Académique Documents
Professionnel Documents
Culture Documents
ESTER
Transféré par
meritnasaTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
ESTER
Transféré par
meritnasaDroits d'auteur :
Formats disponibles
Ester
From Wikipedia, the free encyclopedia
For other uses, see Ester (disambiguation).
This article may be too technical for most readers to understand. Please help improve this article to make it understandable to non-experts, without removing the technical details. The talk page may contain suggestions. (March 2013)
A carboxylate ester. R and R' denote any alkylor aryl group
Esters are chemical compounds consisting of a carbonyl adjacent to an ether linkage. They are derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol.[1] Esters are usually derived from an inorganic acid or organic acid in which at least one -OH (hydroxyl) group is replaced by an -Oalkyl (alkoxy) group, and most commonly from carboxylic acids and alcohols. That is, esters are formed by condensing an acid with an alcohol. Esters are ubiquitous. Most naturally occurring fats and oils (e.g. triglycerides) are the fatty acid esters of glycerol. Esters with low molecular weight are commonly used as fragrances and found in essential oils and pheromones. Phosphoesters form the backbone of DNA molecules. Nitrate esters, such as nitroglycerin, are known for their explosive properties, while polyesters are important plastics, with monomers linked by ester moieties.
Contents
[hide]
1 Nomenclature
o o o o
1.1 Etymology 1.2 IUPAC nomenclature of Esters 1.3 Orthoesters 1.4 "Inorganic esters"
2 Structure and bonding 3 Physical properties and characterization
3.1 Characterization and analysis
4 Applications and occurrence 5 Preparation
o o o o o o o
5.1 Esterification of carboxylic acids 5.2 Alcoholysis of acyl chlorides and acid anhydrides 5.3 Alkylation of carboxylate salts 5.4 Transesterification 5.5 Carbonylation 5.6 Addition of carboxylic acids to alkenes 5.7 Other methods
6 Reactions
o o o o o
6.1 Addition of nucleophiles at carbonyl 6.2 Reduction 6.3 Claisen condensation and related reactions 6.4 Other reactions 6.5 Protecting groups
7 List of ester odorants 8 See also 9 References 10 External links
Nomenclature[edit]
Etymology[edit]
The word 'ester' was coined in 1848 by German chemist Leopold Gmelin,[2] probably as a contraction of the German Essigther - acetic ether.
IUPAC nomenclature of Esters[edit]
Main article: IUPAC nomenclature of organic chemistry#Esters Ester names are derived from the parent alcohol and the parent acid, where the latter may be an organic or an inorganic acid. Esters derived from the simplest carboxylic acids are commonly named according to the more traditional, so-called "trivial names" e.g. as formate, acetate, propionate, and butyrate, as opposed to the IUPAC nomenclature methanoate, ethanoate, propanoate and butanoate. Esters derived from more complex carboxylic acids are, on the other hand, more frequently named using the systematic IUPAC name, based on the name for the acid followed by the suffix -oate. For example the ester hexyl octanoate, also known under the trivial name hexyl caprylate, has the formula CH3(CH2)6CO2(CH2)5CH3.
Ethyl acetate derived from an alcohol (blue) and an acyl group (yellow) derived from a carboxylic acid.
The chemical formulas of organic esters are typically written in the format of RCO2R', where R and R' are the hydrocarbon parts of the carboxylic acid and alcohol, respectively. For example butyl acetate (systematically butyl ethanoate), derived frombutanol and acetic acid (systematically ethanoic acid) would be written CH3CO2C4H9. Alternative presentations are common including BuOAc and CH3COOC4H9. Cyclic esters are called lactones, regardless of whether they are derived from an organic or an inorganic acid. One example of a (organic) lactone is gamma-valerolactone.
Orthoesters[edit]
An uncommon class of organic esters are the orthoesters, which have the formula RC(OR')3. Triethylorthoformate (HC(OC2H5)3) is derived, in terms of its name (but not its synthesis) from orthoformic acid (HC(OH)3) and ethanol.
