Académique Documents
Professionnel Documents
Culture Documents
Spermatogenesis
Transféré par
Qurrotul A'yunCopyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Spermatogenesis
Transféré par
Qurrotul A'yunDroits d'auteur :
Formats disponibles
Spermatogenesis Spermatogenesis begins at puberty with a primitive germ cell, the spermatogonium (Gr.
sperma + gone, generation), a relatively small round cell, about 12 m in diameter. These cells are located basally in the epithelium next to the basement membrane (Figures 215 and 216) and different stages of their development are recognized mainly by the shape and staining properties of their nuclei. Spermatogonia with dark, ovoid nuclei act as stem cells, dividing infrequently and giving rise both to new stem cells and to cells with more pale-staining, ovoid nuclei that divide more rapidly as transit amplifying (progenitor) cells (Figure 217). These type A spermatogonia each undergo several unique clonal divisions, remaining interconnected as a syncytium (see below), and form type B spermatogonia, which have more spherical pale nuclei.
Figure 21.6
Seminiferous tubules: Sertoli cells and spermatogenesis. In the two cross-sections of seminiferous tubules shown, most of the associated cell types can be seen. Outside the tubules are myoid cells (M) and fibroblasts (F). Inside near the basement membrane are many prominent spermatogonia (SG), small cells which divide mitotically but give rise to a population that enters meiosis. The meiotic cells grow and undergo chromosomal synapsis to become primary spermatocytes (PS), arrested for 3 weeks in prophase of the first meiotic division during which recombination occurs. Primary spermatocytes are the largest spermatogenic cells and are usually abundant at all levels between the basement membrane and the lumen. Each divides to form two secondary spermatocytes, which are seldom seen in sections because they undergo the second meiotic division almost immediately to form two haploid spermatids. Newly formed round spermatids (RS) differentiate and lose volume in becoming late spermatids (LS) and finally motile, highly specialized sperm cells. All stages of spermatogenesis and spermiogenesis occur with the cells intimately associated with the surfaces of adjacent Sertoli cells (SC) which perform several supportive functions. Both X750. H&E.
Figure 21.7
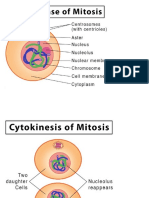
Clonal nature of spermatogenesis. The diagram shows the clonal nature of the germ cells during spermatogenesis. A subpopulation of type A spermatogonia act as stem cells, dividing to produce new stem cells and other type A spermatogonia that undergo transit amplification as progenitor cells for spermatocytes. Mitosis in these cells occurs with incomplete cytokinesis, leaving the cytoplasm of most or all of these cells connected by intercellular bridges. Type A spermatogonia divide mitotically two or three more times, then differentiate as type B spermatogonia which undergo a final round of mitosis to form the cells that then enter meiosis and become a primary spermatocytes (two are shown), with their cytoplasm still interconnected. The intercellular bridges persist during the first and second meiotic divisions and are finally lost as the haploid spermatids complete their differentiation into sperm (spermiogenesis). During differentiation each spermatid sheds excess cytoplasm as a residual body which is phagocytosed by Sertoli cells, and any germ cells that cannot complete this process and degenerate. The interconnected state of these spermatogonia
and the sperm to which they give rise allows free intercellular communication and facilitates their coordinated progress through meiosis and spermiogenesis.
