Académique Documents
Professionnel Documents
Culture Documents
Leishmania Sis
Transféré par
Shailendra YadavDescription originale:
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Leishmania Sis
Transféré par
Shailendra YadavDroits d'auteur :
Formats disponibles
Leishmaniasis
http://www.austincc.edu/microbio/2321/ld.htm
Leishmaniasis
By Leo E. Larios
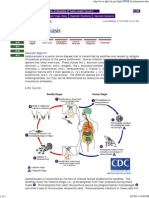
Disease Etiologic Agent Leishmaniasis (leesh-muh-nahy-uh-sis) [1] is a vector-borne tropical/subtropical disease caused by intracellular protozoan species of the genus Leishmania. Infection in humans is caused by 21 different species of Leishmania, all of them morphologically indistinguishable from each other, and all of them causing the spectrum of clinical manifestations (cutaneous/mucosal and visceral) collectively known as Leishmaniasis. [1] [4]
Disease Transmission It is spread through the bite of infective female sandflies of the genus Phlebotomus in the Old World, and of the genus Lutzomyia in the New World [5]. In humans and other mammals infection is produced by the promastigote form of the parasite; once inside the host the protozoan is taken up by macrophages in the host, where it transforms into the amastigote form and multiply inside this leucocyte to later rupture it. The amastigotes released infect other macrophages and the cycle continues when a female sandfly feeds from the infected host [5], [6]. Parasite, host, and other factors affect whether the infection becomes symptomatic and whether cutaneous or visceral (a.k.a. Kala-azar) leishmaniasis results. [4]
1 of 4
8/10/2013 9:37 PM
Leishmaniasis
http://www.austincc.edu/microbio/2321/ld.htm
Reservoir
Both anthroponotic and zoonotic foci exist; humans are the reservoir in anthroponotic cutaneous leishmaniasis; in zoonotic cases, wild rodents, marsupials and dogs are the reservoir for the species of Leishmania. In visceral leishmaniasis humans, wild members of canidae and dogs [4],[6] are reservoirs. In Bangladesh the only known reservoir for visceral leishmaniasis are humans. [6] Specific Microbial Characteristics The genus Leishmania is an obligate intracellular parasite, member of the trypanosomatid protozoa whose distinguishing feature is the possession of a single flagellum [5]. The variety of Leishmania species that cause leishmaniasis is one of the important factors that determine the type of leishmania infection (mucocutaneous or visceral) that can manifest in the host. [6] In the eastern hemisphere L. tropica, L. major, L. aethiopica are prevalent, and in the western hemisphere the L.mexicana and the L. braziliensis complexes are responsible for infection. Members of the L. braziliensis complex are likely to cause the mucosal form of the disease; and L. tropica causes usually the cutaneous form. The visceral manifestation is produced in the eastern hemisphere by members of the L. donovani complex and in the western hemisphere by L. chagasi/infantum. Taxonomy and groups are summarized in the two tables below. [6] [7] [8]
Specific Tests for Identification Diagnosis is through microscopic identification of the intracellular amastigote form in Giemsa stained slides from lesions, and through culture of the motile promastigote in suitable media [6]. Antibody detection can prove useful in visceral leishmaniasis but is of limited value in cutaneous disease, where most patients do not develop a significant antibody response [6].
