Académique Documents
Professionnel Documents
Culture Documents
C NMR Spectroscopy Worksheet (30 Points) Due 2/24/11 in Lecture
Transféré par
Nurillahi Febria LeswanaTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
C NMR Spectroscopy Worksheet (30 Points) Due 2/24/11 in Lecture
Transféré par
Nurillahi Febria LeswanaDroits d'auteur :
Formats disponibles
13
CNMRspectroscopyworksheet(30points)
AI:
due2/24/11inlecture
Name
1. (18points)ProvideviablestructuresforthefollowingNMRsbasedonthefollowingmolecularstructures.Giventhe molecularformula,itwouldbesmarttostarttheproblembycalculatingthedegreeofunsaturationfirst.Labelyour structureappropriatelyonyourstructureandtheNMRclearlysothatyourAIcangradeiteasily.
e
a b c d
OH
a. C5H10O2(inCDCl3):
13
pentanoic acid
CNMRsignals:181,34,27,22,4ppm
c
Cl
1-chloro-2,2- dimethyl propane b
a b
Cl
b. C5H11Cl(inCDCl3):
13
acceptable answer, but b too deshielded
CNMRsignals:57,33,27ppm
c b a
Accetpable answers:
O
b c a
OH
a b
c. C5H10O(inCDCl3):
13
CNMRsignals:69,31,19ppm (Asignallike19ppmissignificantlydifferentthan31suchthatitwould needtobeamethylversusamethylene.)
d c
b
O O O O
d. C8H12O4(inCDCl3):
13
diethyl cis-butenedioate
CNMRsignals:165,131,61,14ppm
(cisortransstructurecouldbeprovidedabove)
Onceyoufigureoutthestructure,proposewhatthesmallimpurityat167ppmmightbe:
O
Thestereoisomer(cisortrans)istheimpurity.
a b
c
d
OH
methacrylic acid
O
a d c
OH
e. C4H6O2(inCDCl3):
13
b accetpable answer
CNMRsignals:174,136,128,18ppm
O
c b d e f
b&c
a acetophenone
O
a
b
c d e
H
f e d
f
a partial credit, except not two ipso carbons would be observed in the NMR
f.
C8H8O(inCDCl3):
13
CNMRsignals:198,137,133,128.7,128.4,26ppm
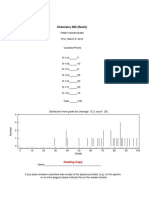
2. (6 points) The two 13C NMR spectra match two of the compounds below. Determine which isomer fits the each NMRbelowandthenassignthepeaks.(Generatedspectrashownosolventpeaksbelow.)
For this top spectrum, you need seven signals, no aliphatic CO signals (i.e. alkoxides), an ipso carbon attached to an oxygen and an ethyl group that gives signals that provide aliphatics that are around 30 and 10 ppm. This leads you to thebottomtwoleftcompounds.However,thefinalpiecethatleadsyoutothephenolicketoneisthatthearenesignals havealargerdisparityduetothephenolicOHbeingelectrondonatingandplacingmoreelectrondensityontothering. TheketoneandthephenolicOHhaveoppositeeffects:electronwithdrawinganddonating,leadingtodisparatearene signals.
