Académique Documents
Professionnel Documents
Culture Documents
Chemical Composition of The Essential Oil of Phlomis Linearis Boiss. & Bal., and Biological Effects On The CAM-Assay: A Safety Evaluation
Transféré par
Sonia SahnounDescription originale:
Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Chemical Composition of The Essential Oil of Phlomis Linearis Boiss. & Bal., and Biological Effects On The CAM-Assay: A Safety Evaluation
Transféré par
Sonia SahnounDroits d'auteur :
Formats disponibles
Chemical Composition of the Essential Oil of Phlomis linearis Boiss. & Bal.
, and Biological Effects on the CAM-Assay: A Safety Evaluation
Betl Demircia,b,*, Mehmet Y. Dadandc, Dietrich H. Paperb, Gerhard Franzb, and Kemal Hsn Can Bas era
a b c
Department of Pharmacognosy, Faculty of Pharmacy, Anadolu University, 26470 Eskis ehir, Turkey. E-mail: bdemirca@anadolu.edu.tr Department of Pharmaceutical Biology, Faculty of Chemistry and Pharmacy, University of Regensburg, 93040 Regensburg, Germany Department of Biology, Faculty of Science and Letters, Erciyes University, 38039 Kayseri, Turkey
* Author for correspondence and reprint requests Z. Naturforsch. 58 c, 826829 (2003); received June 20/August 15, 2003 Phlomis linearis Boiss. & Bal. of the Lamiaceae family growing in central, east and southeast Anatolia is an endemic species for Turkey. The essential oil obtained from the aerial parts by hydro distillation was subsequently analyzed by GC/MS. The main components of the oil were found as -caryophyllene (24.2%), germacrene D (22.3%) and caryophyllene oxide (9.2%), among 49 identified compounds, representing 94.5% of the total essential oil. The overall biological activity of the essential oil (100 g/pellet) was tested on the chorioallantoic membrane (CAM) of the fertilized hens egg in order to examine the anti-angiogenic and anti-inflammatory activity. None of the tests showed pronounced activity, toxicity or irritation at the tested concentration. Key words: Phlomis linearis, Essential Oil, CAM-Assay
Introduction The genus Phlomis as perennial herbs or shrubs of Lamiaceae are represented by thirty four species of which twenty one are endemic for Turkey (Huber-Morath, 1982). The leaves and flowers of Phlomis species are used as aromatic amara, carminative, stimulant and are locally known as Ballkotu, Calba, C alba, S alba in Turkish traditional medicine (Baytop, 1997, 1999). The endemic species Phlomis linearis Boiss. & Bal. grows in steps, rocky igneous slopes, volcanic rubble at 1400 to 2400 m and has a distribution area from central to east and southeast Anatolia. This species is distinguished by a wide tubular calyx with a leave size 4 to 8 times longer than the width. The calyx and bracteoles also contain intense long hairs, the corolla is two lipped, generally in yellow and the upper lip is occasionally brownish (Dadand, 2002) Chemical constituents of Phlomis linearis have been investigated by C and co-workers (1990, als 1991); new glycosides, iridoids, neolignans, and their derivatives have been isolated. Some Phlomis species have also been investigated for both
09395075/2003/11000826 $ 06.00
their biological activities and essential oil compositions by different groups (Mirza and Nik, 2003; Couladis et al., 2000; Sokovic et al., 2002a,b; Ristic et al., 2000) The in vivo application on the chorioallantoic membrane of the fertilized egg (CAM-assay) is a useful and well established method to determine anti-angiogenic and anti-inflammatory activity of individual compounds or complex plant extracts. It can be used for testing natural compounds in small amounts for revealing various modes of action and the complex mechanisms related to angiogenesis and inflammation. Furthermore, possible side effects such as membrane irritation, toxic and anticoagulant properties of the investigated material in question can be revealed (Paper, 1998; DArcy and Howard, 1967; Brgermeister et al., 2002; and references cited herein). In this study, we have isolated the volatiles by water distillation from the aerial parts of P. linearis. The essential oil was then analyzed by gas chromatography/mass spectroscopy (GC/MS). To evaluate the essential oil and its potential anti-angiogenic and anti-inflammatory activity, the CAMassay was employed.
