Académique Documents
Professionnel Documents
Culture Documents
New Microsoft Office Word Document
Transféré par
sandipkadoliCopyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
New Microsoft Office Word Document
Transféré par
sandipkadoliDroits d'auteur :
Formats disponibles
Que. No.
1) Choose Correct Answer
(25)
1) The rate of a reaction increases, if the concentration of reactants is a) Increased b) decreased c) Always unaffected e) uncertain
2) An exothermic reaction is that in which the reacting substances a) Have more energy than the products b) have less energy than products c) have a much energy as the products d) are at a higher temp. than products 3) Chemical kinetics can predict the --a) Rate of reaction c) Both a and b 4) Molecularity of a reaction --a) May not be equal to the order of reaction c) both a and b b) cant have a fractional value d) None of these b) feasibility of reaction d) none of these
5) Transition state theory relates the above quantities are --a) k e E/RT c) k T 6) In autocatalytic reactions --a) one of the reactants acts as a catalyst b) one of the products acts as a catalyst c) catalysts have very high selectivity 7) Equilibrium state is --a) Dynamic c) Neither dynamic nor static b) static d) sometimes static & sometimes dynamic d) no catalyst is used b) k T. e E/RT d) k T 0.5
8) For a zero order reaction, concentration of product increases with --a) increase of reaction time c) total pressure b) increase in initial concentration d) decrease in total pressure
9) A batch reactor is characterized by ------a) constant residence time b) the variation in extent of reaction and propert
ies of the reaction mixture with time c) variation in reactor volume d) very low conversion 10) Space velocity ---a) describes the extensive operating characteristics of a tubular flow reactor b) is the maximum feed rate pre unit volume of reactor for a given conversion c) is a measure of the ease of the reaction 11) Space time equals the mean residence time --a) when the density of the reaction mixture is constant reactor c) for narrow dia. tubular reactor b) for large dia. tubular d) for CSTR d) all of those
12) In a homogeneous chemical reaction, the rate of reaction can be affected by a) temperature only c) both temp. and pressure b) pressure only d) temp. pressure and composition
13) A space velocity of 5 hr-1 means that --a) five reactor volumes of feed (at specified conditions) are being fed into the reactor per hour b) after every 5 hours, rector is being filled with feed
c) cent per cent conversion can be achieved in at least 5 hours d) a fixed conversion of a given batch of feed takes 5 hours 14) The ratio of moles of a reactant converted into the desired product to that converted into unwanted product is called ---a) operational yield c) selectivity b) relative yield d) None of these
15) The performance of a cascade of CSTR,s can be improved by adding ---
a) a P.F.reactor in series c) more CSTRs in series
b) a P.F.reactor in parallel d) more CSTRs in parallel
16) The most suitable reactor for carrying out an auto thermal reaction is --a) batch reactor c) semi batch reactor 17) Promoter -----a) initiates a chemical reaction and is a catalyst by itself b) atlers the position of equilibrium in a reversible reaction c) increases the number of active centres by increasing the unevenness of catalyst surface and by creating discontinuities in the crystals d) None of these 18) A catalyst --a) initiates a reaction b) lower the activation energy of reacting molecules c) is capable of reacting with any one of the reactants d) can not be recovered chemically unchanged at the end of a chemical reaction. 19) The dimensionless (D / uL) called the vessel dispersion number, for plug Flow is equal to --a) b) 0 c) 2100 d) 400 b) CSTR d) plug flow reactor