"Inorganic esters"[edit]
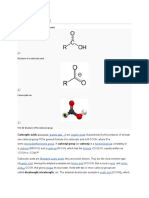
A phosphoric acid ester
Ester is a general term for the product derived from the condensation of an acid and an alcohol. Thus, the nomenclature extends to inorganic oxo acids, e.g. phosphoric acid, sulfuric acid, nitric acid and boric acid. For example, triphenyl phosphateis the ester derived from phosphoric acid and phenol. Organic carbonates, such as ethylene carbonate, are derived from carbonic acid and ethylene glycol.
Structure and bonding[edit]
Esters contain a carbonyl center, which gives rise to 120C-C-O and O-C-O angles. Unlike amides, esters are structurally flexible functional groups because rotation about the C-O-C bonds has a low barrier. Their flexibility and low polarity is manifested in their physical properties; they tend to be less rigid (lower melting point) and more volatile (lower boiling point) than the corresponding amides.[3] The pKa of the alphahydrogens on esters is around 25.[4]
Physical properties and characterization[edit]
Esters are more polar than ethers but less polar than alcohols. They participate in hydrogen bonds as hydrogen-bond acceptors, but cannot act as hydrogen-bond donors, unlike their parent alcohols. This ability to participate in hydrogen bonding confers some water-solubility. Because of their lack of hydrogenbond-donating ability, esters do not self-associate. Consequently esters are more volatile than carboxylic acids of similar molecular weight.[3]
Characterization and analysis[edit]
Esters are usually identified by gas chromatography, taking advantage of their volatility. IR spectra for esters feature an intense sharp band in the range 17301750 cm1 assigned to C=O. This peak changes depending on the functional groups attached to the carbonyl. For example, a benzene ring or double bond in conjugation with the carbonyl will bring the wavenumber down about 30 cm1.
Applications and occurrence[edit]
Esters are widespread in nature and are widely used in industry. In nature, fats are, in general, triesters derived from glycerol and fatty acids.[5] Esters are responsible for the aroma of many fruits, including apples, durians, pears, bananas, pineapples, and strawberries.[6] Several billion kilograms of polyesters are produced industrially annually, important products being polyethylene terephthalate, acrylate esters, and cellulose acetate.[7]
Representative triglyceride found in a linseed oil, a triester (triglyceride) derived oflinoleic acid, alphalinolenic acid, and oleic acid.
Preparation[edit]
Esterification is the general name for a chemical reaction in which two reactants (typically an alcohol and an acid) form an ester as the reaction product. Esters are common in organic chemistry and biological materials, and often have a characteristic pleasant, fruity odor. This leads to their extensive use in the fragrance and flavor industry. Ester bonds are also found in many polymers.
Esterification of carboxylic acids[edit]
The classic synthesis is the Fischer esterification, which involves treating a carboxylic acid with an alcohol in the presence of a dehydrating agent: RCO2H + R'OH RCO2R' + H2O
The equilibrium constant for such reactions is about 5 for typical esters, e.g., ethyl acetate. [8] The reaction is slow in the absence of a catalyst. Sulfuric acid is a typical catalyst for this reaction. Many other acids are also used such as polymeric sulfonic acids. Since esterification is highly reversible, the yield of the ester can be improved using Le Chatelier's principle:
using the alcohol in large excess (i.e., as a solvent) using a dehydrating agent: sulfuric acid not only catalyzes the reaction but sequesters water (a reaction product). Other drying agents such as molecular sieves are also effective.
removal of water by physical means such as distillation as a lowboiling azeotropes with toluene, in conjunction with a Dean-Stark apparatus.