13
CNMR:200,163,130,129,116,32,8ppm
13
CNMR:172,151,129,125,121,27,10ppm
3. (4 points) Compare the expected 13C signals between carbons A and B on the following structure. Use structures to provide a good answer. Then, compare expected signals for carbonsCandD.Finally,comparesignalsEandF. Carbon F is next to an electron withdrawing group, O, which places it around 6080 ppm. By comparison, E is next a less electronwithdrawing group, C=O, where one would expect it to comearound30ppm. Carbon C is attached is an electronwithdrawing substituent (ketone) which would deshield it with respect to an arene; howevercarbon Disattachedtoanelectronnegativeelement, O,whichwould bemore deshielding. CarbonC (ca.140 ppm)versuscarbonD(ca.160ppm). Carbon A is beta is an electron withdrawing group and can participate in resonance which places a partial positive chargeoncarbonA,deshieldingit.CarbonBisbetatoanelectrondonatinggroupwhichplacesapartialnegativeonit, shieldingwithrespecttocarbonA. 4. (2 points) Explain how to make up an NMR sample. Explain why deuterated solvent is used (what is meant by a residualpeak?). Most NMR spectra are recorded for compounds dissolved in a solvent. Therefore, signals will be observed for the solventandthismustbeaccountedforinsolvingspectralproblems.AnNMRsampleismadeupbydissolvingca.20 mg of sample into 0.5 1.0 mL deuterated solvent in an NMR tube, and it is then capped. To avoid spectra dominated bythesolvent signal,most1HNMRspectraarerecordedinadeuteratedsolvent.However, deuteration is not "100%", therefore, signals for the residual protons are observed and used for calibration. In chloroform solvent (CDCl3), this corresponds to CHCl3, so a singlet signal is observed at 7.26 ppm. For methanol solvent, this correspondstoCHD2OD,soasignalisobservedat3.31ppm. 13 C NMR is six thousand times less sensitive than 1H NMR, and a good rule of thumb is to provide as much material aswillgiveasaturatedsolutionifpossible.
Vous aimerez peut-être aussi
- 1-Phenylethanol H-NMR PDFDocument2 pages1-Phenylethanol H-NMR PDFkobir960% (1)
- Atomic Structure 1Document25 pagesAtomic Structure 1Gowri ShankarPas encore d'évaluation
- RMN ProblemsDocument7 pagesRMN ProblemsAnonymous llSDP0tPas encore d'évaluation
- Thermodynamics TutorialDocument2 pagesThermodynamics TutorialMuhamad Hazim Zaaba0% (1)
- Recitation - Analytical Chemistry 1Document23 pagesRecitation - Analytical Chemistry 1LORRAINE ABIGAIL KHOZAPas encore d'évaluation
- Tutorial # 1 - KineticsDocument7 pagesTutorial # 1 - KineticsbebsybiswezPas encore d'évaluation
- Chem Stoichio QnsDocument9 pagesChem Stoichio Qnsemily_liu_5Pas encore d'évaluation
- 460 Bai Tap Dien HoaDocument69 pages460 Bai Tap Dien Hoavanhiepk52a100% (1)
- KMÜ 308 Take Home Examination - 2021-SpringDocument1 pageKMÜ 308 Take Home Examination - 2021-Springhatice yanıkPas encore d'évaluation
- Chemical Kinetics of Complex Reactions - Sample Solution - Question 2Document3 pagesChemical Kinetics of Complex Reactions - Sample Solution - Question 2Trung VõPas encore d'évaluation
- Analytical Methods for Major and Modified Nucleosides - HPLC, GC, MS, NMR, UV and FT-IRD'EverandAnalytical Methods for Major and Modified Nucleosides - HPLC, GC, MS, NMR, UV and FT-IRPas encore d'évaluation
