Nodules (Rhizobium Legumes) PDFmanoj_rkl_07Pas encore d'évaluation
- Metabolism Engineering Internship:: Health Bars For Disaster ReliefDocument19 pagesMetabolism Engineering Internship:: Health Bars For Disaster ReliefRick WuPas encore d'évaluation
- Kinetic Modeling of Hyaluronic Acid Production in Palmyra PalmDocument31 pagesKinetic Modeling of Hyaluronic Acid Production in Palmyra PalmIngrid CabralPas encore d'évaluation
- Biology Unit 2 For Cape ExaminationsDocument11 pagesBiology Unit 2 For Cape ExaminationsTrishannPas encore d'évaluation
- Mitochondrial Medicine: Volume 2: Assessing MitochondriaDocument455 pagesMitochondrial Medicine: Volume 2: Assessing MitochondriaHenrique OliveiraPas encore d'évaluation
- Fatty Acid Synthesis by Prof DR Abdalla Jarari 2nd Year For VIDEOSDocument66 pagesFatty Acid Synthesis by Prof DR Abdalla Jarari 2nd Year For VIDEOSnoran alfaitoryPas encore d'évaluation
- Ketone BodiesDocument16 pagesKetone BodiesAsim Ali100% (1)
- Notas Curso HerbalismoDocument149 pagesNotas Curso HerbalismomilkykiwidevPas encore d'évaluation
- The Indian School Darsait: NameDocument4 pagesThe Indian School Darsait: Namesubnair100% (1)
- Module 5Document8 pagesModule 5mirai desuPas encore d'évaluation
- 01A-Energy Partitioning Broiler Chickens PDFDocument8 pages01A-Energy Partitioning Broiler Chickens PDFMarcos AntonioPas encore d'évaluation
- (Derita, Marcos G. Rai, Mahendra Zacchino, Susan (B-Ok - Xyz) PDFDocument327 pages(Derita, Marcos G. Rai, Mahendra Zacchino, Susan (B-Ok - Xyz) PDFKima MadPas encore d'évaluation
- Citric Acid CycleDocument2 pagesCitric Acid CyclePablo MaldonadoPas encore d'évaluation
- Microbiology PhysiologyDocument6 pagesMicrobiology PhysiologyLady DaniellePas encore d'évaluation
- Energy and Respiration - 2Document39 pagesEnergy and Respiration - 2Rohan PaneruPas encore d'évaluation
- 2.7 (BIOCHEMISTRY) Gluconeogenesis - Better PicturesDocument12 pages2.7 (BIOCHEMISTRY) Gluconeogenesis - Better Pictureslovelots1234Pas encore d'évaluation
- Recent Advances in Lactic Acid Production by Microbial Fermentation ProcessesDocument26 pagesRecent Advances in Lactic Acid Production by Microbial Fermentation ProcessesJuan Camilo Camacho100% (1)
- BDSDocument80 pagesBDSAbhishakHackZPas encore d'évaluation
- Peroxisome Diversity and EvolutionDocument10 pagesPeroxisome Diversity and EvolutionPaulius SungailaPas encore d'évaluation
- PhotosynthesisDocument4 pagesPhotosynthesisRishar bokPas encore d'évaluation
- SanMilan Inigo Cycling Physiology and Physiological TestingDocument67 pagesSanMilan Inigo Cycling Physiology and Physiological Testingjesus.clemente.90Pas encore d'évaluation
- 04-Nutrition COURSE PLANDocument5 pages04-Nutrition COURSE PLANPNT EXAM 2016Pas encore d'évaluation
- Anabolism: The Use of Energy in Biosynthesis: Chapter OverviewDocument5 pagesAnabolism: The Use of Energy in Biosynthesis: Chapter OverviewElishae SamontePas encore d'évaluation
- 8787 37898 1 PBDocument4 pages8787 37898 1 PBGabriella NaomiPas encore d'évaluation
- Glycogenolysis BreakdownDocument6 pagesGlycogenolysis BreakdownManila MedPas encore d'évaluation
- NEPHAR 305 Metabolism - 12Document61 pagesNEPHAR 305 Metabolism - 12Ra'fat RaheemPas encore d'évaluation
- '-Daftar Harga Reagen LabtestDocument1 page'-Daftar Harga Reagen LabtestUPT Labkes R/LPas encore d'évaluation
- Aerobic - Anaerobic - Importance: of RespirationDocument22 pagesAerobic - Anaerobic - Importance: of RespirationLizzy AnthonyPas encore d'évaluation
- GluconeogenesisDocument48 pagesGluconeogenesisQshyanPas encore d'évaluation