2003 Verlag der Zeitschrift fr Naturforschung, Tbingen http://www.znaturforsch.com D
B. Demirci et al. Essential Oil of Phlomis linearis
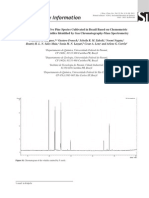
827 Table I. The composition of the essential oil of Phlomis linearis. RRI Compound 1479 1497 1528 1535 1553 1589 1600 1612 1650 1668 1687 1704 1706 1726 1740 1755 1773 1776 1808 1827 1838 1857 1868 1900 1958 2001 2008 2045 2046 2071 2095 2131 2179 2198 2209 2239 2255 2300 2324 2384 2389 2392 2400 2500 2600 2622 2655 2700 2900 %
Methods and Materials Plant material Aerial parts of the plant were collected from a step area in Kayseri, Erciyes Mountain, at about 2000 m, on July 24, 2002, by one of us (M.Y.D.) and M. G. Halc. A voucher specimen (MYD1621) was kept at the Herbarium of Erciyes University, Faculty of Science and Letters. Isolation of the essential oil The aerial parts were dried in shade at room temperature. The essential oil was obtained by water distillation using a Clevenger type apparatus for 3 h having a yield of 0.05% (w/v). Gas chromatography mass spectrometry The essential oil was analysed by gas chromatography/mass spectrometry using a HewlettPackard GCD system. Innowax FSC column (60 m 0.25 mm, 0.25 m film thickness) was used with helium as carrier gas. GC oven temperature was kept at 60 C for 10 min and programmed to 220 C at a rate of 4 C/min, and then kept constant at 220 C for 10 min and programmed to 240 C at a rate of 1 C/min. Split flow was adjusted at 50 ml/min. The injector temperature was 250 C. Mass spectra were recorded at 70 eV. Mass range was from m/z 35 to 425. n-Alkanes were used as reference points in the calculation of relative retention indices (RRI). Relative percentages of the characterized components were as listed in Table I. Library search was carried out using both Wiley GC/MS Library and Baser Library of Essential Oil Constituents. CAM-assay Sample preparation: The essential oil (10 mg/ml) was dissolved in a 2.5% (w/v) agarose (Merck, Darmstadt) solution. For ease of application pellets of these solutions (10 l) were prepared and applied drop wise on circular teflon supports of 3 mm diameter, cooled to room temperature for solidification and applied on to the chorioallantoic membrane (CAM). Preparation of the test medium: The fertilized hens eggs were previously incubated for 6572 h at 37 C and a relative humidity of 80%. The eggs were positioned in a horizontal position and ro-
-Elemene tr -Copaene 0.3 -Bourbonene tr -Bourbonene 1.4 Linalool 0.6 -Ylangene 0.5 -Elemene 0.7 -Caryophyllene 24.2 -Elemene 0.1 (Z)--Farnesene 6.6 -Humulene 3.5 -Muurolene 0.4 -Terpineol 0.2 Germacrene D 22.3 -Muurolene 1.4 Bicyclogermacrene 1.1 -Cadinene 1.0 -Cadinene tr Nerol tr (E,E)-2,4-Decadienal 0.2 (E)--Damascenone 0.3 Geraniol 0.5 (E)-Geranyl acetone 0.5 Nonadecane 0.2 (E)--Ionone 0.6 Isocaryophyllene oxide 0.8 Caryophyllene oxide 9.2 Humulene epoxide-I tr Norbourbonone 0.1 Humulene epoxide-II 0.7 Hexyl benzoate 0.2 Hexahydrofarnesyl acetone 5.8 3,4-Dimethyl-5-pentylidene-2(5H)-furanone 0.6 Thymol 0.5 T-Muurolol 0.3 Carvacrol 1.2 -Cadinol 1.1 Tricosane 0.6 Caryophylla-2(12),6(13)-dien-5-ol 0.4 (Caryophylladienol II) Hexadecanol 0.9 Caryophylla-2(12),6-dien-5-ol 0.1 (Caryophyllenol I) Caryophylla-2(12),6-dien-5-ol 1.1 (Caryophyllenol II) Tetracosane 0.1 Pentacosane 1.3 Hexacosane tr Phytol 0.1 Benzyl benzoate 0.2 Heptacosane 1.7 Nonacosane 0.8 Total 94.5
RRI: Relative retention indices calculated against n-alkanes. %: calculated from TIC. tr: Trace (< 0.1%).