- Quiz Show FileDocument18 pagesQuiz Show FileLeaniel SilvaPas encore d'évaluation
- LAB5Document1 pageLAB5Tarmizi Al-AminPas encore d'évaluation
- Ch516 Chemical & Catalytic Reaction Engineering Assignment 5Document3 pagesCh516 Chemical & Catalytic Reaction Engineering Assignment 5Janaki Devi Parrat0% (1)
- Problem Set 1Document3 pagesProblem Set 1Lu JunqueiraPas encore d'évaluation
- (1970) Differential Binding of Alkylaing Fluorochrome in Human ChromosomesDocument5 pages(1970) Differential Binding of Alkylaing Fluorochrome in Human Chromosomesmaulia praditaPas encore d'évaluation
- Synthesis of Methyl SalicylateDocument3 pagesSynthesis of Methyl SalicylateDike FahiraPas encore d'évaluation
- Exp 5Document8 pagesExp 5Azli AzmanPas encore d'évaluation
- Adama Science and Technology University School of Applied Natural Science Department of Applied MathematicsDocument9 pagesAdama Science and Technology University School of Applied Natural Science Department of Applied MathematicsALEMAYEHUPas encore d'évaluation
- Lecture 9Document4 pagesLecture 9Asif AliPas encore d'évaluation
- Soal-Soal Kimia AnorganikDocument5 pagesSoal-Soal Kimia AnorganikSalsaLinaSinasaPas encore d'évaluation
- A Novel Reverse Flow Strategy For Ethylbenzene Dehydrogenation in A Packed-Bed ReactorDocument17 pagesA Novel Reverse Flow Strategy For Ethylbenzene Dehydrogenation in A Packed-Bed ReactorMuhammad Akbar FahleviPas encore d'évaluation
- Sample Test Exam One CH201Document7 pagesSample Test Exam One CH201Ashly PhilipPas encore d'évaluation
- Synthesis of Isobutyl AcetateDocument7 pagesSynthesis of Isobutyl AcetateRandy DavenportPas encore d'évaluation
- Synt432 PrepCuA4H2ODocument12 pagesSynt432 PrepCuA4H2OWisi Wasi100% (1)
- Practice Chapter 18 Carboxylic AcidsDocument0 pagePractice Chapter 18 Carboxylic AcidsRochelle BartiletPas encore d'évaluation
- Partial Molar VolumeDocument6 pagesPartial Molar VolumeAaron Chris GonzalesPas encore d'évaluation
- Workshop IDocument6 pagesWorkshop IValentina GonzálezPas encore d'évaluation
- Price List - Chemicals SolventsDocument10 pagesPrice List - Chemicals SolventsVo Hoang Anh100% (1)
- Functional Group TestsDocument2 pagesFunctional Group TestsSalman HusainPas encore d'évaluation
- Ccb2053 Tutorial 1Document1 pageCcb2053 Tutorial 1eja70Pas encore d'évaluation
- A Study of The Equilibrium Between Ferric and Thiocyanate IonsDocument4 pagesA Study of The Equilibrium Between Ferric and Thiocyanate IonsWombatNZ0% (1)
- AspirinDocument3 pagesAspirinMAYUR CHATURPas encore d'évaluation
- Amperometric TitrationsDocument5 pagesAmperometric Titrationsarun231187100% (1)
- Bioprocess BasicsDocument365 pagesBioprocess BasicssaveenaPas encore d'évaluation
- Some Basic Concepts of ChemistryDocument12 pagesSome Basic Concepts of ChemistryNikhil BhattPas encore d'évaluation
- Exercise CH 1 (New)Document22 pagesExercise CH 1 (New)Wengki MuhammadPas encore d'évaluation
- HW8 Soln PDFDocument9 pagesHW8 Soln PDFPatricia de Leon100% (1)
- Synthesis and Determination of A Cobalt Bromide Ammine Complex - YCZengDocument15 pagesSynthesis and Determination of A Cobalt Bromide Ammine Complex - YCZengJack Zeng100% (1)