Reagents are known that drive the dehydration of mixtures of alcohols and carboxylic acids. One example is the Steglich esterification, which is a method of forming esters under mild conditions. The method is popular in peptide synthesis, where the substrates are sensitive to harsh conditions like high heat. DCC (dicyclohexylcarbodiimide) is used to activate the carboxylic acid to further reaction. DMAP (4-dimethylaminopyridine) is used as an acyl-transfer catalyst.[9]
Another method for the dehydration of mixtures of alcohols and carboxylic acids is the Mitsunobu reaction: RCO2H + R'OH + P(C6H5)3 + R2N2 RCO2R' + OP(C6H5)3 + R2N2H2 Carboxylic acids can be esterified using diazomethane: RCO2H + CH2N2 RCO2CH3 + N2 Using this diazomethane, mixtures of carboxylic acids can be converted to their methyl esters in near quantitative yields, e.g., for analysis by gas chromatography. The method is useful in specialized organic synthetic operations but is considered too expensive for large scale applications.
Alcoholysis of acyl chlorides and acid anhydrides[edit]
Alcohols react with acyl chlorides and acid anhydrides to give esters: RCOCl + R'OH RCO2R' + HCl
(RCO)2O + R'OH RCO2R' + RCO2H The reactions are irreversible simplifying work-up. Since acyl chlorides and acid anhydrides also react with water, anhydrous conditions are preferred. The analogous acylations of amines to give amides are less sensitive because amines are stronger nucleophiles and react more rapidly than does water. This method is employed only for laboratory-scale procedures, as it is expensive.
Alkylation of carboxylate salts[edit]
Although not widely employed for esterifications, salts of carboxylate anions can be alkylating agent with alkyl halides to give esters. In the case that an alkyl chloride is used, an iodide salt can catalyze the reaction (Finkelstein reaction). The carboxylate salt is often generated in situ. In difficult cases, the silver carboxylate may be used, since the silver ion coordinates to the halide aiding its departure and improving the reaction rate. This reaction can suffer from anion availability problems and, therefore, can benefit from the addition of phase transfer catalysts or highly polaraprotic solvents such as DMF.
Transesterification[edit]
Transesterification, which involves changing one ester into another one, is widely practiced: RCO2R' + CH3OH RCO2CH3 + R'OH Like the hydrolysation, transesterification is catalysed by acids and bases. The reaction is widely used for degrading triglycerides, e.g. in the production of fatty acid esters and alcohols. Poly(ethylene terephthalate) is produced by the transesterification of dimethyl terephthalate and ethylene glycol:[7] (C6H4)(CO2CH3)2 + 2 C2H4(OH)2 1/n {(C6H4)(CO2)2(C2H4)}n + 2 CH3OH
Carbonylation[edit]
Alkenes undergo "hydroesterification" in the presence of metal carbonyl catalysts. Esters of propionic acid are produced commercially by this method: C2H4 + ROH + CO C2H5CO2R The carbonylation of methanol yields methyl formate, which is the main commercial source of formic acid. The reaction is catalyzed by sodium methoxide:
CH3OH + CO CH3O2CH
Addition of carboxylic acids to alkenes[edit]
In the presence of palladium-based catalysts, ethylene, acetic acid, and oxygen react to give vinyl acetate: C2H4 + CH3CO2H + 1/2 O2 C2H3O2CCH3 + H2O Direct routes to this same ester are not possible because vinyl alcohol is unstable.
Other methods[edit]
Favorskii rearrangement of -haloketones in presence of base
Baeyer-Villiger oxidation of ketones with peroxides
Pinner reaction of nitriles with an alcohol Nucleophilic abstraction of a metal-acyl complex
Hydrolysis of orthoesters in aqueous acid Cellulolysis via esterification [10]
Reactions[edit]
Esters react with nucleophiles at the carbonyl carbon. The carbonyl is weakly electrophilic but is attacked by strong nucleophilies (amines, alkoxides, hydride sources, organolithium compounds, etc.). The C-H bonds adjacent to the carbonyl are weakly acidic but undergo deprotonation with strong bases. This process is the one that usually initiates condensation reactions. The carbonyl oxygen is weakly basic (less so than in amides) but forms adducts.