828
B. Demirci et al. Essential Oil of Phlomis linearis
tated several times. Then the eggs were opened on the snub side. Before the opening 1015 ml of albumin were aspirated from a hole on the pointed side. At two third of the height (from the pointed side) the eggs were traced with a scalpel and after that the shells were removed with forceps. The cavity was covered with film and the eggs were incubated at 37 C at a relative humidity of 80% for further 75 h. If the formed CAM had approximately a diameter of 2 cm one pellet (1 pellet/egg) was placed on it. The eggs were incubated for one further day and then evaluated under the stereomicroscope. For every test compound 1015 eggs were utilized. Evaluation and scoring: For the evaluation of the effects, a scoring system was used (see Tables II and III). In brief; score: 0.5, no anti-angiogenic effect; score: 0.51, weak to medium anti-angiogenic effect; score: 1, medium to strong anti-angiogenic effect. As controls suramin (Merck, Darmstadt) and sodium dodecyl sulphate (SDS) (Merck, Darmstadt) at the concentration of 50 g/pellet were also tested. As blank, CAMs treated with solidified agarose-solution in pellet form (2.5%, w/v) were also included. Each experiment was performed in triplicate (DArcy and Howard, 1967; Brgermeister et al., 2002). Results and Discussion The chemical compositions of various Phlomis species have been investigated. However, to the best of our knowledge this is the first investigation of the volatile fraction of P. linearis. The hydrodistillation of the aerial parts yielded a yellowish essential oil with a pleasant odour, however in a poor yield. Subsequent GC/MS analysis resulted in detection and identification of 49 components
representing 94.5% of the total oil composition (Table I). Sesquiterpene hydrocarbons were the major volatiles with -caryophyllene (24.2%), germacrene D (22.3%), and (Z)--farnesene (6.6%), respectively. Oxygenated sesquiterpenes were present mostly as epoxides like caryophyllene oxide (9.2%). Noteworthy was the presence of various caryophyllene derivatives in different amounts that probably contribute to the characteristic odour of the oil. Oxygenated monoterpenes and monoterpene hydrocarbons were the minor portion of the whole oil which certainly also have impacts to the odour. Due to the ethno-botanical use and various biological activities of Phlomis species we have also subjected the essential oil for potential anti-angiogenic and anti-inflammatory activity evaluation using the CAM-assay. At 100 g/pellet the essential oil showed a very weak angiogenic effect compared to standard substances (Table II and III). When applied to the CAM the essential oil showed at this concentration neither toxic, nor irritant effects. The essential oil composition of Phlomis olivieri Benth. from Iran was very recently reported, sesquiterpenes were among the major components with germacrene D (28.1%) and -caryophyllene (16.1%) of fifteen identified compounds (Mirza and Nik, 2003). Phlomis lanata Willd. essential oil was analyzed to reveal forty-eight compounds representing 97% of the oil where -pinene (25.4%), limonene (15.7%) and trans-caryophyllene (8.8%) were found as major components (Couladis et al., 2000). Another study was on the essential oils from the leaves of Phlomis fruticosa L. Forty components were identified, -caryophyllene (12%), (E)-methyl isoeugenol (15.3%) and -asarone
Table II. Effects of Phlomis linearis essential oil in the CAM-assay. Test Sample Phlomis linearis oil Agarose Suramin Sodium dodecyl sulphate (SDS) * Formula for scoring: Average score = Number of eggs (score 2) 2 + number of eggs (score 1) 1 Total number of eggs (score 0, 1, 2) Score* 0.2 0.2 0.2 0.2 0.5 0.2 0 Irritation (%) 85 5 100 g/pellet Blanc (2.5%, w/v) Positive control (50 g/pellet) Negative control (50 g/pellet)
B. Demirci et al. Essential Oil of Phlomis linearis Table III. Score values for the evaluation of the anti-angiogenic effect on the CAM. Score 0 0.5 1 2 Effect no very weak weak medium strong Observation/Interpretation
829
Normal embryo formation. No capillary free area. Area with reduced density of capillaries around the pellet not larger than its own area. Small capillary free area or area with significantly reduced density of capillaries. Effects not larger than double the size of the pellet. Capillary free area around the pellet at least double the size of the pellet.
(10.9%) as major compounds, respectively (Sokovic et al., 2002a). Phlomis fruticosa L. from two different localities in Yugoslavia were analyzed and investigated for their antimicrobial activities. Here, -pinene (3957%) was found to be the major constituent in both samples (Ristic et al., 2000).
Phlomis species are widely distributed in Turkey, however, composition of the essential oil has not been investigated thoroughly. This is also the case for their biological activities. Using the CAMassay other essential oils and major common components will be also tested in further work. Our studies on medicinal and aromatic Phlomis species are ongoing.