2 of 4
8/10/2013 9:37 PM
Leishmaniasis
http://www.austincc.edu/microbio/2321/ld.htm
Species identification is done through monoclonal antibody testing, DNA techniques and biochemical isoenzyme assays. [4] Signs and Symptoms The most common forms of leishmaniasis are two, these are known as mucocutaneous leishmaniasis which causes skin sores, and visceral leishmaniasis which affects several internal organs (usually spleen, liver, and bone marrow) [6]. Cutaneous infection is localized and diffuse [5], it starts with a macule, then a papule and enlarges to a painful or painless ulcer. Lesion may be single or multiple, and these lesions may heal spontaneously within weeks or several months, sometimes lasting a year or more [6]. Visceral leishmaniasis is a chronic disease characterized by fever of sudden or gradual onset, liver and spleen enlargement, anemia, leukopenia, emaciation and weakness [6]. This form of the disease is usually fatal if left untreated. Historical Information The parasite was named in 1903 after the Scottish pathologist William Boog Leishman, who developed one of the earliest stains of the parasite in 1903 [9] [3]. Pre-Incan pottery in the first century AD depicts lesions consistent with mucocutaneous leishmaniasis; in Africa and India texts dating from the eighteenth century describe visceral leishmaniasis [3]. The origin of the genus Leishmania is uncertain. A proposed origin, evolution and dispersal of Leishmania, suggests a predecessor of L. donovani group and L. major would have evolved from parasites of insects in South America between 46 and 36 million years ago, and migrated to Asia via the Bering strait. The ancestor of the L. donovani complex diverged from other Leishmania species approximately between 14 and 24 million years ago. This predecessor arrived in central Asia and approx. 1 million years ago and later diverged into L. infantum, and L. donovani . Leishmania infantum was later introduced in South America by the European colonization. [10]
Virulence Factors Susceptibility is general [6]. Leishmania virulence is generally consequence of 1- Intrinsic virulence (drug resistance components, surface secretory components, and efficiency of the parasitic housekeeping system); 2- parasite burden in host (consequence in part of intrinsic virulence); 3- Host factors (immune response). [11] These components interact with each other and determine the global virulence of Leishmania. Parasites drug resistance, type of strain, and human host coinfection with HIV are examples of how these factors can affect virulence. Control and Treatment Patient treatment, periodic spraying with insecticides, destruction of rodent population in zoonotic areas and insecticide impregnated collars in the canine population foci are effective control measures. In anthroponotic foci, prompt and effective chemotherapy treatment of patients can help control transmission. [6] In mucocutaneous infections, pentavalent antimonials is the drug of choice. Pentamidine is used as a second line drug. In visceral or kala-azar leishmaniasis the most effective and expensive chemotherapy is amphotericin B. In developing countries the high cost of amphotericin B makes the pentavalent antimonials (Sb5) first line treatment of choice. [6] Prevention/Vaccines Currently there are no vaccines or drugs that prevent infection with Leishmania. Infection confers lifelong immunity; therefore a vaccine might be an effective method for prevention in the future. Protection from sand fly bites is best to avoid infection. Limiting outdoor activities in endemic areas from dusk to dawn, minimizing area of uncovered skin, use of insect repellant, and the use of bed nets treated with pyrethroids are practices that minimize exposure to the vector. Effective