Académique Documents
Professionnel Documents
Culture Documents
Wittig Reaction Report
Transféré par
johnjohnrocksCopyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Wittig Reaction Report
Transféré par
johnjohnrocksDroits d'auteur :
Formats disponibles
Aldol Condensation Diels-Alder Name: John OConnor Lab: F 8:00 a.m.
. 420 CE Teaching Assistant: Emily Vojt Submitted: March 22, 2013
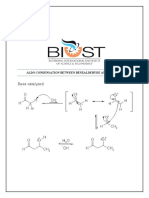
Purpose: To synthesize 2,3,4,5-tetraphenylcyclopentadienone from benzil and dibenzyl ketone via an aldol concentration reaction and use the resulting product along with isoamyl nitrite and anthranilic acid to synthesize 1,2,3,4tetraphenylnaphthalene via a Diels-Alder reaction. Reaction Scheme (aldol condensation):
Synthesis of 2,3,4,5-tetraphenylcyclopentadienone:1 A solution of benzil (1.9643 g, 9.3436 mmol), dibenzyl ketone (1.6710 g, 7.9469 mmol), and anhydrous ethanol (15 mL) was heated at 70 C under a reflux condenser for five minutes. A solution of potassium hydroxide (0.5 g) and anhydrous ethanol (5 mL) was added to the benzil solution in two equal portions, and the solution was heated for 15 minutes at 78 C. The product was crystallized out of solution by cooling to 0 C. The suspension was vacuum filtered, resulting in a deep purple crystalline product, 2,3,4,5-tetraphenylnaphthalene (2.4511 g, 6.3753 mmol, 80%): mp 218-219 C (lit2 mp 219-220 C).
Reaction Scheme (Diels-Alder):
Synthesis of 1,2,3,4-tetraphenylnaphthalene:3 A solution of tetraphenylcyclopentadienone (0.9460 g, 2.461 mmol) and dimethoxyethane (7.5 mL) was heated to 85 C with a reflux condenser attached. While refluxing, a solution of anthranilic acid (0.3761 g, 2.743 mmol) and dimethoxyethane (3.5 mL) was added through a Claisen adapter simultaneously with a solution of isoamyl nitrite (0.463 g, 0.500 mL, 3.72 mmol) and dimethoxyethane (3.5 mL) added through the reflux condenser. The two solutions were added in .1 mL portions over 45 minutes, and the reaction refluxed at 85 C for an additional 15 minutes. The suspension was evaporated using a rotovap, and the resulting solid crude product was triturated in methanol. The product was vacuum filtered, resulting in a white-yellow powder product, 1,2,3,4tetraphenylnaphthalene (0.8364 g, 1.934 mmol, 79%): mp 202-204 C (lit4 mp 196199 C or 203-205 C). Discussion: The synthesis of 1,2,3,4-tetraphenylnaphthalene was accomplished through a twostep procedure. In the first part of the procedure, benzil and dibenzyl ketone were reacted in ethanol in the presence of a basic catalyst, KOH, to yield 2,3,4,5tetraphenylcyclopentadienone as a purple crystalline solid in 80% yield. The melting point range of the product was determined to be 218-219 C, which borders the reported literature range and supports the identity of the product as 2,3,4,5tetraphenylcyclopentadienone. The narrow melting point range indicates the product is relatively free of impurities. In the second part of the procedure, anthranilic acid, and isoamyl nitrite were reacted in dimethoxyethane to generate