20) Reaction rate of a first order reaction which is half completed in 23 minutes will be -----a) 0.03 sec-1 b) 0.03 min-1 c) 0.03hr-1 d) 0.05 min-1
21) What is the response curve for a step input signal from a reactor ---a) S - curve b) C - curve c) I - curve d) None
22) Autocatalytic reactions are best carried out in a ------a) CSTR c) plug flow reactor b) SCTR in series d) recycle reactor
23) The response curve for a step input signal from a reactor is called C-curve The variance of C-curve in a tanks in series model comprising of m tanks Is equal to -----a) m b) 1 / m c) m d) m2
24) If given irreversible reaction A + B = 2C is elementary, then rate of reaction is defined as -----25) For a zero order solid catalyzed reactor having a Thiele modulus equal to One, one value of effectiveness factor is -----a) 0 b) 1 c) 2 d) None of these
Section II
Answer the following
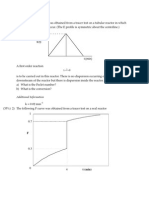
1)The data given below represents a continuous response to a pulse input into a closed vessel. Calculate the mean residence time of fluid in vessel t , Calculate Variance, Calculate vessel dispersion number. (3x2)
t, min Cpulse,g/lit
0 0
5 3
10 5
15 5
20 4
25 2
30 1
35 0
2) The vessel is to be used as a reactor for decomposition of liquid A with the stoichiometry : A = Products And rate -rA = KCA , K = 0.307 min -1
Refer the table in Q.1 for concentration readings and estimate a) the fraction of reactant unconverted in real reactor b) Compare this with fraction unconverted in PFR of same size. (2x2) 3) On doubling the concentration of reactant the rate of reaction triples. Find the reaction
order. Given : -rA = KCAn (2x1) 4) The activation energy of a bimolecular reaction is about 9150 cal/mol. How much faster is this reaction takes place at 500k than at 400k (2x1) 5) At 500k the rate of a bimolecular reaction is ten times the rate at 400k. Find the activation energy for this reaction from collision theory. (2x1) 6) Decomposition of a gas is second order. When the initial concentration of gas is 5x10-4 mol/lit, it is 40% decomposed in 50 min. Calculate the value of rate constant. (2x1) 7) The half life period for certain first order reaction is 2.5 x 103 S. How long will take for of the reactant to be left behind. (2x1) 8) In case of first order reaction show that the time required for 75% conversion is double the time required for 50%conversion. (2x1) 9) Find the first order reaction rate constant of the gas reaction 2A = P if on holding the Pressure constant, the volume of reaction mixture starting with 80 mole % A & 20 Mole % inerts , decreases by 20% in 3 minutes. (2x1) 10) Define the following terms a) Molecularity of reaction b) Order of a reaction (2x2)
11) Write the performance equation of the following reactors a) Batch reactor b) Plug flow reactor c) Mixed flow reactor (3x2)
12) In an isothermal batch reactor the conversion of a liquid reactant A is 70% in 13 Min. Find the space time necessary to effect this conversion in plug flow reactor And mixed flow reactor. Consider first order kinetics. (2x2) 13) Discuss in detail the significance of following a) E Curve b) F Curve c) C- Curve (3x2)
14) Milk is pasteurized if it is heated to 36*C for 30 minutes, but if it is heated to 74*C , it needs 15 sec. for the same result. Find the activation energy for Sterilization process.
(1x2) 15) Define the following terms ---
a) Effectiveness factor b) Residence time distribution (RTD) (2x2)
Vous aimerez peut-être aussi
- Introduction To Particle PhysicsDocument101 pagesIntroduction To Particle PhysicsДарко Симић100% (6)
- Jean Zinn-Justin - Quantum Field Theory and Critical Phenomena-Oxford University Press (2021)Document1 074 pagesJean Zinn-Justin - Quantum Field Theory and Critical Phenomena-Oxford University Press (2021)Vi Kem100% (1)