chemotherapy is becoming increasingly difficult due to emergence of drug resistance. [6] [12] [15] Local Cases/Outbreaks Although rare in Texas, native leishmaniasis infections have occurred in the past. From 1903 to 1989 a total of 27 native cases have been identified [13]. The distribution of these cases closely matches that of the wood rat (Neotoma micropus). In 2006-2007 nine cases were identified over the north Texas area, mostly rural locations in proximity to wooded or brushy areas where wild rodents, such as the wood rat, live. [14] Global Cases/Outbreaks The disease is a significant health problem in many parts of the world resulting in an estimated 12 million new total leishmania cases each year [6] [15]. There are 2 million new cases of mucocutaneous leishmania infections worldwide each year. Visceral leishmaniasis cases amount to an incidence of 500,000 cases, with over 90% of the burden in India, Nepal, Sudan and Brazil [6]. Worldwide distribution maps for cutaneous and visceral leishmaniasis are shown below. [2] [3] a) Cutaneous Leishmaniasis distribution. [3]
3 of 4
8/10/2013 9:37 PM
Leishmaniasis
http://www.austincc.edu/microbio/2321/ld.htm
b) Visceral Leishmaniasis distribution. [2]
References [1] "Leishmaniasis." Dictionary.com Unabridged. Random House, Inc, n.d. Web. 13 Feb. 2013. <Dictionary.com http://dictionary.reference.com/browse/leishmaniasis>. [2] Anon. "Essential leishmaniasis maps." World Health Organization Leishmaniasis home. World Health Organization, n.d. Web. 14 Feb 2013. <http://www.who.int/leishmaniasis /leishmaniasis_maps/en/index1.html>. [3] Narciso, Heather, and Josuel Ruelan. "Leishmaniasis." Parasites & Pestilence:ParaSite Webpages. Stanford University, n.d. Web. 14 Feb 2013. <http://www.stanford.edu/class /humbio103/ParaSites2006/Leishmaniasis/Index.htm>. [4] Anon. "Leishmaniasis." DPDx-Laboratory Identification of Parasites of Public Health Concern. Centers for Disease Control and Prevention (CDC), 20 Jul 2009. Web. 14 Feb 2013. <http://dpd.cdc.gov/dpdx/HTML/Leishmaniasis.htm>. [5] Wikipedia contributors. "Trypanosomatid." Wikipedia, The Free Encyclopedia. Wikipedia, The Free Encyclopedia, 12 Feb. 2013. Web. 25 Feb 2013. <http://en.wikipedia.org /w/index.php?title=Trypanosomatid&oldid=537900951> [6] Heymann M.D., David L. United States. American Public Health Association and WHO. Control of Communicable Diseases Manual. Washington, DC: American Public Health Association, 2008. Print. [7] McCall L-I, Zhang W-W, Matlashewski G (2013) Determinants for the Development of Visceral Leishmaniasis Disease. PLoS Pathog 9(1): e1003053. doi:10.1371/journal.ppat.1003053. Web.18 Feb 2013. <http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3536654/> [8] Bhuwan B. Mishra, Raju R. Kale, Rakesh K. Singh, Vinod K. Tiwari. Alkaloids: Future prospective to combat leishmaniasis. Fitoterapia, Volume 80, Issue 2, March 2009, Pages 8190. Web.23 Feb 2013. <http://dx.doi.org/10.1016/j.fitote.2008.10.009> [9] Wikipedia contributors. "Leishmania." Wikipedia, The Free Encyclopedia. Wikipedia, The Free Encyclopedia, 24 Feb. 2013. Web. 25 Feb 2013. <http://en.wikipedia.org /wiki/Leishmania> [10] Julius Lukes et al. Evolutionary and geographical history of the Leishmania donovani complex with a revision of current taxonomy PNAS 2007 104 (22) 9375-9380; published ahead of print May 21, 2007,doi:10.1073/pnas.0703678104. Web. 22 Feb 2013. <http://www.pnas.org/content/104/22/9375.abstract> [11] Luis Rivas et al. Virulence and disease in leishmaniasis: what is relevant for the patient?. Trends in Parasitology, Volume 20, Issue 7, 1 July 2004, Pages 297301. Web. 22 Feb 2013. <http://dx.doi.org/10.1016/j.pt.2004.05.005> [12] Anon. United States. Centers for Disease Control and Prevention. Leishmaniasis. CDC, Update 01/10/2013. Web. Accessed 24 Feb 2013.