benzyne in situ. Benzyne then reacted with 2,3,4,5-tetraphenylcyclopentadienone to yield 1,2,3,4-tetraphenylnaphthalene as a white-yellow powder solid in 79% yield. The melting point range of the product was determined to be 202-204 C, which is partially within the reported literature range and supports the identity of the product as 1,2,3,4-tetraphenylnaphthalene. The narrow melting point range indicates the product is relatively free of impurities. The aldol reaction in the first step produced only one product in significant yield for two reasons. First, benzils lack of an alpha proton prevents it from forming an enolate, therefore all enolate formation was by dibenzyl ketone. Second, the dibenzyl ketone enolate was not very prone to self-condensation due to the benzil carbonyl groups being more electrophilic. Yield for this step could have been improved by generating the product through the reaction of 1,2,3,4-tetraphenyl-1,4-dilithio-1,3-diene and carbon dioxide. This reaction possesses two advantages. While side product generation was minimized in the aldol condensation, this procedure offers even more negligible side product generation. Also, this reaction is not an equilibrium reaction, so unreacted products are less prevalent5. In step two, anthranilic acid and isoamyl nitrite were added in small simultaneous quantities because benzyne readily dimerizes. In order to minimize benzyne dimerization, it needed to be present in much smaller quantities than 2,3,4,5-tetraphenylcyclopentadienone. As the cyclopentadienone reactant was used up, additional benzyne was more likely to dimerize. Therefore, yield could have been improved by using benzyne (either anthranilic acid or isoamyl nitrite) as the limiting reagent, rather than 1,2,3,4cyclopentadienone. References: (1) Callam, C.S.; Paul, N.M. Aldol Condensation. Chemistry 2550 Organic Chemistry Lab 2012 Academic Year, McGraw-Hill: United States, 2012; pp 113-114 (2) Sigma-Aldrich Chemical Catalog Online, http://www.sigmaaldrich.com/unitedstates.html (accessed Feb 2013); Search: Product No. T25801 (3) Callam, C.S.; Paul, N.M. Diels Alder. Chemistry 2550 Organic Chemistry Lab 2012 Academic Year, McGraw-Hill: United States, 2012; pp 122-124 (4) Sigma-Aldrich Chemical Catalog Online, http://www.sigmaaldrich.com/unitedstates.html (accessed Feb 2013); Search: Product No. 149799 (5) Xi, Z.; Song, Q. Efficient Synthesis of Cyclopentadienone Derivatives by the Reaction of Carbon Dioxide with 1,4-Dilithio-1,3-dienes. Journal of Organic Chemistry. 2000. 65 (260). 9157-9159.
Vous aimerez peut-être aussi
- Bio-Based SolventsD'EverandBio-Based SolventsFrançois JérômePas encore d'évaluation
- Prac 5 Chem 220Document9 pagesPrac 5 Chem 220Mxokzah CmohPas encore d'évaluation
- Sustainable synthesis of ciclopentene derivatives through multicomponent reactions in continuous flow regimeD'EverandSustainable synthesis of ciclopentene derivatives through multicomponent reactions in continuous flow regimePas encore d'évaluation
- Chem 305 Lab 5 Benzoil.Document4 pagesChem 305 Lab 5 Benzoil.Gobe JamPas encore d'évaluation
- Formal LabDocument4 pagesFormal Labljeanja2100% (1)
- Chm557 Laboratory Report: Experiment 4 The Aldol Condensation Reaction: Preparation of DibenzalacetoneDocument17 pagesChm557 Laboratory Report: Experiment 4 The Aldol Condensation Reaction: Preparation of DibenzalacetonesyafPas encore d'évaluation
- Exp 3 OtwwDocument3 pagesExp 3 Otwwexpido tapologoPas encore d'évaluation
