Académique Documents
Professionnel Documents
Culture Documents
00018
Transféré par
Rico Fernando TCopyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
00018
Transféré par
Rico Fernando TDroits d'auteur :
Formats disponibles
IJRPC 2011, 1(3)
Yashwanth Kumar et al.
ISSN: 2231 2781
INTERNATIONAL JOURNAL OF RESEARCH IN PHARMACY AND CHEMISTRY
Available online at www.ijrpc.com
Research Article
DEVELOPMENT AND VALIDATION OF RP-HPLC METHOD FOR ESTIMATION OF DIACEREIN BULK AND PHARMACEUTICAL DOSAGE FORMULATION Yashwanth Kumar D1*., Rohit Reddy T2., Someshwar K. and Neelima KSSN.
1Prist 2Nalanda
University, Thanjavur, Tamilnadu, India. College of Pharmacy, Nalgonda, Andhra Pradesh, India.
*Corresponding Author: yashwanth.pharma@gmail.com
ABSTRACT A simple, selective and rapid reverse phase high performance liquid chromatographic (RPHPLC) method for the analysis of Diacerein in bulk and in tablet dosage form has been developed and validated. Sample was resolved on a Agillent TC C18 (150mm X 4.6 mm i.d., particle size 5) column. The mobile phase consisted of Buffer and Acetonitrile were mixed in the ratio of 25:75 V/V and sonicated to degas was delivered at a flow rate of 1.2 ml/min at ambient temperature and the retention time was about 4 minutes. Studies were performed on an HPLC system equipped with a UV/Visible detector at 257nm. The method is specific to Diacerein and able to resolve the drug peak from formulation excipients. The calibration curve was linear over the concentration range of 1-10 g/ml (R=0.9996).The results of analysis of formulation was found to be 99.350.5205. The lower limits detection for Diacerein was found to be 0.001g/ml and the quantification limit was about 0.0466g/ml. The proposed method is applicable to routine analysis of Diacerein in bulk and in tablet dosage form. Keywords: Diacerein, RP-HPLC, Validation, Pharmaceutical dosage form.
INTRODUCTION Chemically Diacerein1 (DIA) is known as 4, 5Bis(acetyloxy)-9, 10-dioxo-2-anthracene carboxylic acid (Fig.1). Diacerein is a novel anti-inflammatory drug and used for the treatment of not only Osteoarthritis but also used for rheumatoid arthritis when used in combination2,3. Diacerein is a di-acetylated derivative of rhein, a molecule with an anthraquinone ring which is actually the active metabolite of Diacerein4. DIA is a selective inhibitor of interleukin-1 having protective effect on granuloma-induced cartilage breakdown by a reduction in the concentration range of proinflammatory cytokines5,6. However DIA lacks cyclooxygenase inhibitory activity and hence
shows no effect on prostaglandin synthesis. Therefore it has been considered as a slowacting antiarthritic drug. Analytical methods play a vital role in new drug development, preformulation and formulation studies, stability studies, quality control testing and quality assurance programmes. So analysts are always in search of developing rapid and accurate new methods of analysis that are able to exist in routine analytical work. Diacerein is not official in IP, USP and BP. The review of literature reveals that only few chromatographic methods have been reported for the estimation of Diacerein like LCMS, LCNMR MS, capillary gas chromatography,
397
IJRPC 2011, 1(3)
Yashwanth Kumar et al.
ISSN: 2231 2781
HPLC7, 8. There is a need for developing newer methods in HPLC for developing a simple and economic method and so we proceeded with HPLC and validated as per the ICH guidelines. The present analytical work comprises of simple, precise, rapid, sensitive and accurate methods for the estimation of Diacerein bulk and dosage form. EXPERIMENTAL Instrumental specification HPLC - AGILLENT-Model NO.1120 LC Compact System Consisting of Agillent TCC18 ODS column output signal was monitored and integrated using Lab monitor diagnosis and EZ-chrome. MATERIALS AND METHODS Diacerein working Standard was obtained from SAIN Medicaments Pvt, Ltd, Hyderabad and commercial dosage forms containing the studied drug were obtained from local market. All the reagents used were analytical reagents, solvents were of HPLC grade. Buffer Preparation Dissolve 0.57ml of Acetic Acid in 1000ml of HPLC water and 0.205gm of Sodium acetate in 250ml of water then take fresh beaker add 847ml of 0.01M of Acetic acid and 153ml of 0.01M Sodium acetate, Filter through 0.45m membrane filter and degas. Mobile Phase Buffer and Acetonitirile were mixed in the ratio of 50:50 V/V and sonicated to degas. Preparation of Standard Solution Stock solution of Diacerein sample was prepared by dissolving accurately weighed 10 mg of drug initially in Tetrahydrafuran in 10 ml volumetric flask and further dilutions were made by taking 0.1 ml of stock solution and made up with methanol and degassed by ultra bath sonicator for 30 min. Finally passed through 0.45 m filter (The final concentration is 10g/ml). Preparation of sample stock solution Diacerein 5 tablets were accurately weighed and powdered. Amount equivalent to 0.3074 gm of Diacerein from the powdered formulation were accurately weighed and dissolved in Tetrahydrafuran and future dilution of 10g/ml were made by taking
0.1ml from stock solution and made with methanol in 10 ml of volumetric flask and degassed by ultra bath sonicator for 30 min. Finally passed through 0.45 m filter (The final concentration is 10g/ml).