<http://www.cdc.gov/parasites /leishmaniasis/epi.html>. [13] McHugh, CP. "Leishmaniasis in Texas: epidemiology and clinical aspects of human cases." Am J Trop Med Hyg. Nov 55.5 (1996): 547-55. Web. 24 Feb. 2013. <http://www.ncbi.nlm.nih.gov/pubmed/8940988>. [14] Jacobson, Sherry. NEWSclips." Rare, nonfatal skin infection found in N. Texas. Dallas Morning News, 28 Jun 2010. Web. 24 Feb 2013. <http://online.dshs.state.tx.us/Consumerand-External-Affairs/newsclips/September-2007/Rare,-nonfatal-skin-infection-found-in-N--Texans.aspx> [15] L. KEDZIERSKI, Y. ZHU and E. HANDMAN (2006). Leishmania vaccines: progress and problems. Parasitology, 133, pp S87-S112. doi:10.1017/S0031182006001831. Web. 2011. 24 Feb 2013. <http://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=680888>
http://www.google.com/url?q=http%3A%2F%2Fdx.doi.org%2F10.1016%2Fj.pt.2004.05.005&sa=D&sntz=1&usg=AFQjCNHz0QJAvO3TC7E0Ex4uuLD4I5cjLA
4 of 4
8/10/2013 9:37 PM
Vous aimerez peut-être aussi
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (121)
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (588)
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (400)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (266)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5794)
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (345)
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (895)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (74)
- Carnatic Music NotationDocument6 pagesCarnatic Music Notationksenthil kumar100% (1)
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- 555LDocument8 pages555LVictor Mamani VargasPas encore d'évaluation
- Broken Squares - Unfreezing Team Building ExerciseDocument4 pagesBroken Squares - Unfreezing Team Building Exerciselilywhite786Pas encore d'évaluation
- Ventures Onsite Market Awards 22062023 64935868dDocument163 pagesVentures Onsite Market Awards 22062023 64935868dhamzarababa21Pas encore d'évaluation
- HOPE 2A MODULE 1 Introduction To SportsDocument11 pagesHOPE 2A MODULE 1 Introduction To SportsChristian Ray Lucnagan ReyesPas encore d'évaluation
- Giant Panda: 1 DescriptionDocument18 pagesGiant Panda: 1 DescriptionMsKarolyPas encore d'évaluation
- Understanding The School Curriculum Close Encounter With The School Curriculum SPARK Your InterestDocument12 pagesUnderstanding The School Curriculum Close Encounter With The School Curriculum SPARK Your InterestJoshua Lander Soquita CadayonaPas encore d'évaluation
- FICCI-BCG Report On Railway Station RedevelopmentDocument47 pagesFICCI-BCG Report On Railway Station RedevelopmentRahul MehrotraPas encore d'évaluation
- ADVOCATE ACT - Smart Notes PDFDocument30 pagesADVOCATE ACT - Smart Notes PDFAnonymous n7rLIWi7100% (2)
- Organic Agriculture Gr12 - Module2.final For StudentDocument20 pagesOrganic Agriculture Gr12 - Module2.final For Studentapril jean cahoyPas encore d'évaluation
- How To Write A Scholarship Motivation Letter - ScholarshipPortalDocument3 pagesHow To Write A Scholarship Motivation Letter - ScholarshipPortalShailendra Yadav100% (2)
- Physiology of Adrenergic ReceptorsDocument3 pagesPhysiology of Adrenergic ReceptorsShailendra YadavPas encore d'évaluation
- Cell Counting With A Hemocytometer - Easy As 1, 2, 3 - Bitesize Bio PDFDocument10 pagesCell Counting With A Hemocytometer - Easy As 1, 2, 3 - Bitesize Bio PDFShailendra YadavPas encore d'évaluation
- Counting Cells in A Hemacytometer - Life TechnologiesDocument1 pageCounting Cells in A Hemacytometer - Life TechnologiesShailendra YadavPas encore d'évaluation
- List of Parasites of Humans - Wikipedia, The Free EncyclopediaDocument12 pagesList of Parasites of Humans - Wikipedia, The Free EncyclopediaShailendra YadavPas encore d'évaluation