- Chem 305 Lab OneDocument6 pagesChem 305 Lab OneGobe JamPas encore d'évaluation
- Aldol Condensation Reaction Lab ReportDocument4 pagesAldol Condensation Reaction Lab ReportAvril Watson100% (3)
- 2,3,4,5 TetraphenylcyclopentadienoneDocument6 pages2,3,4,5 TetraphenylcyclopentadienoneDenisse Watt Cuarteros100% (2)
- STEP 1: Synthesis of N-Phenyl-1,2-Benzenediamine: Experimental ProdedureDocument2 pagesSTEP 1: Synthesis of N-Phenyl-1,2-Benzenediamine: Experimental ProdedureKonstantina MsPas encore d'évaluation
- Experiment 8Document10 pagesExperiment 8Chong songheePas encore d'évaluation
- Exer 9b Post Lab ReportDocument6 pagesExer 9b Post Lab ReportKatrinne Clea PincaPas encore d'évaluation
- Aldol Condensation Reaction PDFDocument6 pagesAldol Condensation Reaction PDFaizatPas encore d'évaluation
- The Aldol Condensation Reaction: Preparation of Benzalacetophenones (Chalcones)Document7 pagesThe Aldol Condensation Reaction: Preparation of Benzalacetophenones (Chalcones)ohhiPas encore d'évaluation
- 14 Diphenyl 13 ButadieneDocument7 pages14 Diphenyl 13 ButadienesaiPas encore d'évaluation
- Pre LabghvyicDocument6 pagesPre LabghvyicGobe JamPas encore d'évaluation
- Chitosan Catalyzed Synthesis of IminesDocument6 pagesChitosan Catalyzed Synthesis of IminesaustingoewertPas encore d'évaluation
- EXP 2 Advance Organic ChemistryDocument7 pagesEXP 2 Advance Organic ChemistryMay Lee100% (1)
- Katch UmbelliferoneffrDocument9 pagesKatch Umbelliferoneffrapi-456902531Pas encore d'évaluation
- Benzoin Exp7Document4 pagesBenzoin Exp7Liz Hackett0% (1)
- Adol Condensation of DibenzalacetoneDocument2 pagesAdol Condensation of DibenzalacetoneMohammad Bazrouk100% (1)
- Case StudyDocument10 pagesCase StudyRebekka PacquingPas encore d'évaluation
- Experiment # 8 Six-Step Synthesis Aniline To 1-Bromo-3cholor-5iodobenzeneDocument10 pagesExperiment # 8 Six-Step Synthesis Aniline To 1-Bromo-3cholor-5iodobenzeneColin ChePas encore d'évaluation
- Synthesis of 4-IodonitrobenzeneDocument2 pagesSynthesis of 4-IodonitrobenzeneJulian Felipe Peña RamirezPas encore d'évaluation
- AromaticDocument173 pagesAromaticParom Waikasikarn100% (1)
- Experiment 32Document14 pagesExperiment 32Morgan Elizabeth Lepley100% (6)
- Report1 s1057552Document6 pagesReport1 s1057552Revencu DanaPas encore d'évaluation
- Experiment 17B Lab ReportDocument3 pagesExperiment 17B Lab ReportMikey ZhitnitskyPas encore d'évaluation
- Preparation of Novolacs Using Phenolic Rich Components As Partial Substitute of Phenol From Biomass Pyrolysis OilsDocument7 pagesPreparation of Novolacs Using Phenolic Rich Components As Partial Substitute of Phenol From Biomass Pyrolysis OilsMohammad Mofeez AlamPas encore d'évaluation
- FAR 113-Prac 3Document9 pagesFAR 113-Prac 3Arvin KumarPas encore d'évaluation
- 1 s2.0 S0926860X98000210 MainDocument11 pages1 s2.0 S0926860X98000210 MainOwen KhosashiPas encore d'évaluation
- Aldol Condensation Reaction: BenzalacetophenoneDocument12 pagesAldol Condensation Reaction: Benzalacetophenoneberjalankehadapan100% (1)
- 2,3 Dimethoxy 4,5 Methylenedioxy Phenylacetone From IsodillapioleDocument2 pages2,3 Dimethoxy 4,5 Methylenedioxy Phenylacetone From IsodillapiolejolouisPas encore d'évaluation
- Suzuki Coupling Lab ExperimentDocument4 pagesSuzuki Coupling Lab Experimentkblack301Pas encore d'évaluation