Fig. 1: Chemical Structure of Diacerein (DIA)
Fig. 2: RP-HPLC data of Diacerein
RESULTS METHOD VALIDATION The described method has been validated for the assay of Diacerein using following parameter9-11. Accuracy (Recovery): Demonstrated the accuracy of the test method by preparing recovery samples (i. e. spiking formulation with known quantities of API.) at the level of 50 %, 100 %, and 150 % of target concentration. Prepare the recovery sample in triplicate in each level. The sample was prepared as like
398
IJRPC 2011, 1(3)
Yashwanth Kumar et al.
ISSN: 2231 2781
assay method. The results were tabulated in Table 1. Method Precision: Prepared and analyzed six replicate sample preparations as per method. And calculate the % of assay value. The results were tabulated in Table 2. System Precision: Prepared and analyzed six replicate sample preparations as per method. And calculate the %RSD of area of response. The results were tabulated in Table 3. Ruggedness: The ruggedness of an analytical method was determined by analysis of aliquots from homogenous lots by different analyst using operational and environmental conditions that may differ but were still within the specified parameters of the assay. The degree of reproducibility of the test results was then determined as a function of the assay variables. This reproducibility was assayed under normal conditions to obtain a measure of the ruggedness of the analytical method. Inter day precision: Prepared and analyzed three replicate for day-1 and day-2 sample preparations as per method. And calculate the %RSD of assay level. The results were tabulated in Table 4. Robustness: The robustness of an analytical method was determined by analysis of aliquots from homogenous lots by differing physical parameters that may differ but were still within the specified parameters of the assay by changing physical parameters like flow rate mobile phase and pH. HPLC system was set with small but deliberate change in method as mentioned below and their effect on system suitability test and the values were given in Table 5. Specificity: Prepare blank, diluted standard preparation and sample preparation as per method.20l of the above mentioned solution was injected into HPLC system. Values were tabulated in Table 6 Linearity: The linearity of an analytical method is to elite test results that are directly, of by well defined mathematical transformation, proportional to the concentration of analyte in sample within a
given working range. The results were tabulated in Table 7 Limit of detection and quantification (LOD and LOQ) From the linearity data calculate the limit of detection and quantitation, using the following formula. LOD= 3.3 S
= standard deviation of the response S = slope of the caliberation curve of the analyte.
LOQ = 10 S
= standard deviation of the response S = slope of the caliberation curve of the analyte.
DISCUSSION The analytical method was developed by studying different parameters. First of all, maximum absorbance was found to be at 257nm and the peak purity was excellent. Injection volume was selected to be 20l which gave a good peak area (figure 2). The column used for study on Diacerein, Zorabax C18, 250, 5m was chosen good peak shape. Ambient temperature was found to be suitable for the nature of drug solution. The flow rate was fixed at 1.2ml/min because of good peak area and satisfactory retention time. Different pH and ratios of mobile phase were studied, mobile phase with ratio of 50:50 Acetate buffer: Acetonitrile was fixed due to good symmetrical peak. So this mobile phase was used for the proposed study. Diluent 1Tetarhydrofuron, Diluent 2 methanol was selected because of maximum extraction, sonication time was fixed to be 10min at which all the drug particles were completely soluble and showed good recovery. Run time was selected to be 12min because analyze gave peak around 4.0 and also to reduce the total run time. The percent recovery was found to be 98.96101.77 was linear and precise over the same range. Both system and method precision was found to be accurate and well within range. In specificity study all degradant impurity and excipient peaks were separated from the analyte peak. Detection limit was found to be 0.001g/ml. Linearity study was, correlation coefficient and curve fitting was found to be 10g/ml. The analytical method was found linearity over the range of 2-10g/ml of the
399
IJRPC 2011, 1(3)
Yashwanth Kumar et al.