Addition of nucleophiles at carbonyl[edit]
Esterification is a reversible reaction. Esters undergo hydrolysis under acid and basic conditions. Under acidic conditions, the reaction is the reverse
reaction of the Fischer esterification. Under basic conditions, hydroxide acts as a nucleophile, while an alkoxide is the leaving group. This reaction, saponification, is the basis of soap making.
The alkoxide group may also be displaced by stronger nucleophiles such as ammonia or primary or secondary amines to give amides: RCO2R' + NH2R" RCONHR" + R'OH This reaction is not usually reversible. Hydrazines and hydroxylamine can be used in place of amines. Esters can be converted to isocyanates through intermediate hydroxamic acids in the Lossen rearrangement. Sources of carbon nucleophiles, e.g., Grignard reagents and organolithium compounds, add readily to the carbonyl.
Reduction[edit]
Compared to ketones and aldehydes, esters are relatively resistant to reduction. The introduction of catalytic hydrogenation in the early part of the 20th century was a breakthrough; esters of fatty acids are hydrogenated to fatty alcohols. RCO2R' + 2 H2 RCH2OH + R'OH A typical catalyst is copper chromite. Prior to the development of catalytic hydrogenation, esters were reduced on a large scale using the BouveaultBlanc reduction. This method, which is
largely obsolete, uses sodium in the presence of proton sources. Especially for fine chemical syntheses, lithium aluminium hydride is used to reduce esters to two primary alcohols. The related reagent sodium borohydride is slow in this reaction. DIBAH reduces esters to aldehydes.[11]
Claisen condensation and related reactions[edit]
As for aldehyde and aldehydes, the hydrogen atoms on the carbon adjacent (" to") the carboxyl group in esters are sufficiently acidic to undergo deprotonation, which in turn leads to a variety of useful reactions. Deprotonation requires relatively strong bases, such as alkoxides. Deprotonation gives a nucleophilic enolate, which can further react, e.g., the Claisen condensation and its intramolecular equivalent, the Dieckmann condensation. This conversion is exploited in the malonic ester synthesis, wherein the diester of malonic acid reacts with an electrophile (e.g.,alkyl halide), and is subsequently decarboxylated. Another variation is the FrterSeebach alkylation.
Other reactions[edit]
Phenyl esters react to hydroxyarylketones in the Fries rearrangement.
Specific esters are functionalized with an -hydroxyl group in the Chan rearrangement.
Esters with -hydrogen atoms can be converted to alkenes in ester pyrolysis.
Protecting groups[edit]
As a class, esters serve as protecting groups for carboxylic acids. Protecting a carboxylic acid is useful in peptide synthesis, to prevent self-reactions of the bifunctional amino acids. Methyl and ethyl esters are commonly available for many amino acids; the tbutyl ester tends to be more expensive. However, t-butyl esters are particularly useful because, under strongly acidic conditions, the t-butyl esters undergo elimination to give the carboxylic acid and isobutylene, simplifying work-up.
List of ester odorants[edit]
Many esters have distinctive fruit-like odors, and many occur naturally in the essential oils of plants. This has also led to their commonplace use in artificial flavorings and fragrances when those odors aim to be mimicked.