- Pku 2018 Analitik IV Era 085Document10 pagesPku 2018 Analitik IV Era 085Era MelaniaPas encore d'évaluation
- 4-Nonelementary Reaction KineticsDocument23 pages4-Nonelementary Reaction KineticsFajri AmrullahPas encore d'évaluation
- Fisicoquimica CCCCDocument24 pagesFisicoquimica CCCCCarlos de la CruzPas encore d'évaluation
- Chapter 17 Fatty Acid Catabolism: Multiple Choice QuestionsDocument5 pagesChapter 17 Fatty Acid Catabolism: Multiple Choice QuestionsSheelendra Mangal BhattPas encore d'évaluation
- Chaper 4 Non-Reactive Multi Units ProcessDocument48 pagesChaper 4 Non-Reactive Multi Units Processجنات الغبيراءPas encore d'évaluation
- Combines Spectra ExerciseDocument76 pagesCombines Spectra ExerciseIbtihal noorPas encore d'évaluation
- Complejos OrganometálicosDocument14 pagesComplejos OrganometálicosJuan Felipe SanabriaPas encore d'évaluation
- Tugas Simulasi OptimasiDocument3 pagesTugas Simulasi Optimasidimas wPas encore d'évaluation
- Ponchon Savarit - Open Steam, Double Feed, Side StreamDocument15 pagesPonchon Savarit - Open Steam, Double Feed, Side StreamDiah MuslimawatiPas encore d'évaluation
- Titration WorksheetDocument1 pageTitration Worksheetp bergerPas encore d'évaluation
- MATLAB ProjectsDocument2 pagesMATLAB ProjectsNidhi ChauhanPas encore d'évaluation
- Tutorial 4 Solution PDFDocument6 pagesTutorial 4 Solution PDFSalihah AbdullahPas encore d'évaluation
- PMHDocument1 pagePMHMohammed AltahirPas encore d'évaluation
- TesDocument6 pagesTesDanika PutriPas encore d'évaluation
- Developing and Using Stio Tables NotesDocument27 pagesDeveloping and Using Stio Tables NotesThabangPas encore d'évaluation
- PhyChem 2 Problem Set Chemical KineticsDocument1 pagePhyChem 2 Problem Set Chemical KineticsEugenie Chavez100% (1)
- Precipitation Titrimetry-221Document11 pagesPrecipitation Titrimetry-221HudzaifiPas encore d'évaluation
- Semibatch UniDocument22 pagesSemibatch UniMelgi159100% (1)
- Notes 14C CNMRDocument5 pagesNotes 14C CNMRTux BsdPas encore d'évaluation
- C-13 NMR and DEPTDocument41 pagesC-13 NMR and DEPTV G Viju Kumar100% (1)
- Adi & Husna: 04 Desember 2016 Sakinah Mawadah WarahmahDocument1 pageAdi & Husna: 04 Desember 2016 Sakinah Mawadah WarahmahNurillahi Febria LeswanaPas encore d'évaluation
- 8630-Article Text-23760-1-10-20221120Document9 pages8630-Article Text-23760-1-10-20221120Nurillahi Febria LeswanaPas encore d'évaluation
- Photochemical Degradation of Selected SurfactantsDocument12 pagesPhotochemical Degradation of Selected SurfactantsJúlio Gabriel Queiroz dos SantosPas encore d'évaluation
- 2 - in Situ Cross-Linked Polymerization Toward Poly (Ether Sulfone) Ie5028809Document10 pages2 - in Situ Cross-Linked Polymerization Toward Poly (Ether Sulfone) Ie5028809Nurillahi Febria LeswanaPas encore d'évaluation
- Nama Hari Dalam Bahasa InggrisDocument5 pagesNama Hari Dalam Bahasa InggrisTorpin KibaidPas encore d'évaluation
- BenzingerS 2010-3 BODYDocument47 pagesBenzingerS 2010-3 BODYNurillahi Febria LeswanaPas encore d'évaluation
- Referensi FotofentonDocument5 pagesReferensi FotofentonNurillahi Febria LeswanaPas encore d'évaluation