- C 781 - 02 - QZC4MQDocument8 pagesC 781 - 02 - QZC4MQmarkPas encore d'évaluation
- O Level Biology Practice Questions And Answers EnzymesD'EverandO Level Biology Practice Questions And Answers EnzymesÉvaluation : 5 sur 5 étoiles5/5 (1)
- Chemical Reaction Engineering IDocument42 pagesChemical Reaction Engineering IMuthu UmayalPas encore d'évaluation
- Bioprocess Engineering QuestionsDocument13 pagesBioprocess Engineering QuestionsPalanisamy Selvamani100% (1)
- Tut1 2016 QDocument5 pagesTut1 2016 QAbhishek SardaPas encore d'évaluation
- ASTM E-542 Standard Practice For Calibration of Laboratory Volumetric Apparatus PDFDocument7 pagesASTM E-542 Standard Practice For Calibration of Laboratory Volumetric Apparatus PDFAngel Alvarez CarrilloPas encore d'évaluation
- ChE Objective Type Questions Compilation - Dean MedinaDocument130 pagesChE Objective Type Questions Compilation - Dean MedinaPatrick GoPas encore d'évaluation
- Chemical Engineering Objective Type Questions Reaction KineticsDocument18 pagesChemical Engineering Objective Type Questions Reaction KineticsSaakshi Sharma67% (3)
- ChE Objective Type Questions Compilation-Dean Medina 8-28-10Document191 pagesChE Objective Type Questions Compilation-Dean Medina 8-28-10Airra IlaganPas encore d'évaluation
- Kinetics Ans Key Master FileDocument10 pagesKinetics Ans Key Master FileJOANA RHEA SAGPAEYPas encore d'évaluation
- Kinetics PretestDocument4 pagesKinetics PretestAngeline SmithPas encore d'évaluation
- Suggesion CRE 2022Document14 pagesSuggesion CRE 2022Soumyodeep ChowdhuryPas encore d'évaluation
- Semester-6 3360503 CRE MCQ KRD PDFDocument9 pagesSemester-6 3360503 CRE MCQ KRD PDFDhruv RanaPas encore d'évaluation
- Dean Medina CompilationDocument165 pagesDean Medina CompilationArlene F. MontalboPas encore d'évaluation
- Cre MCQDocument17 pagesCre MCQAditya WaghPas encore d'évaluation
- CN2116 QZ1Document31 pagesCN2116 QZ1Wang ShenghaoPas encore d'évaluation
- CHE3044F, 2013: Reactor Design 1: TUTORIAL 6Document4 pagesCHE3044F, 2013: Reactor Design 1: TUTORIAL 6nmhatityePas encore d'évaluation
- CRE PYQ (1988-2020) : BY Shailendra Sir (SKS50)Document60 pagesCRE PYQ (1988-2020) : BY Shailendra Sir (SKS50)Romil GandhiPas encore d'évaluation
- Advanced Chemical Reaction EngineeringDocument1 pageAdvanced Chemical Reaction EngineeringIbmWasuserPas encore d'évaluation
- Tutorial 11Document5 pagesTutorial 11Aakash R RajwaniPas encore d'évaluation
- ChE Objective Type Questions Compilation Dean Medina 1 8 1 11 PDFDocument187 pagesChE Objective Type Questions Compilation Dean Medina 1 8 1 11 PDFJom SanzPas encore d'évaluation
- Chemistry (Maninagar-Target) Section-I (Only One Option Correct)Document4 pagesChemistry (Maninagar-Target) Section-I (Only One Option Correct)Rajeev GangwarPas encore d'évaluation
- 9A23502 Biochemical Reaction Engineering IDocument8 pages9A23502 Biochemical Reaction Engineering IsivabharathamurthyPas encore d'évaluation
- Chemical Kinetics RevisionDocument2 pagesChemical Kinetics RevisionShubham KumarPas encore d'évaluation
- Cn2116 2010 Quiz1 Solutions (Set 1)Document6 pagesCn2116 2010 Quiz1 Solutions (Set 1)Wang Xin YiPas encore d'évaluation
- DQE January 2001: Additional InformationDocument12 pagesDQE January 2001: Additional InformationryezhuPas encore d'évaluation
- Cbse+2 Chemistry 1mark Bits 2023-2024Document41 pagesCbse+2 Chemistry 1mark Bits 2023-2024lama lamaPas encore d'évaluation
- Chemical Kinetics FinalDocument7 pagesChemical Kinetics Finalaxiliya6Pas encore d'évaluation
- 3 - QP - Chemical KineticsDocument5 pages3 - QP - Chemical Kineticsssheeladevi84Pas encore d'évaluation
- Chapter 3 Cre MCQDocument10 pagesChapter 3 Cre MCQRohit Ramesh KalePas encore d'évaluation
- Tutorial 5drtuhDocument2 pagesTutorial 5drtuhFikrie MuhdPas encore d'évaluation