- RT PCR PDFDocument2 pagesRT PCR PDFShailendra YadavPas encore d'évaluation
- Overview of ELISADocument5 pagesOverview of ELISAShailendra YadavPas encore d'évaluation
- RT PCR PDFDocument2 pagesRT PCR PDFShailendra YadavPas encore d'évaluation
- λ DNA-HindIII Digest - New England BiolabsDocument3 pagesλ DNA-HindIII Digest - New England BiolabsShailendra YadavPas encore d'évaluation
- Far-Eastern Blotting - Wikipedia, The Free EncyclopediaDocument2 pagesFar-Eastern Blotting - Wikipedia, The Free EncyclopediaShailendra YadavPas encore d'évaluation
- Ultracentrifuge - Wikipedia, The Free EncyclopediaDocument3 pagesUltracentrifuge - Wikipedia, The Free EncyclopediaShailendra YadavPas encore d'évaluation
- Protein SequencingDocument17 pagesProtein SequencingShiv KumarPas encore d'évaluation
- Centrosome - Wikipedia, The Free EncyclopediaDocument6 pagesCentrosome - Wikipedia, The Free EncyclopediaShailendra YadavPas encore d'évaluation
- Klenow Fragment - Wikipedia, The Free EncyclopediaDocument2 pagesKlenow Fragment - Wikipedia, The Free EncyclopediaShailendra YadavPas encore d'évaluation
- Gel Electrophoresis - Wikipedia, The Free EncyclopediaDocument10 pagesGel Electrophoresis - Wikipedia, The Free EncyclopediaShailendra YadavPas encore d'évaluation
- Molecular Pathology - Cesium Chloride (CSCL) Density Gradient CentrifugationDocument2 pagesMolecular Pathology - Cesium Chloride (CSCL) Density Gradient CentrifugationShailendra YadavPas encore d'évaluation
- DPDX - LeishmaniasisDocument1 pageDPDX - LeishmaniasisShailendra YadavPas encore d'évaluation
- Toyota Corolla AE80 - 2 - 3 01 - 85-08 - 86 Corolla (PDFDrive)Document107 pagesToyota Corolla AE80 - 2 - 3 01 - 85-08 - 86 Corolla (PDFDrive)Abhay Kumar Sharma BOODHOOPas encore d'évaluation
- Dela Cruz vs. Atty. DimaanoDocument8 pagesDela Cruz vs. Atty. DimaanoMarga CastilloPas encore d'évaluation
- Traverse AdjustmentDocument22 pagesTraverse AdjustmenthabtePas encore d'évaluation
- 32 EM GreenTechDocument45 pages32 EM GreenTechMark Lester RealPas encore d'évaluation
- DS ClozapineDocument3 pagesDS ClozapineMiggsPas encore d'évaluation
- Mouth Tongue and Salivary GlandsDocument52 pagesMouth Tongue and Salivary GlandsIrfan FalahPas encore d'évaluation
- Monthly Film Bulletin: 1T1IcqDocument12 pagesMonthly Film Bulletin: 1T1IcqAlfred_HitzkopfPas encore d'évaluation
- BA 238. Berita Acara XCMGDocument3 pagesBA 238. Berita Acara XCMGRizkiRamadhanPas encore d'évaluation
- MSPM Clark UniversityDocument27 pagesMSPM Clark Universitytushar gargPas encore d'évaluation
- CABE Space - A Guide To Producing Park and Green Space Management PlansDocument48 pagesCABE Space - A Guide To Producing Park and Green Space Management PlansbenconnolleyPas encore d'évaluation
- Unit 2 Listening SkillDocument7 pagesUnit 2 Listening SkillSbgacc SojitraPas encore d'évaluation
- 1416490317Document2 pages1416490317Anonymous sRkitXPas encore d'évaluation
- MLOG GX CMXA75 v4.05 322985e0 UM-EN PDFDocument342 pagesMLOG GX CMXA75 v4.05 322985e0 UM-EN PDFGandalf cimarillonPas encore d'évaluation
- Aalborg Engineering Sg8 EngDocument4 pagesAalborg Engineering Sg8 EngHenrique de OliveiraPas encore d'évaluation
- Different Varieties of English C1 WSDocument5 pagesDifferent Varieties of English C1 WSLaurie WPas encore d'évaluation
- Or SuperDocument2 pagesOr SuperJac BacPas encore d'évaluation
- Problem+Set+ 3+ Spring+2014,+0930Document8 pagesProblem+Set+ 3+ Spring+2014,+0930jessica_1292Pas encore d'évaluation
- Tucker CarlsonDocument4 pagesTucker CarlsonDai ZPas encore d'évaluation
- Examples of Literacy Narrative Thesis StatementDocument6 pagesExamples of Literacy Narrative Thesis Statementzxtccvgld100% (2)
- MNDCS-2024 New3 - 231101 - 003728Document3 pagesMNDCS-2024 New3 - 231101 - 003728Dr. Farida Ashraf AliPas encore d'évaluation