(DictioBaytop T. (1997), Trkc e Bitki Adlar Szlg nary of Turkish Plant Names), TDK Yay., No. 280, Ankara, Turkey. Baytop T. (1999), Trkiyede Bitkiler ile Tedavi, Gec mis te ve Bugn (Therapy with Medicinal Plants in Turkey, Past and Present). Nobel Tp Basmevi, Istanbul, Turkey, 2nd ed. Brgermeister J., Paper D. H., Vogl H., Linhardt R. J., and Franz G. (2002), LaPSvS1, a (153)--galactan sulfate and its effect on angiogenesis in vivo and in vitro. Carbohydrate Res. 337, 14591466. Couladis M., Tanimanidis A., Tzakou O., Chinou I. B., and Harvala C. (2000), Essential oil of Phlomis lanata growing in Greece: Chemical composition and antimicrobial activity. Planta Med. 66, 670672. lu I., Sticher O., and C I., Bas aran A. A., Sarac og als Redi P. (1990), Phlinosides A, B and C, three phenylpropanoid glycosides from Phlomis linearis. Phytochemistry 29, 12531257. lu I., Sticher O., and C I., Bas aran A. A., Sarac og als Redi P. (1991), Phlinosides D and E, phenylpropanoid glycosides, and iridoids from Phlomis linearis. Phytochemistry 30, 30733076. DArcy P. F. and Howard E. M. (1967), A new antiinflammatory test, utilizing the chorioallantoic membrane of the chick embryo. Br. J. Pharmac. Chemother. 29, 378387.
Dadand M. Y. (2002), Trkiyenin Phlomis L. (Lamiaceae) Cinsi Revizyonu [The Revision of the Phlomis L. (Lamiaceae) of Turkey]. Ph. D. Thesis, Institute of Science and Technology, Gazi University, Ankara, Turkey. Huber-Morath A. (1982), Phlomis L. In: Flora of Turkey and East Aegean Islands Davis P. H., ed.). Edinburgh Univ. Press, Edinburgh, Vol. 7, pp. 102126. Mirza M. and Nik Z. B. (2003), Volatile constituents of Phlomis olivieri Benth. from Iran. Flavour Fragr. J. 18, 131132. Paper D. H. (1998), Natural Products as angiogenesis inhibitors. Planta Med. 64, 686695. Ristic M. D., Duletic-Lausevic S., Knezevic-Vukcevic J., Marin P. D., Simic D., Vukojevic J., Janackovic P., and Vajs V. (2000), Antimicrobial activity of essential oils and ethanol extract of Phlomis fruticosa L. (Lamiaceae). Phytother. Res. 14, 267271. Sokovic M. D., Marin P. D., Janackovic P., Vajs V., Milosavljevic S., Dokovic D., Tesevic V., and Petrovic S. (2002a), Composition of the essential oils of Phlomis fruticosa L. (Lamiaceae). J. Essent. Oil Res. 14, 167 168. Sokovic M. D., Marin P. D., Simic D., Knezevic-Vukcevic J., Vajs V., and Petrovic S. (2002b), Antimutagenic activity of essential oil and crude extract of Phlomis fruticosa. Pharm. Biol. 40, 311314.
Vous aimerez peut-être aussi
- THE DOSE, Issue 1 (Tokyo)Document142 pagesTHE DOSE, Issue 1 (Tokyo)Damage85% (20)
- ISO 13485-2016 - DR - Pack - Control of Non Conforming ProductsDocument4 pagesISO 13485-2016 - DR - Pack - Control of Non Conforming ProductskmasanPas encore d'évaluation
- Ansible Playbook for BeginnersDocument101 pagesAnsible Playbook for BeginnersFelix Andres Baquero Cubillos100% (1)
- Chemical Compositon Ocimum GratissimumDocument5 pagesChemical Compositon Ocimum GratissimumJosafat TarazonaPas encore d'évaluation
- Essential Oil Components of German Chamomile Cultivated in Firoozabad IranDocument3 pagesEssential Oil Components of German Chamomile Cultivated in Firoozabad IranDavid Meza CarbajalPas encore d'évaluation
- Chemical Variability and Antioxidant Activity ofDocument11 pagesChemical Variability and Antioxidant Activity ofleonorgcl9362Pas encore d'évaluation
- K. H Usn U Can Ba Ser, Bet Ul Demirci, Nurhayat Tabanca, Temel Ozek and Nezhun G OrenDocument6 pagesK. H Usn U Can Ba Ser, Bet Ul Demirci, Nurhayat Tabanca, Temel Ozek and Nezhun G OrenРусланPas encore d'évaluation
- 1007 PDFDocument3 pages1007 PDFDana Iulia MoraruPas encore d'évaluation
- Dashtianeh Et AlDocument7 pagesDashtianeh Et AlWicky WallayPas encore d'évaluation
- 11 Azadirachta IndicaDocument6 pages11 Azadirachta IndicaTrần Thanh TríPas encore d'évaluation