ISSN: 2231 2781
target concentration. The analytical passed both robustness and ruggedness tests. On both cases, relative standard deviation was all satisfactory. CONCLUSION A simple, rapid and reproducible HPLC method was developed and validated for the estimation of Diacerein in tablet dosage form. Agilent TC-C18 ODS column, in gradient mode with mobile phase containing Acetronitrile and Acetate buffer pH4.0 (50:50v/v) was used. The flow rate was 1.2ml/min and the analyte was monitored at 257 nm. The retention time for Diacerein was 4.00 minutes. The system was validated for system suitability, precision, accuracy, linearity,
ruggedness and robustness. The system suitability parameter were within the limit, hence it was concluded that the system was suitable to perform the assay. Linearity was obtained in the concentration range of 2g/ml to 10g/ml with correlation coefficient of 0.999. The percentage recovery of Diacerein was found to be in the range of 98% -102%. The method was robust and rugged as observed from insignificant variation in the results of analysis by changes in flow rate, Mobile phase composition separately and analysis being performed by different day with different columns respectively. Therefore it was concluded that the proposed method can be used for routine analysis of Diacerein in its tablet dosage form.
Table 1: Recovery of Diacerein
Level Level-1 50% Level-2 100% Level-3 150% g/ml recovered 4.99mcg 4.96mcg 5.04mcg 9.98mcg 9.89mcg 101.77mcg 15.08mcg 15.05mcg 14.91mcg g/ml added 5g 5g 5g 10g 10g 100g 15g 15g 15g Recovery% 99.84 99.29 100.89 99.80 98.96 101.77 100.57 100.34 99.43 Mean% 100.00 %RSD 0.8129
100.1
1.4439
100.1
0.6030
Table 2: Method Precision
Sample Preparation Number 1 2 3 4 5 6 Mean %RSD Diacerein Assay % 99.39 99.03 99.42 99.59 99.28 100.43 99.35 0.5205
Table 3: System Precision
Standard Preparation Number 1 2 3 4 5 6 Mean %R.S.D Standard Response 18516904 18638271 18724032 19637412 18918043 18421791 112856453 1.4751
400
IJRPC 2011, 1(3)
Yashwanth Kumar et al.
ISSN: 2231 2781
Table 4: Ruggedness
S. No. 1 2 3 Mean %RSD Day-1 97.98 98.88 100.24 99.03 1.1488 Assay % of Diacerein Day-2 Analyst-1 Analyst-2 97.04 100.44 10.69 100.67 97.05 100.87 100.24 97.37 99.17 99.67 98.43 100.2 2.0 1.73 0.9334
Table 5: Robustness
Test Flow rate 1.4ml/min Flow rate 1ml/min Mobile phase Composition ACN:Buffer (52:48) 15588070 1.6580 Mobile phase Composition ACN:Buffer (48:52) 15089173 0.23 pH 4.2 pH.3.8
Mean standard area %RSD
3322789 0.67
31179637 1.3091
18882689 0.13
18361019 0.58
Table 6: Specificity
S. No. 1 2 3 Sample Name Blank Formulation Standard Area ---------18479518 18678942 Rt -------4.001 4.007
Table 7: Linearity of Diacerein
S. No. Concentration (g/ml) Area of Diacerein 3695903 7412806 11087709 14583612 184563484 0.999 480876.03 0.2346 X
1 2 2 4 3 6 4 8 5 10 Correlation coefficient (r) Slope y-intercept
REFERENCES 1. Tamura T, Shirai T, Kosaka N, Ohmori K and Takafumi N. Eur J Pharmacol. 2002;448:81-87. 2. Toegel S, Huang W, Piana C, Unger FM, Wirth M, Gold ring MB et al. BMC Molecular Biology. 2007;8:13. 3. Nicolas P, Tod M, Padoin C and Petitjean O. Clin Pharmacokinetic. 1998;35:347-359. 4. Tamura T and Ohmori K. J Pharmacol. 2001;85:101-104. 5. Pelletier JP, Mineau F, Fernandes JC, Duval N and Martel-Pelletier J. J Rheumatol. 1998;25:2417-2424. 6. Zawilla NH, Mohammad M, Abdul A, El Kousy NM, Ali SM and EI
Moghazy. J Pharm Biomed Anal. 2002:243-251. 7. Giannellini V, Salvatore F, Bartolucci G, Coran SA and Bambagiotti-Alberti M. J Pharm Biomed Anal. 2005:776780. 8. Rao J, Chauhan K, Mahadik KR and Kadam SS. Indian J Pharm Sci. 2009:24-29. 9. Sethi PD. Quantitative chemical analysis of drugs in pharmaceutical formulations, 2nd edition. CBS publishers and distributors, New Delhi. p: 150. 10. Organisation of Pharmaceutical procedures of India; validation Chapter 7.0. [cited Saturday, February
401
IJRPC 2011, 1(3)
Yashwanth Kumar et al.