- Analisa KadarDocument11 pagesAnalisa KadarNurillahi Febria LeswanaPas encore d'évaluation
- Daftar PustakaDocument4 pagesDaftar PustakaNurillahi Febria LeswanaPas encore d'évaluation
- Reactivo de FentonDocument12 pagesReactivo de FentonFranklinCcoylloCcantoPas encore d'évaluation
- 5 QM in One DimensionDocument11 pages5 QM in One Dimensionxaviour111Pas encore d'évaluation
- 2013 Research Methodology Slide 02 Finish TranslaeDocument30 pages2013 Research Methodology Slide 02 Finish TranslaeNurillahi Febria LeswanaPas encore d'évaluation
- Polystyrene in To AzoDocument9 pagesPolystyrene in To AzoNurillahi Febria LeswanaPas encore d'évaluation
- Nagoya UniversityDocument7 pagesNagoya UniversityNurillahi Febria LeswanaPas encore d'évaluation
- Wk14-Reactions of Metal ComplexesDocument41 pagesWk14-Reactions of Metal ComplexesDian Eka FajriyantoPas encore d'évaluation
- Radical SederhanaDocument9 pagesRadical SederhanaNurillahi Febria LeswanaPas encore d'évaluation
- Transisi Logam Dalam Fenton PentingDocument18 pagesTransisi Logam Dalam Fenton PentingNurillahi Febria LeswanaPas encore d'évaluation
- Jurnal AspartamDocument6 pagesJurnal AspartamNurillahi Febria LeswanaPas encore d'évaluation
- Degradasi Triazole Dengan FotofentonDocument7 pagesDegradasi Triazole Dengan FotofentonNurillahi Febria LeswanaPas encore d'évaluation
- Penjelasan Fenton Dan Foto FentonDocument8 pagesPenjelasan Fenton Dan Foto FentonNurillahi Febria LeswanaPas encore d'évaluation
- CooperDocument16 pagesCooperNurillahi Febria LeswanaPas encore d'évaluation
- 1 s2.0 S0956053X12000906 MainDocument6 pages1 s2.0 S0956053X12000906 MainNurillahi Febria LeswanaPas encore d'évaluation
- Sensitive Determination of Trace Mercury by UV-visible Diffuse Re Ectance Spectroscopy After Complexation and Membrane Filtration-EnrichmentDocument6 pagesSensitive Determination of Trace Mercury by UV-visible Diffuse Re Ectance Spectroscopy After Complexation and Membrane Filtration-EnrichmentNurillahi Febria LeswanaPas encore d'évaluation
- Patents: Publicati On NumberDocument10 pagesPatents: Publicati On NumberNurillahi Febria LeswanaPas encore d'évaluation
- Ajinomoto Scholarship For ASEAN International Students 2014Document1 pageAjinomoto Scholarship For ASEAN International Students 2014Nurillahi Febria LeswanaPas encore d'évaluation
- C NMR Spectroscopy Worksheet (30 Points) Due 2/24/11 in LectureDocument4 pagesC NMR Spectroscopy Worksheet (30 Points) Due 2/24/11 in LectureNurillahi Febria LeswanaPas encore d'évaluation
- BA Penetapan Hasil Seleksi Proposal KKN PPM As 2014 FixDocument11 pagesBA Penetapan Hasil Seleksi Proposal KKN PPM As 2014 FixNurillahi Febria LeswanaPas encore d'évaluation
- Solubility As A Function of TemperatureDocument2 pagesSolubility As A Function of TemperatureNurillahi Febria LeswanaPas encore d'évaluation
- ITS Master 15629 Bibliography 2Document6 pagesITS Master 15629 Bibliography 2Nurillahi Febria LeswanaPas encore d'évaluation
- Rates of Diffusion-Controlled ReactionsDocument6 pagesRates of Diffusion-Controlled Reactionsantonius_naibahoPas encore d'évaluation
- NMRDocument30 pagesNMRBharatula Suryamani Shankar ee19b013Pas encore d'évaluation