Each type B spermatogonium then undergoes a final mitotic division to produce two cells that grow in size and become primary spermatocytes, which are spherical cells with euchromatic nuclei (Figures 216 and 217). Primary spermatocytes replicate their DNA, so each chromosome consists of duplicate chromatids, and enter meiosis, during which homologous chromosomes come together in synapsis, DNA recombination occurs, and two rapid cell divisions produce haploid cells (Chapter 3). The primary spermatocyte has 46 (44 + XY) chromosomes, the diploid number, and a DNA content of 4N. (The letter N denotes either the haploid number of chromosomes 23 in humans, or the amount of DNA in this set.) Soon after their formation, these cells enter the first meiotic prophase which lasts about 22 days. Most spermatocytes seen in sections are in this phase of meiosis. The primary spermatocytes are the largest cells of the spermatogenic lineage and are characterized by the presence of partially condensed chromosomes in various stages of synapsis and recombination (Figure 216) Homologous chromosomes separate in the first meiotic division, which produces smaller cells called secondary spermatocytes (Figures 215 and 217) with only 23 chromosomes (22 + X or 22 + Y), but each still consists of two chromatids so the amount of DNA is 2N (Chapter 3). Secondary spermatocytes are rare in testis sections because they are short-lived cells that remain in interphase only very briefly and quickly undergo the second meiotic division. Division of each secondary spermatocyte separates the chromatids of each chromosome and produces two haploid cells called spermatids that each contain 23 chromosomes (Figures 215, 216, and 217). Because no S phase (DNA replication) occurs between the first and second meiotic divisions, the amount of DNA per cell is reduced by half when the chromatids separate and the cells formed are haploid (1N). With fertilization, a haploid ovum and sperm produced by meiosis unite and the normal diploid number for the species is restored. Interstitial Tissue The interstitial tissue of the testis is the site of androgen production. The spaces between the seminiferous tubules are filled with connective tissue that contains mast cells, macrophages, nerves, lymphatics, and blood vessels including fenestrated capillaries. During puberty, interstitial, or Leydig, cells become apparent as either rounded or polygonal cells with central nuclei and eosinophilic cytoplasm rich in small lipid droplets (Figures 214). These cells produce the male hormone testosterone , which is responsible for the development of the secondary male sex characteristics. Testosterone is synthesized by enzymes present in mitochondria and the smooth ER in a system similar to that of adrenal cortical cells. Just as Sertoli cells are stimulated by FSH, testosterone secretion by interstitial cells is promoted by the other gonadotropic hormone of the pituitary, luteinizing hormone (LH), which is also called interstitial cell stimulating hormone (ICSH). Testosterone synthesis thus begins
at puberty, when the hypothalamus begins producing gonadotropin-releasing hormone. In the late embryonic testes gonadotropic hormone from the placenta stimulates interstitial cells to synthesize the testosterone needed for development of the ducts and other parts of the male reproductive system. These fetal interstitial cells are very active during the third and fourth months of pregnancy, then regress and become quiescent cells resembling fibroblasts until puberty when they resume testosterone synthesis in response to the pituitary gonadotropin. Intratesticular Ducts The intratesticular genital ducts are the straight tubules (tubuli recti), the rete testis, and the efferent ductules (Figure 212). These ducts carry spermatozoa and liquid from the seminiferous tubules to the duct of the epididymis. Most seminiferous tubules are in the form of loops, both ends of which join the rete testis by the short straight tubules. These tubules are recognized by the gradual loss of spermatogenic cells, with an initial segment in which the walls are lined only by Sertoli cells (Figures 214a and 219), followed by a main segment consisting of cuboidal epithelium supported by a dense connective tissue sheath. All the straight tubules empty into the rete testis, an interconnected network of channels lined with cuboidal epithelium. The channels of the rete testis are embedded within the connective tissue of the mediastinum (Figure 219).
Straight tubules and rete testis. (a): The micrograph shows the long, looping seminiferous tubule (S) that drains into a short straight tubule (T), called a tubulus rectus. X120. H&E. (b): A higher magnification of one such junction shows that the transition to the straight tubule (T) is characterized by many tall Sertoli cells devoid of germ cells. The straight
tubules all empty into the rete testis (R), a network of interconnected channels embedded along with blood vessels (V) in the connective tissue (CT) of the mediastinum. Channels of the rete testis are lined with simple cuboidal epithelium.
The rete testis drains into about 20 efferent ductules (Figure 2110). They are lined by an unusual epithelium with groups of nonciliated cuboidal cells alternating with groups of taller ciliated cells. This gives the epithelium a characteristic scalloped appearance (Figure 2110c). The nonciliated cells absorb most of the fluid secreted by the seminiferous tubules. This absorption and the ciliary activity create a fluid flow that sweeps sperm toward the epididymis. A thin layer of circularly oriented smooth muscle cells is seen outside the basal lamina of the epithelium which aids movement of the sperm. The efferent ductules empty into the duct of the epididymis.