ISSN: 2231 2781
14, 2009] Available at http://www.indiaoppi.com/publicati on.asp 11. Text on validation of analytical procedures ICH harmonised tripartite
guidelines. [cited Saturday, February 14, 2009] Available at http://www.ich.org/cache/compo/3 63-272-1.html
402
Vous aimerez peut-être aussi
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (119)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (265)
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (399)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (587)
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5794)
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2219)
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (344)
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (894)
- Food AllergyDocument38 pagesFood AllergyOsama100% (2)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (73)
- STEM Pre-Calculus Curriculum GuideDocument4 pagesSTEM Pre-Calculus Curriculum GuideJan Alexis Monsalud75% (8)
- Differential Equations Midterm 1 v1 SolutionsDocument6 pagesDifferential Equations Midterm 1 v1 SolutionsRodney HughesPas encore d'évaluation
- Cinderella WebsiteSamplerDocument70 pagesCinderella WebsiteSamplerRico Fernando TPas encore d'évaluation
- Code of Practice on Food Allergen ManagementDocument20 pagesCode of Practice on Food Allergen ManagementMiquelinaPas encore d'évaluation
- Taccp/Vaccp: Maltsters' Association of Great BritainDocument16 pagesTaccp/Vaccp: Maltsters' Association of Great BritainMohamedPas encore d'évaluation
- MVDE Summer 2015Document5 pagesMVDE Summer 2015IsaiahGerardo100% (1)
- Food Safety ChecklistDocument4 pagesFood Safety Checklistjalali007Pas encore d'évaluation
- SYLLABUS IN ADVANCED STATISTICS PHDDocument2 pagesSYLLABUS IN ADVANCED STATISTICS PHDchiara maye notarte100% (3)
- HACCP Audit ChecklistDocument8 pagesHACCP Audit ChecklistJaykishan Parmar100% (3)
- Report No 2 Auditing PrerequisitesDocument32 pagesReport No 2 Auditing PrerequisitesI Kadek Tony Adi SetiawanPas encore d'évaluation
- Poster brcv8 A3 RequirementsDocument1 pagePoster brcv8 A3 RequirementsRico Fernando TPas encore d'évaluation
- Prerequisite Programmes For Food Safety in Food Retail - SpecificationDocument26 pagesPrerequisite Programmes For Food Safety in Food Retail - SpecificationRico Fernando T100% (1)
- Introduction To Six SigmaDocument30 pagesIntroduction To Six SigmaadysubagyaPas encore d'évaluation
- Report No 2 Auditing PrerequisitesDocument32 pagesReport No 2 Auditing PrerequisitesI Kadek Tony Adi SetiawanPas encore d'évaluation
- Food Safety Self-Inspection ChecklistDocument3 pagesFood Safety Self-Inspection ChecklistRico Fernando TPas encore d'évaluation
- Food Safety Self-Inspection ChecklistDocument3 pagesFood Safety Self-Inspection ChecklistRico Fernando TPas encore d'évaluation
- Implementation Haccp Food Safety System: Create and Prepare by Irwan Arief Irwan - Arief87@yahoo - Co.idDocument40 pagesImplementation Haccp Food Safety System: Create and Prepare by Irwan Arief Irwan - Arief87@yahoo - Co.idRico Fernando TPas encore d'évaluation
- To Go: Safe FoodDocument16 pagesTo Go: Safe FoodRico Fernando TPas encore d'évaluation
- NDSU EXTENSION FOOD STORAGE GUIDEDocument16 pagesNDSU EXTENSION FOOD STORAGE GUIDERico Fernando TPas encore d'évaluation
- Food Safety Self-Inspection ChecklistDocument3 pagesFood Safety Self-Inspection ChecklistRico Fernando TPas encore d'évaluation
- Food Safety ChecklistDocument3 pagesFood Safety ChecklistRico Fernando TPas encore d'évaluation