- Chee4367 HW051231Document2 pagesChee4367 HW051231kimhoang_16927574Pas encore d'évaluation
- R05320802chemicalreactionengineeringiiDocument8 pagesR05320802chemicalreactionengineeringiiSanthosh KumarPas encore d'évaluation
- (Section A) - Answer The Following For 1 Mark.Document6 pages(Section A) - Answer The Following For 1 Mark.vivek davePas encore d'évaluation
- Cre 2020CDocument7 pagesCre 2020CRitul RajbangshiPas encore d'évaluation
- Senior - 2020 - Class - 12 - Chemistry - Objective Questions - Chemical KineticsDocument5 pagesSenior - 2020 - Class - 12 - Chemistry - Objective Questions - Chemical Kineticsblaise.denzil.rodriguesPas encore d'évaluation
- Exam I Sem I 2011 12 Cheng 323Document7 pagesExam I Sem I 2011 12 Cheng 323Faisal MumtazPas encore d'évaluation
- Che 605B - 2009Document7 pagesChe 605B - 2009shubhang2392Pas encore d'évaluation
- Delhi Public School: Nacharam/ Mahendra Hills/ NadergulDocument3 pagesDelhi Public School: Nacharam/ Mahendra Hills/ Naderguleeshwar saagarPas encore d'évaluation
- Cre Jntu Question PaperDocument8 pagesCre Jntu Question PaperNikhil Kumar ChennuriPas encore d'évaluation
- CDB2043 - Reaction EngineeringDocument6 pagesCDB2043 - Reaction EngineeringXin-YiWoonPas encore d'évaluation
- CHCE 3004 CHEG 333 Chemical Reaction Engineering I QP 2020 SUPPLIMENTRY - AM PDFDocument2 pagesCHCE 3004 CHEG 333 Chemical Reaction Engineering I QP 2020 SUPPLIMENTRY - AM PDFLGK KlanPas encore d'évaluation
- Tutorial For Chapter 23Document9 pagesTutorial For Chapter 23Thurgah VshinyPas encore d'évaluation
- Assignment 4Document5 pagesAssignment 4Yi Hong LowPas encore d'évaluation
- Midterm Quiz 1 March 9.2021 QDocument5 pagesMidterm Quiz 1 March 9.2021 QThalia RodriguezPas encore d'évaluation
- Chemical Kinetics Question BankDocument5 pagesChemical Kinetics Question BankShivam kumarPas encore d'évaluation
- TALYDocument3 pagesTALYJose David D SPas encore d'évaluation
- NR-320802 Chemical Reaction Engineering-IDocument8 pagesNR-320802 Chemical Reaction Engineering-ISrinivasa Rao G100% (1)
- CRE Previous Year QuestionsDocument14 pagesCRE Previous Year QuestionsAbhishek GadhwalPas encore d'évaluation
- Chemical Reaction Engineering Ph. D. Qualifier Examination Open Book (Scott Fogler) ExamDocument3 pagesChemical Reaction Engineering Ph. D. Qualifier Examination Open Book (Scott Fogler) ExamNicole Anne BorromeoPas encore d'évaluation
- 1st Year Chemistry Revision Assignment For Test 11Document6 pages1st Year Chemistry Revision Assignment For Test 11Syed Moeen NaqviPas encore d'évaluation
- Cro Tut8Document13 pagesCro Tut8Ernst SmitPas encore d'évaluation
- EErcDocument108 pagesEErcRohit Ramesh KalePas encore d'évaluation
- Kinetics ConceptsDocument7 pagesKinetics ConceptsJane Guiron AballaPas encore d'évaluation
- Microsoft Word - 6 - Prob RTD-Non Id React 11-12 61-78 - EnglishDocument9 pagesMicrosoft Word - 6 - Prob RTD-Non Id React 11-12 61-78 - EnglishPavithra Sivaraja100% (1)
- 3K4 2013 Assignment 2Document4 pages3K4 2013 Assignment 2Khalil LasferPas encore d'évaluation
- CH-4 Kinetics MaterialDocument18 pagesCH-4 Kinetics MaterialBishal MishraPas encore d'évaluation
- Chemical Reaction Engineering Exam QuestionDocument2 pagesChemical Reaction Engineering Exam QuestionnadyahginicePas encore d'évaluation
- Entry Exam - M.Sc. / 2017-2018 Chemical Engineering Department University of Baghdad Date 25/6/2018 (1 Attempt) Time: 3 HrsDocument5 pagesEntry Exam - M.Sc. / 2017-2018 Chemical Engineering Department University of Baghdad Date 25/6/2018 (1 Attempt) Time: 3 Hrshiba thamirPas encore d'évaluation
- A Modern Course in Statistical PhysicsD'EverandA Modern Course in Statistical PhysicsÉvaluation : 3.5 sur 5 étoiles3.5/5 (2)
- Analytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportD'EverandAnalytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportPas encore d'évaluation