a
Rete testis and efferent ductules. (a): The micrograph shows the channels of the rete testis (R) drained by efferent ductules (E). A transition from rete testis to an efferent ductule is seen (arrows) and blood vessels (V) are seen in the mediastinum connective tissue (CT).(b): The micrograph shows simple cuboidal epithelium that lines the rete testis. Mallory trichrome. (c): The efferent ductules (E) are lined by a simple epithelium with a characteristic scalloped appearance in section, consisting of patches of cuboidal cells with water-absorbing microvilli alternating with patches of taller cells with cilia. This epithelium creates a fluid flow that, together with contractile activity of the thin muscularis around the efferent ductules, carries sperm toward the epididymis
Vous aimerez peut-être aussi
- The Male Reproductive SystemDocument76 pagesThe Male Reproductive SystemteklayPas encore d'évaluation
- Reproductive Systems A. B. C. DDocument6 pagesReproductive Systems A. B. C. DJamie BagundolPas encore d'évaluation
- Zoology Lecture 5Document7 pagesZoology Lecture 5Stephen OcheaPas encore d'évaluation
- Embryology of The Female Reproductive SystemDocument5 pagesEmbryology of The Female Reproductive SystemAndrew LukmanPas encore d'évaluation
- The Cell CycleDocument9 pagesThe Cell Cyclesalahuddin_md5935Pas encore d'évaluation
- Bone MarrowDocument4 pagesBone MarrowSamra AwanPas encore d'évaluation
- CELL CYCLE + (General Biology)Document17 pagesCELL CYCLE + (General Biology)Jona RodicaPas encore d'évaluation
- BiologyDocument17 pagesBiologyMuskan ShreePas encore d'évaluation
- Chapter 20: The Cardiovascular System: Blood: Embryology Atlas HematopoiesisDocument8 pagesChapter 20: The Cardiovascular System: Blood: Embryology Atlas Hematopoiesisfjestefania6892Pas encore d'évaluation
- Biosciweek6 11Document43 pagesBiosciweek6 11Jesalyn AbrinicaPas encore d'évaluation
- Hema TransesDocument25 pagesHema TransesNikoh Anthony EwayanPas encore d'évaluation
- Bio 001 Cell DivisionDocument8 pagesBio 001 Cell DivisionKnick KnackPas encore d'évaluation
- Reproductive System NotesDocument12 pagesReproductive System NotesTanaya PujarePas encore d'évaluation
- Mitochondria Has A Double Wall Membranous StructureDocument8 pagesMitochondria Has A Double Wall Membranous StructureLalitha BalajiPas encore d'évaluation
- The Male Genital SystemDocument20 pagesThe Male Genital SystemLittleChenaPas encore d'évaluation
- Week 1 - Study QuestionsDocument13 pagesWeek 1 - Study Questionsjess waldenPas encore d'évaluation
- Reduction DivisionaaaaaaDocument2 pagesReduction DivisionaaaaaakimuyyyyPas encore d'évaluation
- Gastrulation and Segmentation ofDocument20 pagesGastrulation and Segmentation ofAina AdesolaPas encore d'évaluation
- Reproductive SystemDocument14 pagesReproductive SystemMichael NyaongoPas encore d'évaluation
- 11 MaleDocument14 pages11 MaleJasmine KaurPas encore d'évaluation
- Cell CycleDocument111 pagesCell Cyclepreyaaa pascuaPas encore d'évaluation
- Emt318 Cytology Note3 2021Document10 pagesEmt318 Cytology Note3 2021Ehigie promisePas encore d'évaluation
- Cell CycleDocument6 pagesCell Cyclewebpixel servicesPas encore d'évaluation
- MeiosisDocument8 pagesMeiosisJohn Chiyu Garde-Labite Azuki100% (1)
- Cell DivisionDocument118 pagesCell DivisionNora AfidaPas encore d'évaluation
- Batterjee Medical College (BMC) FAST (Preparatory Year) Biology Department 2012-2013Document14 pagesBatterjee Medical College (BMC) FAST (Preparatory Year) Biology Department 2012-2013edain84Pas encore d'évaluation
- The ChromosomeHandout - sci8.Q4.LESSON2Document8 pagesThe ChromosomeHandout - sci8.Q4.LESSON2Russ CastilloPas encore d'évaluation