- Sarisan From Leaves of Piper Affinis Hispidinervum C. DC (Long Pepper)Document3 pagesSarisan From Leaves of Piper Affinis Hispidinervum C. DC (Long Pepper)РусланPas encore d'évaluation
- Chemical Composition and Insecticidal Activities of Essential Oils of Two Algerian Endemic Plants: Ferula Vesceritensis Coss. Et Dur. andDocument8 pagesChemical Composition and Insecticidal Activities of Essential Oils of Two Algerian Endemic Plants: Ferula Vesceritensis Coss. Et Dur. andTJPRC PublicationsPas encore d'évaluation
- A Influence of Drying andDocument5 pagesA Influence of Drying andkiper.valPas encore d'évaluation
- Chemical Composition of Saudi Arabian Sukkari Variety of Date Seed Oil and Extracts Obtained by Slow PyrolysisDocument12 pagesChemical Composition of Saudi Arabian Sukkari Variety of Date Seed Oil and Extracts Obtained by Slow Pyrolysisfarkad rawiPas encore d'évaluation
- Chemical Constituents of Rose Oil and WaterDocument6 pagesChemical Constituents of Rose Oil and Wateramitaggarwal78Pas encore d'évaluation
- Chemical Components in Volatile Oil From: Blumea Balsamifera (L.) DCDocument3 pagesChemical Components in Volatile Oil From: Blumea Balsamifera (L.) DCMartin WemanPas encore d'évaluation
- Indicus R. BR.: Chemical Composition of The Volatiles of HemidesmusDocument3 pagesIndicus R. BR.: Chemical Composition of The Volatiles of HemidesmusРусланPas encore d'évaluation
- Essential Oil Composition of Thymus Vulgaris L. and Their UsesDocument12 pagesEssential Oil Composition of Thymus Vulgaris L. and Their UsesAlejandro 20Pas encore d'évaluation
- A Simple Capillary Column GC Method For Analysis of Palm Oil-Based Polyol EstersDocument6 pagesA Simple Capillary Column GC Method For Analysis of Palm Oil-Based Polyol Estersanuradha.d.bhat9860Pas encore d'évaluation
- 1111 Chemical Composition of Berry Essential Oils From Juniperus Communis L. From MACEDONIA PDFDocument10 pages1111 Chemical Composition of Berry Essential Oils From Juniperus Communis L. From MACEDONIA PDFGaston CassaroPas encore d'évaluation
- Damascena Rose Oil ChromatographyDocument10 pagesDamascena Rose Oil ChromatographyMartinaPas encore d'évaluation
- Argyropoulou 2007Document7 pagesArgyropoulou 2007Fabio PicassoPas encore d'évaluation
- Multivariate Statistical Analysis of The Essential Oil Composition of Thymus Praecox Opiz Ssp. Polytrichus (Kern. Ex Borb.) Ronn. Collected in The Tyrolean AlpsDocument6 pagesMultivariate Statistical Analysis of The Essential Oil Composition of Thymus Praecox Opiz Ssp. Polytrichus (Kern. Ex Borb.) Ronn. Collected in The Tyrolean AlpsРусланPas encore d'évaluation
- Comparison of Microwave-Assisted Hydro Distillation Withthe Traditional Hydro Distillation Method in The Extraction of Essential Oils From Thymus Vulgaris L.Document6 pagesComparison of Microwave-Assisted Hydro Distillation Withthe Traditional Hydro Distillation Method in The Extraction of Essential Oils From Thymus Vulgaris L.myrtarom100% (4)
- Comparative Study of Essential Oil Composition of Leaves and Rhizomes of Alpinia Conchigera Griff. at Different Post - Harvest Drying PeriodsDocument6 pagesComparative Study of Essential Oil Composition of Leaves and Rhizomes of Alpinia Conchigera Griff. at Different Post - Harvest Drying Periodssambinu1Pas encore d'évaluation
- Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of Extracted Oil From Whole Garden Cress (Rashaad) SeedsDocument7 pagesGas Chromatography-Mass Spectrometry (GC-MS) Analysis of Extracted Oil From Whole Garden Cress (Rashaad) SeedsAli Mohammad Abu-RummanPas encore d'évaluation
- Kim 34 5 13 1002 62 PDFDocument10 pagesKim 34 5 13 1002 62 PDFmadinatul khujjahPas encore d'évaluation
- Supercritical Fluid Extraction 2017Document6 pagesSupercritical Fluid Extraction 2017jajangsamsungPas encore d'évaluation