- Chapter 1 Atomic StructureDocument210 pagesChapter 1 Atomic StructureHarshit GoyalPas encore d'évaluation
- Chapter 2-Material Structure and Bonding NEWDocument34 pagesChapter 2-Material Structure and Bonding NEWfayrell100% (2)
- FTS-01 (Code-B) @aakash - Test - Papers - 2023Document21 pagesFTS-01 (Code-B) @aakash - Test - Papers - 2023chiragmali8652Pas encore d'évaluation
- Books About Spectroscopic MethodsDocument3 pagesBooks About Spectroscopic Methodschem_dream10Pas encore d'évaluation
- Intro To Bonding SlideshowDocument10 pagesIntro To Bonding SlideshowAndreaMarkhamPas encore d'évaluation
- 2 Supplementary Exercise Microscopic World I (Question)Document147 pages2 Supplementary Exercise Microscopic World I (Question)RyanPas encore d'évaluation
- Chapter 9 - Covalent Bonding - OrbitalsDocument59 pagesChapter 9 - Covalent Bonding - OrbitalsToka TariqPas encore d'évaluation
- Absorptiom and EmissionDocument48 pagesAbsorptiom and EmissionashishPas encore d'évaluation
- 03 Chapter 1Document45 pages03 Chapter 1hymerchmidt100% (1)
- RF 5301PCDocument3 pagesRF 5301PCNikhil VijayPas encore d'évaluation
- Chemical Bonding LectureDocument7 pagesChemical Bonding LectureSymonette OcturaPas encore d'évaluation
- STPM Chemistry Form 6 NotesDocument5 pagesSTPM Chemistry Form 6 NotesAfz Min100% (3)
- 4A F16 Exp 04 Absorbance and Fluorescence Spectros PDFDocument27 pages4A F16 Exp 04 Absorbance and Fluorescence Spectros PDFWanqing HePas encore d'évaluation
- Pract 1 AnsDocument11 pagesPract 1 AnsNita ListianiPas encore d'évaluation
- 3.1 Jar, JMRDocument16 pages3.1 Jar, JMRNurImanPas encore d'évaluation
- 09 - Electron MicrosDocument63 pages09 - Electron Microsnayonaega100% (2)
- Chalcogenide LettersDocument6 pagesChalcogenide LettersnomitaPas encore d'évaluation
- Atomic Structure Revision Grade 8 Term 1Document11 pagesAtomic Structure Revision Grade 8 Term 1dimondPas encore d'évaluation
- Elements of Physics Atoms and MoleculesDocument33 pagesElements of Physics Atoms and MoleculesAlberto ReyesPas encore d'évaluation
- 1 BhattiAcademy - Com Chemistry 7. ILmi (Objective)Document17 pages1 BhattiAcademy - Com Chemistry 7. ILmi (Objective)Muhammad RizwanPas encore d'évaluation
- Chapter 64 Multiple-Choice QuestionsDocument12 pagesChapter 64 Multiple-Choice QuestionsytPas encore d'évaluation
- Part 2Document15 pagesPart 2john doePas encore d'évaluation
- EEE 132 / ETE 132 Introduction To Materials and ChemistryDocument29 pagesEEE 132 / ETE 132 Introduction To Materials and ChemistryMD. SHAEKH ZAHAB CHOWDHURYPas encore d'évaluation
- Electronic Spectroscopy of Transition Metal CompoundsDocument24 pagesElectronic Spectroscopy of Transition Metal CompoundsBruno Ramos de LimaPas encore d'évaluation
- Chapter 3 Matter and Atomic StructureDocument4 pagesChapter 3 Matter and Atomic StructureMari LarryPas encore d'évaluation
- Chapter 8Document56 pagesChapter 8VatshallaPas encore d'évaluation
- 1.1! Describing Chemical Bonds - Valence Bond TheoryDocument4 pages1.1! Describing Chemical Bonds - Valence Bond TheorySadeeq ArtxzPas encore d'évaluation
- STPM Chemistry Form 6Document5 pagesSTPM Chemistry Form 6BabasChong100% (1)
- IsotopesDocument35 pagesIsotopesAnonymous hqIwzXOkwUPas encore d'évaluation