- What Is Meiosis?Document5 pagesWhat Is Meiosis?James DazPas encore d'évaluation
- GametogenesisDocument3 pagesGametogenesisWai KikiPas encore d'évaluation
- Module 2 Meiosis and MitosisDocument88 pagesModule 2 Meiosis and MitosisSevdred CadelinaPas encore d'évaluation
- Cellular Reproduction and GeneticsDocument47 pagesCellular Reproduction and GeneticsJean Marie Macadaeg OrdinarioPas encore d'évaluation
- Embryology GastrulationDocument9 pagesEmbryology GastrulationRivera Gómez América LucíaPas encore d'évaluation
- HAPP Supplementary Notes - Reproductive SystemDocument15 pagesHAPP Supplementary Notes - Reproductive SystemZuhri PorzaPas encore d'évaluation
- Biotechnology NotesDocument21 pagesBiotechnology NotesRommel BauzaPas encore d'évaluation
- Male Histology Trans-Batch 2023Document11 pagesMale Histology Trans-Batch 2023Hanako Sasaki AranillaPas encore d'évaluation
- Mitosis Is A Process Where A Single Cell Divides Into Two Identical Daughter Cells (Cell Division)Document10 pagesMitosis Is A Process Where A Single Cell Divides Into Two Identical Daughter Cells (Cell Division)Holly Mc Kenzie Layson DalmanPas encore d'évaluation
- 2.1.6 Biology Ocr ADocument7 pages2.1.6 Biology Ocr AFatima AfifiPas encore d'évaluation
- Cell CycleDocument19 pagesCell CycleMoffat KaprezzoPas encore d'évaluation
- Cell DivisionDocument2 pagesCell DivisionedukahonPas encore d'évaluation
- Embryology: DR - Kumar Satish Ravi Assist. Prof. & HeadDocument45 pagesEmbryology: DR - Kumar Satish Ravi Assist. Prof. & HeadDr.Kumar Satish RaviPas encore d'évaluation
- SZL B206 Principles of GeneticsDocument28 pagesSZL B206 Principles of Geneticsawuors249Pas encore d'évaluation
- Weeks 3 - 8Document32 pagesWeeks 3 - 8Stefan HutsonPas encore d'évaluation
- MitosisDocument3 pagesMitosisLea Mari JavierPas encore d'évaluation
- Spermatogenesis Is The Process by Which: Seminiferous Tubule With Maturing Sperm.Document2 pagesSpermatogenesis Is The Process by Which: Seminiferous Tubule With Maturing Sperm.Garcia AzirPas encore d'évaluation
- Development of Cardiovascular System PDFDocument58 pagesDevelopment of Cardiovascular System PDFVizhiPas encore d'évaluation
- What Is Mitosis & MeiosisDocument7 pagesWhat Is Mitosis & MeiosisMuhammad VaizPas encore d'évaluation
- InterphaseDocument9 pagesInterphaseJeazel MosendoPas encore d'évaluation
- Organ Reproduksi Laki-Laki: (Organa Genitalia Masculina)Document32 pagesOrgan Reproduksi Laki-Laki: (Organa Genitalia Masculina)Timotius Gatma Buntori PurbaPas encore d'évaluation
- Tdn°2 103019Document9 pagesTdn°2 103019selmi bouzidPas encore d'évaluation
- Meiosis.-Meiosis (del griego μείωσις meíōsis 'disminución')Document6 pagesMeiosis.-Meiosis (del griego μείωσις meíōsis 'disminución')julioPas encore d'évaluation
- Cell Cycle and MitosisDocument49 pagesCell Cycle and Mitosishazharomar958Pas encore d'évaluation
- Male ReproductivehistoDocument9 pagesMale ReproductivehistoaikaPas encore d'évaluation
- Mitosis & Meiosis NotesDocument6 pagesMitosis & Meiosis NotesChris_Barber09100% (1)
- Penugasan Dr. Agung Dewanto, SpOG (K) - Azrul RoeDocument4 pagesPenugasan Dr. Agung Dewanto, SpOG (K) - Azrul RoeAzrul MDPas encore d'évaluation
- Readings Ch04Document11 pagesReadings Ch04Adrian GuzmanPas encore d'évaluation
- Lab Ex1-2 and Chick OrganogenesisiDocument20 pagesLab Ex1-2 and Chick OrganogenesisiLardel CarayPas encore d'évaluation
- 4.2 Cell As A Unit of LifeDocument7 pages4.2 Cell As A Unit of Lifehola adiosPas encore d'évaluation
- Lecture Outline: See Separate Powerpoint Slides For All Figures and Tables Pre-Inserted Into Powerpoint Without NotesDocument58 pagesLecture Outline: See Separate Powerpoint Slides For All Figures and Tables Pre-Inserted Into Powerpoint Without NotesJharaPas encore d'évaluation