- Particle SizeDocument7 pagesParticle SizeyohannesPas encore d'évaluation
- Alet 2012 Chemical Constituents of Essential Oils From Resin and Bark of Agathis BorneensisDocument5 pagesAlet 2012 Chemical Constituents of Essential Oils From Resin and Bark of Agathis BorneensisYuliana Sabarina LewarPas encore d'évaluation
- Hemijski Sastav I Imunomodulatorna Aktivnost Ulja TamjanaDocument9 pagesHemijski Sastav I Imunomodulatorna Aktivnost Ulja TamjanaDjole ProlecePas encore d'évaluation
- Aboutabl Et Al. (2002) - Phytochemical and Pharmacological Studies On Sideritis Taurica Stephan Ex Wild.Document8 pagesAboutabl Et Al. (2002) - Phytochemical and Pharmacological Studies On Sideritis Taurica Stephan Ex Wild.Pavlos RigasPas encore d'évaluation
- Chemical Composition and Antibacterial Activity of Essential Oil of OcimumDocument4 pagesChemical Composition and Antibacterial Activity of Essential Oil of OcimumMedika WisataPas encore d'évaluation
- Food Chemistry: Thomas S.C. Li, Thomas H.J. Beveridge, John C.G. DroverDocument7 pagesFood Chemistry: Thomas S.C. Li, Thomas H.J. Beveridge, John C.G. DroverwxcvbnnbvcxwPas encore d'évaluation
- Razafimamonjison essential oil of clove from Indonesia Madagascar and Zanzibar.Document22 pagesRazafimamonjison essential oil of clove from Indonesia Madagascar and Zanzibar.mohsen fatemiPas encore d'évaluation
- Artikel Antimicrobial Activity of Turkish Citrus Peel OilsDocument6 pagesArtikel Antimicrobial Activity of Turkish Citrus Peel OilsYonathan PalliPas encore d'évaluation
- GraceDocument7 pagesGracePaola JaraPas encore d'évaluation
- Fuel Processing TechnologyDocument6 pagesFuel Processing Technologybayu_sophPas encore d'évaluation
- 2.- chemical composition of essnetial oilsDocument6 pages2.- chemical composition of essnetial oilsguoi910409hmcrrs00Pas encore d'évaluation
- Essential Oil Composition of Two Basil SpeciesDocument6 pagesEssential Oil Composition of Two Basil SpeciesremonrhezaPas encore d'évaluation
- Cyperus Scariosus 1Document4 pagesCyperus Scariosus 1Tony RamirezPas encore d'évaluation
- Analysis of Oxidised Diterpenoid Acids Using Methylation With TMAHDocument17 pagesAnalysis of Oxidised Diterpenoid Acids Using Methylation With TMAHjgPas encore d'évaluation
- Constituents of The Essential Oil of Six Helichrysum Species From MadagascarDocument4 pagesConstituents of The Essential Oil of Six Helichrysum Species From MadagascarРусланPas encore d'évaluation
- Flavour Fragr J 1997 v12 p173Document4 pagesFlavour Fragr J 1997 v12 p173Kharisul IhsanPas encore d'évaluation
- Full Characterisation of Crambe Abyssinica Hochst. Seed OilDocument6 pagesFull Characterisation of Crambe Abyssinica Hochst. Seed Oilwillian01Pas encore d'évaluation
- JNatProd66 (2003) 1101Document3 pagesJNatProd66 (2003) 1101Ana Paula SantosPas encore d'évaluation
- Phytochemical Analysis, Isolation and Identification of Flavan-3ol From Syrian Pinus HalepensisDocument7 pagesPhytochemical Analysis, Isolation and Identification of Flavan-3ol From Syrian Pinus HalepensisリファイPas encore d'évaluation
- Antioxidant Activity of Chamomile Essential Oil and Main Components (#455019) - 526559Document6 pagesAntioxidant Activity of Chamomile Essential Oil and Main Components (#455019) - 526559Catherine GultomPas encore d'évaluation
- Malhotra 2012Document8 pagesMalhotra 2012Noor FatimahPas encore d'évaluation
- Almeida2005 (Curcumin 3)Document6 pagesAlmeida2005 (Curcumin 3)chezia priscillaPas encore d'évaluation
- Research Paper: Extraction of Ambrette Seed Oil and Isolation of Ambrettolide With Its Characterization by H NMRDocument6 pagesResearch Paper: Extraction of Ambrette Seed Oil and Isolation of Ambrettolide With Its Characterization by H NMRMimbel WimbelPas encore d'évaluation