- Melane Zintle Prac 2Document3 pagesMelane Zintle Prac 2Zintle MelanePas encore d'évaluation
- 54754-Article Text-89653-1-10-20100526Document7 pages54754-Article Text-89653-1-10-20100526nurizzaatiPas encore d'évaluation
- Petrochemical IndustryDocument30 pagesPetrochemical Industrybuhayche14Pas encore d'évaluation
- Synthesis and Characterization of DibenzalacetoneDocument7 pagesSynthesis and Characterization of DibenzalacetoneTan Yong Jie100% (7)
- Aliphatic AminesDocument2 pagesAliphatic AminesAmar PandeyPas encore d'évaluation
- Synthesis of Raspberry and Ginger Ketones by Nickel Boride-Catalyzed Hydrogenation of 4-Arylbut-3-En-2-OnesDocument4 pagesSynthesis of Raspberry and Ginger Ketones by Nickel Boride-Catalyzed Hydrogenation of 4-Arylbut-3-En-2-OnesalinaPas encore d'évaluation
- Study Diphenylamine Reaction Colorimetric Estimation of Deoxyribonucleic AcidDocument9 pagesStudy Diphenylamine Reaction Colorimetric Estimation of Deoxyribonucleic AcidJavier VariscoPas encore d'évaluation
- Synthesis of 5 Cyano IndoleDocument6 pagesSynthesis of 5 Cyano IndolebsureshrajuPas encore d'évaluation
- Submitted by Department of Chemistry, Imam Hossein University, Tehran, IRANDocument4 pagesSubmitted by Department of Chemistry, Imam Hossein University, Tehran, IRANsinaPas encore d'évaluation
- MEDICINAL CHEMISTRY-I - Practicals PDFDocument25 pagesMEDICINAL CHEMISTRY-I - Practicals PDFAnit Dubey100% (1)
- Benzoin Condensation.Document7 pagesBenzoin Condensation.Sam Bina92% (13)
- CHM 457 Exp 2Document9 pagesCHM 457 Exp 2Ayish MataPas encore d'évaluation
- Improved Procedure For The Preparation of 1 - (2-Phenethyl) - 4-PiperidoneDocument5 pagesImproved Procedure For The Preparation of 1 - (2-Phenethyl) - 4-PiperidonejesusPas encore d'évaluation
- Ionic Liquids Catalyzed Biginelli Reaction Under Solvent-Free ConditionsDocument3 pagesIonic Liquids Catalyzed Biginelli Reaction Under Solvent-Free ConditionsnileshsalunkhePas encore d'évaluation
- Aniline From Nitrobenzene 1Document11 pagesAniline From Nitrobenzene 1Neha MiraclePas encore d'évaluation
- Chapter Two Production Methods of Ethyl Benzene 2.1 GeneralDocument13 pagesChapter Two Production Methods of Ethyl Benzene 2.1 Generalنزار الدهاميPas encore d'évaluation
- New Diels SDFSDFSD Diels-Alder Synthesis Lab ReportDocument6 pagesNew Diels SDFSDFSD Diels-Alder Synthesis Lab ReportLiz Hackett100% (1)
- Multistep Synthesis of 2-Chloro-4-BromoanilineDocument9 pagesMultistep Synthesis of 2-Chloro-4-Bromoanilinejcrider2100% (1)
- Aldol CondensationDocument2 pagesAldol CondensationGian Wyatt Gamboa100% (1)
- Chemistry Report 4Document7 pagesChemistry Report 4Lih XuanPas encore d'évaluation
- NCL 2006 Pet. Chem.Document58 pagesNCL 2006 Pet. Chem.madurchinnaPas encore d'évaluation
- Organic ChemistryDocument8 pagesOrganic ChemistryAndré Brincat100% (1)
- Exp 1Document10 pagesExp 1zanjinyadzaPas encore d'évaluation
- 3 Synthesis of AcetaminophenDocument6 pages3 Synthesis of Acetaminophenstevenly1383% (6)
- Organic Experiment 7Document7 pagesOrganic Experiment 7Abdulaziz KaramiPas encore d'évaluation
- TD Altosonic IV en 060424Document8 pagesTD Altosonic IV en 060424Anonymous cui5pddkPas encore d'évaluation