- Spermatogenesis 1Document28 pagesSpermatogenesis 1LellllPas encore d'évaluation
- Anatomy and Physiology of Male Reproductive SystemDocument8 pagesAnatomy and Physiology of Male Reproductive SystemAdor AbuanPas encore d'évaluation
- Anaphy Reviewer FINALSDocument4 pagesAnaphy Reviewer FINALSim. EliasPas encore d'évaluation
- CLASS 12 BIO PROJECT SpermatogenesisDocument19 pagesCLASS 12 BIO PROJECT Spermatogenesisanon_38352456389% (9)
- Male Reproductive Physiology - UpToDate - 2020Document18 pagesMale Reproductive Physiology - UpToDate - 2020Karaca AzizPas encore d'évaluation
- Sexual Reproduction in Humans Notes (CAPE)Document29 pagesSexual Reproduction in Humans Notes (CAPE)Desmond JonesPas encore d'évaluation
- Reproductive SystemDocument219 pagesReproductive Systemtzushka_vipPas encore d'évaluation
- OgenisisDocument52 pagesOgenisisBharat ThapaPas encore d'évaluation
- IB Biology Assessment Statements - DrawDocument33 pagesIB Biology Assessment Statements - DrawSchuyler Huff100% (1)
- Histology 18 Male Reproductive SystemDocument49 pagesHistology 18 Male Reproductive SystemAbdul RahmanPas encore d'évaluation
- Intro To Embryology PDFDocument9 pagesIntro To Embryology PDFAdvin BurkePas encore d'évaluation
- Csir Unit 5 Updated PDFDocument10 pagesCsir Unit 5 Updated PDFAmaldev S K100% (2)
- Bio KVS PaperDocument16 pagesBio KVS PaperSanjanaPas encore d'évaluation
- Spermatogenesis, OogenesisDocument16 pagesSpermatogenesis, Oogenesisannita100% (1)
- IB Biology Higher Level Human and Health Physiology NotesDocument41 pagesIB Biology Higher Level Human and Health Physiology NoteshunarsandhuPas encore d'évaluation
- Male and Female Genital Systems 2Document30 pagesMale and Female Genital Systems 2negmm2226Pas encore d'évaluation
- I.J. Singh, Professor Department of Fishery Biology College of Fisheries, G.B.P.U.A.&T., PantnagarDocument39 pagesI.J. Singh, Professor Department of Fishery Biology College of Fisheries, G.B.P.U.A.&T., Pantnagarjoshigauta100% (1)
- Evaluation of Aphrodisiac Activity of Buchanania Axillaris (Linn.) LeavesDocument8 pagesEvaluation of Aphrodisiac Activity of Buchanania Axillaris (Linn.) LeavesDr. Ramadevi DevarakondaPas encore d'évaluation
- Biochemistry of SemenDocument280 pagesBiochemistry of SemenCopper Jean100% (1)
- On The Morphology of The Chromosome Group in Brachystola MagnaDocument18 pagesOn The Morphology of The Chromosome Group in Brachystola MagnaOya KaptanoğluPas encore d'évaluation
- MCQS Human Reproduction Class 12Document13 pagesMCQS Human Reproduction Class 12Shaashwati TandonPas encore d'évaluation
- EM2.Gametogenesis (At)Document52 pagesEM2.Gametogenesis (At)MUDIN ABDELLAPas encore d'évaluation
- Sistem Reproduksi Pria IkrDocument141 pagesSistem Reproduksi Pria IkrNidiyamilhaPas encore d'évaluation
- CLASS 12 BIO PROJECT SpermatogenesisDocument19 pagesCLASS 12 BIO PROJECT Spermatogenesisanon_383524563Pas encore d'évaluation
- KM GametogenesisDocument42 pagesKM GametogenesisKALAMYA BRIAN BWAYOPas encore d'évaluation
- Modul Pintas Tingkatan 5 Peperiksaan Percubaan SPM 2018 Skema Jawapan Biologi Kertas 1 4551/1Document39 pagesModul Pintas Tingkatan 5 Peperiksaan Percubaan SPM 2018 Skema Jawapan Biologi Kertas 1 4551/1VentusPas encore d'évaluation
- 4 AnnotationsDocument8 pages4 Annotationsapi-243334002Pas encore d'évaluation
- OBG DR Pranav PDFDocument183 pagesOBG DR Pranav PDFVinu Poojapranavam100% (6)
- Chapter 21 The Male Reproductive SystemDocument6 pagesChapter 21 The Male Reproductive SystemEllä Pabustan100% (1)