- Chemical Composition, Antibacterial and Antioxidant Activities ofDocument10 pagesChemical Composition, Antibacterial and Antioxidant Activities ofالذكر الحكيمPas encore d'évaluation
- GC/MS Analysis of Essential Oil Isolated From The Roots of Cymbopogon Winterianus JowittDocument7 pagesGC/MS Analysis of Essential Oil Isolated From The Roots of Cymbopogon Winterianus JowittchemistryjournalPas encore d'évaluation
- Unsaponifiable Lipid Constituents of Some Underutilized Tropical Seed OilsDocument4 pagesUnsaponifiable Lipid Constituents of Some Underutilized Tropical Seed OilsDavidsantiago Murillo AvilaPas encore d'évaluation
- Chemical Components and Biological Activities of Volatile Oil of Kaempferia Galanga LinnDocument5 pagesChemical Components and Biological Activities of Volatile Oil of Kaempferia Galanga LinnGuhan KAPas encore d'évaluation
- Available online essential oil composition of Rhanterium adpressumDocument4 pagesAvailable online essential oil composition of Rhanterium adpressumنورالدين غرافPas encore d'évaluation
- Article1379954058 - Yasin Et AlDocument11 pagesArticle1379954058 - Yasin Et AlMuhammad Imran KhanPas encore d'évaluation
- Jals P74 - 329 332Document5 pagesJals P74 - 329 332NaziiPas encore d'évaluation
- Iridoid and Phenylpropanoid Glycosides From Phlomis Samia, P. Monocephala and P. Carica (#142792) - 124215Document11 pagesIridoid and Phenylpropanoid Glycosides From Phlomis Samia, P. Monocephala and P. Carica (#142792) - 124215Hashemi AkhterPas encore d'évaluation
- Characterization of The Portuguese-Grown Cistus: Ladanifer Essential OilDocument6 pagesCharacterization of The Portuguese-Grown Cistus: Ladanifer Essential OilkhalidPas encore d'évaluation
- Nano Scale Analysis of Eucalyptus camaldulensis Essential OilsDocument4 pagesNano Scale Analysis of Eucalyptus camaldulensis Essential OilsAam AmeliaPas encore d'évaluation
- Essential Oils in Food Processing: Chemistry, Safety and ApplicationsD'EverandEssential Oils in Food Processing: Chemistry, Safety and ApplicationsSeyed Mohammed Bagher HashemiPas encore d'évaluation
- 1 s2.0 S0960852409010876 MainDocument4 pages1 s2.0 S0960852409010876 MainSonia SahnounPas encore d'évaluation
- 2010 551752Document10 pages2010 551752Sonia SahnounPas encore d'évaluation
- Interpretation of Mass Spectra: Choose A Reasonable StructureDocument16 pagesInterpretation of Mass Spectra: Choose A Reasonable Structuredjdr0Pas encore d'évaluation
- Profiling of The Resin GlycosideDocument7 pagesProfiling of The Resin GlycosideSonia SahnounPas encore d'évaluation
- Differentiation of Five Pine Species Cultivated in Brazil Based On ChemometriclDocument8 pagesDifferentiation of Five Pine Species Cultivated in Brazil Based On ChemometriclSonia SahnounPas encore d'évaluation
- Flavonoids and Terpenoids From Helichrysum ForskahliiDocument5 pagesFlavonoids and Terpenoids From Helichrysum ForskahliiSonia SahnounPas encore d'évaluation
- Profiling of The Resin GlycosideDocument7 pagesProfiling of The Resin GlycosideSonia SahnounPas encore d'évaluation
- 1Document7 pages1Sonia SahnounPas encore d'évaluation
- Structural Characterization and Isomer DifferentiationDocument18 pagesStructural Characterization and Isomer DifferentiationSonia SahnounPas encore d'évaluation
- Flavonoid Analysis of Heloniopsis OrientalisDocument5 pagesFlavonoid Analysis of Heloniopsis OrientalisSonia SahnounPas encore d'évaluation
- Chemical Constituents From The Root BarkDocument6 pagesChemical Constituents From The Root BarkSonia SahnounPas encore d'évaluation
- Flavonoid Composition in The Leaves of TwelveDocument8 pagesFlavonoid Composition in The Leaves of TwelveSonia SahnounPas encore d'évaluation
- Flavonoids and Terpenoids From Helichrysum ForskahliiDocument5 pagesFlavonoids and Terpenoids From Helichrysum ForskahliiSonia SahnounPas encore d'évaluation