Ester Name
Form ula
Odor or occurrence
Allyl hexanoa te
pineapple
Benzyl acetate
pear, strawberry, jasmi ne
Bornyl acetate
pine
Butyl acetate
apple, honey bee
Butyl butyrate
pineapple
Ethyl acetate
nail polish remover, model paint, model airplane glue
Ethyl butyrate
banana, pineapple, stra wberry
Ethyl hexanoa te
pineapple, waxy-green banana
Ethyl cinnama te
cinnamon
Ethyl formate
lemon, rum, strawberr y
Ethyl heptano ate
apricot, cherry, grape, raspberry
Ethyl isovaler
apple
ate
Ethyl lactate
butter, cream
Ethyl nonanoa te
grape
Ethyl pentano ate
apple
Geranyl acetate
geranium
Geranyl butyrate
cherry
Geranyl pentano ate
apple
Isobutyl acetate
cherry, raspberry, stra wberry
Isobutyl formate
raspberry
Isoamyl acetate
pear, banana (flavoring in Pear drops)
Isoprop yl acetate
fruity
Linalyl acetate
lavender, sage
Linalyl butyrate
peach
Linalyl formate
apple, peach
Methyl acetate
glue
Methyl anthrani late
grape, jasmine
Methyl benzoat e
fruity, ylang ylang, feijoa
Methyl butyrate (methyl butanoat e)
pineapple, apple, straw berry
Methyl cinnama te
strawberry
Methyl pentano ate (met hyl valerate )
flowery
Methyl phenyla cetate
honey
Methyl salicylat e (oil of wintergr een)
Modern root beer, wintergreen, Ger molene and Ralgex oin tments (UK)
Nonyl caprylat e
orange
Octyl acetate
fruity-orange
Octyl butyrate
parsnip
Amyl acetate ( pentyl acetate)
apple, banana
Pentyl butyrate (amyl butyrate )
apricot, pear, pineappl e
Pentyl hexanoa te (amyl caproate )
apple, pineapple
Pentyl pentano ate (am yl valerate )
apple
Propyl acetate
pear
Propyl hexanoa te
blackberry, pineapple, cheese, wine
Propyl isobutyr ate
rum
Terpeny l butyrate
cherry
See also[edit]
Cyanate ester Oligoester Polyolester Transesterification Transamidification
References
Vous aimerez peut-être aussi
- A 1Document29 pagesA 1Christopher WilliamsPas encore d'évaluation
- EstersDocument5 pagesEstersNevin BakrPas encore d'évaluation
- EsterificareaDocument43 pagesEsterificareaMahagney SalehPas encore d'évaluation
- Ester GroupDocument3 pagesEster GroupAnubhav SwaroopPas encore d'évaluation
- Structure of A Carboxylic AcidDocument7 pagesStructure of A Carboxylic AcidKajal SinghPas encore d'évaluation
- ESTERSDocument36 pagesESTERSAbdihakemPas encore d'évaluation
- Carboxylic AcidDocument6 pagesCarboxylic AcidVishu AgrawalPas encore d'évaluation
- Carboxylic Acid: From Wikipedia, The Free EncyclopediaDocument11 pagesCarboxylic Acid: From Wikipedia, The Free EncyclopediaDipti BagdePas encore d'évaluation
- Carboxylic AcidDocument13 pagesCarboxylic AcidJeremy NayagamPas encore d'évaluation
- EsterDocument5 pagesEsterRonPas encore d'évaluation
- Carboxylic Acid: "COOH" Redirects Here. For The Bulgarian Musician, SeeDocument9 pagesCarboxylic Acid: "COOH" Redirects Here. For The Bulgarian Musician, SeeFighter_ace_97Pas encore d'évaluation
- 4.1.3 Carboxylic Acids & EstersDocument5 pages4.1.3 Carboxylic Acids & EstersFin BrickmanPas encore d'évaluation
- Exp 10Document9 pagesExp 10ChantalDanaPas encore d'évaluation
- Carboxylic Acid and Derivatives PDFDocument12 pagesCarboxylic Acid and Derivatives PDFÏt's RîçkgãrçīäPas encore d'évaluation
- LayoutDocument55 pagesLayoutHenok Moges KassahunPas encore d'évaluation