- Development of Fuel Cell Technology by Syngas Reaction by Concentrating Solar Raditions RepairedDocument4 pagesDevelopment of Fuel Cell Technology by Syngas Reaction by Concentrating Solar Raditions RepairedsandipkadoliPas encore d'évaluation
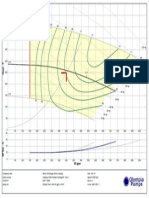
- Pump Report22Document1 pagePump Report22sandipkadoliPas encore d'évaluation
- Sensor On OverlockDocument32 pagesSensor On OverlocksandipkadoliPas encore d'évaluation
- Saturday Aug 31 2013, 10:24:26 Case: G:/distlltin - Usc Flowsheet: Case (Main)Document1 pageSaturday Aug 31 2013, 10:24:26 Case: G:/distlltin - Usc Flowsheet: Case (Main)sandipkadoliPas encore d'évaluation
- UniSimDesign PrintDocument1 pageUniSimDesign PrintsandipkadoliPas encore d'évaluation
- UNIT 1 Introduction To BiopharmaceuticsDocument208 pagesUNIT 1 Introduction To BiopharmaceuticsMamta Pant100% (5)
- ImrulDocument5 pagesImrulJobaer ShaonPas encore d'évaluation
- NMR of Hydrogen (AQA A2 Chemistry) PART 1 OF 2 TOPICSDocument1 pageNMR of Hydrogen (AQA A2 Chemistry) PART 1 OF 2 TOPICSzaheeraPas encore d'évaluation
- Astm 3612-02Document22 pagesAstm 3612-02MarioEnriqueAlcocerÁvila100% (2)
- Presentación 2 - Cinética PDFDocument7 pagesPresentación 2 - Cinética PDFDanny GarcíaPas encore d'évaluation
- Phosphorylation Dependence and Stoichiometry of The Co - 2014 - Molecular - CellDocument14 pagesPhosphorylation Dependence and Stoichiometry of The Co - 2014 - Molecular - CellasdfweafsplashPas encore d'évaluation
- Cambridge International General Certificate of Secondary EducationDocument16 pagesCambridge International General Certificate of Secondary Educationzainab tamerPas encore d'évaluation
- PHE971 SGP EngDocument12 pagesPHE971 SGP EngAndreiPas encore d'évaluation
- Photoelectric Sensor SolutionsDocument13 pagesPhotoelectric Sensor SolutionsHa RbPas encore d'évaluation
- University of Cambridge International Examinations International General Certificate of Secondary EducationDocument16 pagesUniversity of Cambridge International Examinations International General Certificate of Secondary EducationRita SmairatPas encore d'évaluation
- Note 9Document9 pagesNote 9Dr. Aung Ko LattPas encore d'évaluation
- Nature of ResistanceDocument28 pagesNature of Resistancelocutor100% (1)
- Extracting Aluminum From Dross Tailings: A.M. AmerDocument4 pagesExtracting Aluminum From Dross Tailings: A.M. Amermontie3Pas encore d'évaluation
- Ekaland & Vultac® Range For Rubber Application: P P P PDocument1 pageEkaland & Vultac® Range For Rubber Application: P P P Pjulius hasan33Pas encore d'évaluation
- Unit 1 The Solid StateDocument17 pagesUnit 1 The Solid StateSuresh DasaraddiPas encore d'évaluation
- B.SC 1st Year Organic1Document57 pagesB.SC 1st Year Organic1levana dhea lumi100% (1)
- Practice Problems 7Document15 pagesPractice Problems 7Deena RuangchayPas encore d'évaluation
- Agbo 2023 IOP Conf. Ser. Earth Environ. Sci. 1178 012019Document10 pagesAgbo 2023 IOP Conf. Ser. Earth Environ. Sci. 1178 012019Agbo sundayPas encore d'évaluation
- Fuck You DropboxDocument2 pagesFuck You DropboxRebecca DuncanPas encore d'évaluation
- 5.2. Classification of FuelsDocument16 pages5.2. Classification of FuelsadiPas encore d'évaluation
- 2.2.35. OsmolalityDocument1 page2.2.35. OsmolalitySpectre SpectrePas encore d'évaluation
- SURFYNOL® 465 Surfactant: Nonionic Dynamic Wetting AgentDocument2 pagesSURFYNOL® 465 Surfactant: Nonionic Dynamic Wetting AgentJeidy Estefania Serrano MarquinPas encore d'évaluation
- Chlorofluorocarbons: (CFCS)Document4 pagesChlorofluorocarbons: (CFCS)mansikakaniPas encore d'évaluation
- Tugas Pp2 Reny OktaviantiDocument9 pagesTugas Pp2 Reny Oktaviantibimo_alkautsarPas encore d'évaluation
- STPM 2017 Sem 2uDocument8 pagesSTPM 2017 Sem 2uAprillia ChanPas encore d'évaluation