- Campus Piping and Mechanical System NDT - Inspection Presentation To Adn...Document12 pagesCampus Piping and Mechanical System NDT - Inspection Presentation To Adn...Osama LariPas encore d'évaluation
- Foundry Workshop ManualDocument22 pagesFoundry Workshop Manuallakshya100% (1)
- Pharmaceutical Sector Analysis With The Help of Porter's Five Forces ModelDocument4 pagesPharmaceutical Sector Analysis With The Help of Porter's Five Forces ModelBhargavi KharePas encore d'évaluation
- 0620 m23 QP 22-MinDocument14 pages0620 m23 QP 22-Minjelani17fPas encore d'évaluation
- Solid State Sintering in The of Iron Ore Pellets IndurationDocument10 pagesSolid State Sintering in The of Iron Ore Pellets IndurationCamila Barata CavalcantiPas encore d'évaluation
- DrillingDocument21 pagesDrillingNabil RajPas encore d'évaluation
- Chemistry of Kupipakwa RasayanasDocument7 pagesChemistry of Kupipakwa RasayanasSrinivas Naik0% (1)
- Synthesis of Dothiepin and Doxepin by Grignard Reactions in TolueneDocument6 pagesSynthesis of Dothiepin and Doxepin by Grignard Reactions in TolueneJuan Carlos VillotaPas encore d'évaluation
- T 217Document4 pagesT 217macc13Pas encore d'évaluation
- Predicting Production Performance of A Field With Complex Reservoir Heterogeneities Undergoing Water Injection - A Case Study of A Niger-Delta FieldDocument14 pagesPredicting Production Performance of A Field With Complex Reservoir Heterogeneities Undergoing Water Injection - A Case Study of A Niger-Delta FieldnoorPas encore d'évaluation
- Behavioral Based Safety Observation Checklist: ErgonomicsDocument2 pagesBehavioral Based Safety Observation Checklist: ErgonomicsRyan CyrillaPas encore d'évaluation
- Thurmalox 8200 Painting SpecificationDocument2 pagesThurmalox 8200 Painting SpecificationFreddy Carl FredricksenPas encore d'évaluation
- Valve StandardsDocument15 pagesValve StandardsabduPas encore d'évaluation
- Alakliphiles ProjDocument24 pagesAlakliphiles ProjReshmi Jadwani100% (2)
- Licensed Polyolefin Technologies and Services: SpherizoneDocument2 pagesLicensed Polyolefin Technologies and Services: SpherizoneTVCPas encore d'évaluation
- Journal of Trace Elements in Medicine and Biology: NutritionDocument8 pagesJournal of Trace Elements in Medicine and Biology: NutritionCamilla AndreatoPas encore d'évaluation
- LS 100 BrochureDocument2 pagesLS 100 BrochureLun DingPas encore d'évaluation
- 8 Grade Chemistry Unit Review: Name: Teacher: Date: Laylee Taghizadeh Stegemann April 19, 2021Document4 pages8 Grade Chemistry Unit Review: Name: Teacher: Date: Laylee Taghizadeh Stegemann April 19, 2021Laylee TaghizadehPas encore d'évaluation
- Chapter 3-Structure and Stereochemistry of Alkanes: N 2n+2 27 56 N 2n+2 42 86Document18 pagesChapter 3-Structure and Stereochemistry of Alkanes: N 2n+2 27 56 N 2n+2 42 86張湧浩Pas encore d'évaluation
- Plant CompetitionDocument4 pagesPlant CompetitionEugenTutunaruPas encore d'évaluation
- Work Instruction: Jar TestingDocument4 pagesWork Instruction: Jar TestingKadesh Hanah McCarthyPas encore d'évaluation
- Advanced Construction MaterialsDocument37 pagesAdvanced Construction MaterialsZara AliPas encore d'évaluation
- Weldfast™ CL-200 or CL-200QS Adhesive Kit: Storage of Adhesive KitsDocument4 pagesWeldfast™ CL-200 or CL-200QS Adhesive Kit: Storage of Adhesive KitsFernando Cesar PérezPas encore d'évaluation