- EsterificationDocument3 pagesEsterificationKhris Sy-HandumonPas encore d'évaluation
- 5 Hydrocarbon Derivatives 2Document28 pages5 Hydrocarbon Derivatives 2Marivic TayabanPas encore d'évaluation
- A. Experiment Title: The Making of N-Butyl Acetate B. Experiment Started Date: Wednesday, March 4Document21 pagesA. Experiment Title: The Making of N-Butyl Acetate B. Experiment Started Date: Wednesday, March 4Era MelaniaPas encore d'évaluation
- Aldehydes and KetonesDocument25 pagesAldehydes and Ketonesabigail_acostaPas encore d'évaluation
- Alcohols 1Document13 pagesAlcohols 1Suresh VedpathakPas encore d'évaluation
- Carboxylic AcidsDocument15 pagesCarboxylic Acidsmadhu bonamPas encore d'évaluation
- Chem Notes No6Document31 pagesChem Notes No6AnyhaPas encore d'évaluation
- Explain Chemical Reactions of Alcohol CompoundsDocument2 pagesExplain Chemical Reactions of Alcohol CompoundsIrfan DanialPas encore d'évaluation
- Carboxylic 2Document8 pagesCarboxylic 2johnsmithprayPas encore d'évaluation
- Reactions of AlcoholsDocument3 pagesReactions of AlcoholsKimPas encore d'évaluation
- Esters75275254 131207020139 Phpapp02Document19 pagesEsters75275254 131207020139 Phpapp02Mujthaba AdmaniPas encore d'évaluation
- Esters EtcDocument8 pagesEsters EtcBoobalan Maria SusaiPas encore d'évaluation
- AldehydeDocument29 pagesAldehydeJan michael ChivaPas encore d'évaluation
- EsterDocument2 pagesEsterCyrisse MONTANOPas encore d'évaluation
- I. Experiment Title: Aldehyde, Ketone and Carboxylic AcidDocument10 pagesI. Experiment Title: Aldehyde, Ketone and Carboxylic AcidLaily SafitriPas encore d'évaluation
- Alcohols, Phenols and EpoxidesDocument134 pagesAlcohols, Phenols and EpoxidesStudent 365100% (1)
- Class 12 Aldehydes, Ketones and Carboxylic AcidsDocument20 pagesClass 12 Aldehydes, Ketones and Carboxylic Acidsst06082005Pas encore d'évaluation
- IntroductionDocument16 pagesIntroductionAkhtar RazaPas encore d'évaluation
- Ether: Navigation Search AetherDocument7 pagesEther: Navigation Search AetherMuhammad Wahyu Nugraha0% (1)
- Carboxylic Acid 1Document14 pagesCarboxylic Acid 1Junaid KhanPas encore d'évaluation
- 2019 Ch104 Organic AcidsDocument111 pages2019 Ch104 Organic Acidsgenesis gengerPas encore d'évaluation
- AldehydeDocument9 pagesAldehydesenkatuukaPas encore d'évaluation
- Carbonyl CompoundsDocument11 pagesCarbonyl CompoundsAmon RicoPas encore d'évaluation
- A. Title of Experiment: Carboxylic Acid: Thursday, 10 C. Purpose of ExperimentDocument27 pagesA. Title of Experiment: Carboxylic Acid: Thursday, 10 C. Purpose of ExperimentKeyvir AulinzPas encore d'évaluation
- Chem Class12 Chapter 8Document16 pagesChem Class12 Chapter 8rohithardy45Pas encore d'évaluation
- Alcohols LabDocument7 pagesAlcohols Lab7sky7harveyPas encore d'évaluation
- Catboxylic Acids and Their DerivativesDocument45 pagesCatboxylic Acids and Their DerivativesAnil Kumar VermaPas encore d'évaluation
- Go 3 Alcohol, Ether, AminesDocument13 pagesGo 3 Alcohol, Ether, Aminescikaifa100% (1)
- Esters Fats and Oils Pupil NotesDocument19 pagesEsters Fats and Oils Pupil NotesMasumPas encore d'évaluation
- Cac Bo EngliDocument4 pagesCac Bo EngliLinh ĐồngPas encore d'évaluation
- Carboxylic AcidDocument26 pagesCarboxylic AcidDavid ParkPas encore d'évaluation