- 306 806 1 PBDocument7 pages306 806 1 PBRico Fernando TPas encore d'évaluation
- Guia de Interpretación FSSC 22000Document15 pagesGuia de Interpretación FSSC 22000Miguel LemusPas encore d'évaluation
- Penentuan C - TelurDocument5 pagesPenentuan C - TelurRico Fernando TPas encore d'évaluation
- SPSS 19 License UpdateDocument2 pagesSPSS 19 License UpdateWildan RahdyasPas encore d'évaluation
- (Untitled) HHHHDocument1 page(Untitled) HHHHRico Fernando TPas encore d'évaluation
- NFCSO Pesticide Lab Conformity RulesDocument26 pagesNFCSO Pesticide Lab Conformity RulesMauroPas encore d'évaluation
- Chapter 5Document50 pagesChapter 5Aswaja313Pas encore d'évaluation
- Fractal SlideDocument14 pagesFractal SlideMiguel GarciaPas encore d'évaluation
- Data Mining and Warehousing FundamentalsDocument72 pagesData Mining and Warehousing FundamentalsPrakash giriPas encore d'évaluation
- 4 1 C A MathematicalmodelingDocument10 pages4 1 C A Mathematicalmodelingapi-248595624Pas encore d'évaluation
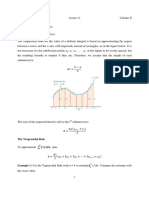
- Civil Engineering Lecture on Numerical IntegrationDocument7 pagesCivil Engineering Lecture on Numerical IntegrationIbrahem HadiPas encore d'évaluation
- W8 Ex 9K Quotient Rule DerivativesDocument2 pagesW8 Ex 9K Quotient Rule DerivativesJessicaPas encore d'évaluation
- Operation of SignalsDocument8 pagesOperation of SignalsBenjhon S. ElarcosaPas encore d'évaluation
- Anfis StructureDocument5 pagesAnfis Structuresecret 31Pas encore d'évaluation
- Range StatDocument5 pagesRange StatDilrukshi FernandoPas encore d'évaluation
- Frequency Distribution, Cross-Tabulation, and Hypothesis TestingDocument76 pagesFrequency Distribution, Cross-Tabulation, and Hypothesis TestingAbdul MateenPas encore d'évaluation
- Multivector Differential Calculus.Document48 pagesMultivector Differential Calculus.kek spinnePas encore d'évaluation
- PDF Official Sat Study Guide About Writing Language TestDocument6 pagesPDF Official Sat Study Guide About Writing Language Testvalentina palominoPas encore d'évaluation
- EE5121: Convex Optimization: Assignment 5Document2 pagesEE5121: Convex Optimization: Assignment 5elleshPas encore d'évaluation
- Chapter 17 PDFDocument10 pagesChapter 17 PDFChirilicoPas encore d'évaluation
- Resume SohamDocument2 pagesResume SohamBubul ChoudhuryPas encore d'évaluation
- Control Theory 1: Performance of Underdamped Second Order Control SystemsDocument5 pagesControl Theory 1: Performance of Underdamped Second Order Control SystemsnctgayarangaPas encore d'évaluation
- Sensityvity AnalysisDocument44 pagesSensityvity AnalysisArnab ShyamalPas encore d'évaluation
- Why intro real analysis teaches bottom-up continuityDocument4 pagesWhy intro real analysis teaches bottom-up continuityMihai PPas encore d'évaluation
- RegressionDocument9 pagesRegressionRatnottar HardikPas encore d'évaluation
- Estimate Measurement UncertaintyDocument29 pagesEstimate Measurement Uncertaintynishu12345Pas encore d'évaluation
- DBA MathsDocument98 pagesDBA Mathsvictor chisengaPas encore d'évaluation
- 3.2 Simple Functions: I I I N I ADocument4 pages3.2 Simple Functions: I I I N I Ajose1200ablePas encore d'évaluation
- ODE Numerial AnalysisDocument25 pagesODE Numerial Analysiscracking khalifPas encore d'évaluation
- What is Data Science? ExplainedDocument21 pagesWhat is Data Science? ExplainedAlt ChinarPas encore d'évaluation
- AC Lesson LectureDocument56 pagesAC Lesson LectureMKPas encore d'évaluation