- Bell & Gosset PumpDocument8 pagesBell & Gosset Pumprogel_ganaPas encore d'évaluation
- Kitchen Safety Awareness: Bureau of Workers' Compensation PA Training For Health & Safety (Paths)Document45 pagesKitchen Safety Awareness: Bureau of Workers' Compensation PA Training For Health & Safety (Paths)Sunil SPas encore d'évaluation
- Slab Bridge DesignDocument21 pagesSlab Bridge DesignMars Villaluna100% (2)
- Recrystallization NotesDocument9 pagesRecrystallization NotesanrychoPas encore d'évaluation
- NEET TEst PapaerDocument11 pagesNEET TEst PapaerBiswajit ChangkakotyPas encore d'évaluation
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincD'EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincÉvaluation : 3.5 sur 5 étoiles3.5/5 (137)
- The Importance of Being Earnest: Classic Tales EditionD'EverandThe Importance of Being Earnest: Classic Tales EditionÉvaluation : 4.5 sur 5 étoiles4.5/5 (44)
- You Can't Joke About That: Why Everything Is Funny, Nothing Is Sacred, and We're All in This TogetherD'EverandYou Can't Joke About That: Why Everything Is Funny, Nothing Is Sacred, and We're All in This TogetherPas encore d'évaluation
- The House at Pooh Corner - Winnie-the-Pooh Book #4 - UnabridgedD'EverandThe House at Pooh Corner - Winnie-the-Pooh Book #4 - UnabridgedÉvaluation : 4.5 sur 5 étoiles4.5/5 (5)
- The Comedians in Cars Getting Coffee BookD'EverandThe Comedians in Cars Getting Coffee BookÉvaluation : 4.5 sur 5 étoiles4.5/5 (8)
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeD'EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeÉvaluation : 5 sur 5 étoiles5/5 (4)
- Welcome to the United States of Anxiety: Observations from a Reforming NeuroticD'EverandWelcome to the United States of Anxiety: Observations from a Reforming NeuroticÉvaluation : 3.5 sur 5 étoiles3.5/5 (10)
- The Inimitable Jeeves [Classic Tales Edition]D'EverandThe Inimitable Jeeves [Classic Tales Edition]Évaluation : 5 sur 5 étoiles5/5 (3)
- The Book of Bad:: Stuff You Should Know Unless You’re a PussyD'EverandThe Book of Bad:: Stuff You Should Know Unless You’re a PussyÉvaluation : 3.5 sur 5 étoiles3.5/5 (3)
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactD'EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactÉvaluation : 5 sur 5 étoiles5/5 (5)
- Cats On The Run: a wickedly funny animal adventureD'EverandCats On The Run: a wickedly funny animal adventureÉvaluation : 4 sur 5 étoiles4/5 (8)
- The Most Forbidden Knowledge: 151 Things NO ONE Should Know How to DoD'EverandThe Most Forbidden Knowledge: 151 Things NO ONE Should Know How to DoÉvaluation : 4.5 sur 5 étoiles4.5/5 (6)
- 1,001 Facts that Will Scare the S#*t Out of You: The Ultimate Bathroom ReaderD'Everand1,001 Facts that Will Scare the S#*t Out of You: The Ultimate Bathroom ReaderÉvaluation : 3.5 sur 5 étoiles3.5/5 (48)
- The Best Joke Book (Period): Hundreds of the Funniest, Silliest, Most Ridiculous Jokes EverD'EverandThe Best Joke Book (Period): Hundreds of the Funniest, Silliest, Most Ridiculous Jokes EverÉvaluation : 3.5 sur 5 étoiles3.5/5 (4)










































































































![The Inimitable Jeeves [Classic Tales Edition]](https://imgv2-1-f.scribdassets.com/img/audiobook_square_badge/711420909/198x198/ba98be6b93/1712018618?v=1)