- Lesson 3 Properties of MonosaccharidesDocument9 pagesLesson 3 Properties of MonosaccharidesMAN'S BEST FRIENDPas encore d'évaluation
- Alcohols From Carbonyl Compounds: Oxidation-Reduction: Central Linking Role of Alcohols and CarbonylsDocument12 pagesAlcohols From Carbonyl Compounds: Oxidation-Reduction: Central Linking Role of Alcohols and CarbonylsAmihanPas encore d'évaluation
- EtherDocument23 pagesEtherOng Kok LeongPas encore d'évaluation
- Laporan Praktikum Kimia Organik Ii Alkohol Absolut: Disusun OlehDocument20 pagesLaporan Praktikum Kimia Organik Ii Alkohol Absolut: Disusun OlehericPas encore d'évaluation
- Functional Groups: Naming of EstersDocument6 pagesFunctional Groups: Naming of EsterspappadakunduPas encore d'évaluation
- Toluenesulfonic Acid: Preparation and HandlingDocument2 pagesToluenesulfonic Acid: Preparation and HandlingMashudi FikriPas encore d'évaluation
- Ethanoic Acid ChemDocument14 pagesEthanoic Acid ChemjasmynefPas encore d'évaluation
- Aldehydes, Ketones, Carboxylic Acids, and EstersDocument11 pagesAldehydes, Ketones, Carboxylic Acids, and EstersNATURE COMPUTERPas encore d'évaluation
- Alcohols ClassDocument29 pagesAlcohols ClassRyan JamesPas encore d'évaluation
- Alcohol Alcohol, Any of A Class of Organic CompoundsDocument4 pagesAlcohol Alcohol, Any of A Class of Organic CompoundsJason Orolfo Salvadora HLPas encore d'évaluation
- Alcohols: Alcohol, Any of A Class ofDocument3 pagesAlcohols: Alcohol, Any of A Class ofCool for the AnimePas encore d'évaluation
- Transition Metal Catalyzed Furans Synthesis: Transition Metal Catalyzed Heterocycle Synthesis SeriesD'EverandTransition Metal Catalyzed Furans Synthesis: Transition Metal Catalyzed Heterocycle Synthesis SeriesPas encore d'évaluation
- Biochemistry Applied to Beer Brewing - General Chemistry of the Raw Materials of Malting and BrewingD'EverandBiochemistry Applied to Beer Brewing - General Chemistry of the Raw Materials of Malting and BrewingÉvaluation : 4 sur 5 étoiles4/5 (1)
- Practice Makes Perfect in Chemistry: Acids, Bases, and SaltsD'EverandPractice Makes Perfect in Chemistry: Acids, Bases, and SaltsPas encore d'évaluation
- Many Villagers Were ThereDocument1 pageMany Villagers Were TheremeritnasaPas encore d'évaluation
- Complex Story Behind ItDocument1 pageComplex Story Behind ItmeritnasaPas encore d'évaluation
- Very Good Asopes Fables Book PG 15Document1 pageVery Good Asopes Fables Book PG 15meritnasaPas encore d'évaluation
- Virtual Graphic Card: Douknowwhatagpuis??Document36 pagesVirtual Graphic Card: Douknowwhatagpuis??meritnasaPas encore d'évaluation
- IsomersDocument1 pageIsomersmeritnasaPas encore d'évaluation
- Anode and CathodeDocument16 pagesAnode and CathodemeritnasaPas encore d'évaluation
- F (1) A, F (2) B, and F (3) C: Answer 11Document2 pagesF (1) A, F (2) B, and F (3) C: Answer 11meritnasaPas encore d'évaluation
- Carbonyl: For Carbonyl As A Ligand, SeeDocument5 pagesCarbonyl: For Carbonyl As A Ligand, SeemeritnasaPas encore d'évaluation
- Ester: For Other Uses, SeeDocument15 pagesEster: For Other Uses, SeemeritnasaPas encore d'évaluation
- TECHNOLOGYDocument1 pageTECHNOLOGYmeritnasaPas encore d'évaluation
- Organic ChemistryDocument1 pageOrganic ChemistrymeritnasaPas encore d'évaluation