- Analytical Separation and Detection Methods For FlavonoidsDocument33 pagesAnalytical Separation and Detection Methods For FlavonoidsrenatacnPas encore d'évaluation
- HPLCDocument6 pagesHPLCSonia SahnounPas encore d'évaluation
- Isolation and Structural Characterization of New Glycolipid EsterDocument16 pagesIsolation and Structural Characterization of New Glycolipid EsterSonia SahnounPas encore d'évaluation
- Quinolizidine Alkaloids As Systematic Markers of The GenisteaeDocument7 pagesQuinolizidine Alkaloids As Systematic Markers of The GenisteaeSonia SahnounPas encore d'évaluation
- JM Guide To ATE Flier (c2020)Document2 pagesJM Guide To ATE Flier (c2020)Maged HegabPas encore d'évaluation
- Paper 4 (A) (I) IGCSE Biology (Time - 30 Mins)Document12 pagesPaper 4 (A) (I) IGCSE Biology (Time - 30 Mins)Hisham AlEnaiziPas encore d'évaluation
- NABARD road inspection report formatDocument24 pagesNABARD road inspection report formatSrinivas PPas encore d'évaluation
- Three Comparison of Homoeopathic MedicinesDocument22 pagesThree Comparison of Homoeopathic MedicinesSayeed AhmadPas encore d'évaluation
- Guide To Raising Capital From Angel Investors Ebook From The Startup Garage PDFDocument20 pagesGuide To Raising Capital From Angel Investors Ebook From The Startup Garage PDFLars VonTurboPas encore d'évaluation
- Sinclair User 1 Apr 1982Document68 pagesSinclair User 1 Apr 1982JasonWhite99Pas encore d'évaluation
- British Universal Steel Columns and Beams PropertiesDocument6 pagesBritish Universal Steel Columns and Beams PropertiesjagvishaPas encore d'évaluation
- EC GATE 2017 Set I Key SolutionDocument21 pagesEC GATE 2017 Set I Key SolutionJeevan Sai MaddiPas encore d'évaluation
- Mrs. Universe PH - Empowering Women, Inspiring ChildrenDocument2 pagesMrs. Universe PH - Empowering Women, Inspiring ChildrenKate PestanasPas encore d'évaluation
- U2 All That You Can't Leave BehindDocument82 pagesU2 All That You Can't Leave BehindFranck UrsiniPas encore d'évaluation
- Efaverenz p1Document4 pagesEfaverenz p1Pragat KumarPas encore d'évaluation
- CFO TagsDocument95 pagesCFO Tagssatyagodfather0% (1)
- Hi-Line Sportsmen Banquet Is February 23rd: A Chip Off The Ol' Puck!Document8 pagesHi-Line Sportsmen Banquet Is February 23rd: A Chip Off The Ol' Puck!BS Central, Inc. "The Buzz"Pas encore d'évaluation
- France Winckler Final Rev 1Document14 pagesFrance Winckler Final Rev 1Luciano Junior100% (1)
- WWW - Commonsensemedia - OrgDocument3 pagesWWW - Commonsensemedia - Orgkbeik001Pas encore d'évaluation
- 4 Influencing Factors of Learners Career Choice Parents Choice Vs Personal DescisionDocument24 pages4 Influencing Factors of Learners Career Choice Parents Choice Vs Personal Descisionmatteo mamaloPas encore d'évaluation
- Grading System The Inconvenient Use of The Computing Grades in PortalDocument5 pagesGrading System The Inconvenient Use of The Computing Grades in PortalJm WhoooPas encore d'évaluation
- DOE Tank Safety Workshop Presentation on Hydrogen Tank TestingDocument36 pagesDOE Tank Safety Workshop Presentation on Hydrogen Tank TestingAlex AbakumovPas encore d'évaluation
- Link Ratio MethodDocument18 pagesLink Ratio MethodLuis ChioPas encore d'évaluation
- Ratio Analysis of PIADocument16 pagesRatio Analysis of PIAMalik Saad Noman100% (5)
- Iphoneos 31Document159 pagesIphoneos 31Ivan VeBoPas encore d'évaluation
- Seminar Course Report ON Food SafetyDocument25 pagesSeminar Course Report ON Food SafetyYanPas encore d'évaluation
- Inventory ControlDocument26 pagesInventory ControlhajarawPas encore d'évaluation
- Easa Management System Assessment ToolDocument40 pagesEasa Management System Assessment ToolAdam Tudor-danielPas encore d'évaluation
- Cell Organelles ColoringDocument2 pagesCell Organelles ColoringThomas Neace-FranklinPas encore d'évaluation
- Ovr IbDocument27 pagesOvr IbAriel CaresPas encore d'évaluation