Académique Documents
Professionnel Documents
Culture Documents
Materials Selection Lecture Notes
Transféré par
Karthick NTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Materials Selection Lecture Notes
Transféré par
Karthick NDroits d'auteur :
Formats disponibles
Materials Selection Lecture otes
These otes is by oway a Replacement of Classroom Attendence !!!
General References: 1- Engineering Materials, Properties and Selection Kenneth G. Budinski Lecture 01 2- Engineering Materials Technology Bill Bolton 3- Lecture notes by professors of international universities. 1- All Exam Question are in English, Answers can be in Arabic, The student should aware of the scientific and engineering idioms. 2- The student final degree depends on Exams and Other Activities, E.g., writing essays and reports; also, attending class lectures is of prime importance. The field of materials science and engineering is often defined by the interrelationship between four topicssynthesis and processing, structure and composition, properties, and performance. # What is the Responsibilities of Materials Engineer? 1Phase -1 of design process Drawing the basic design. Phase -2 of design process Selection of Proper Materials, i.e. section of material according to many parameters e.g. Mechanical loads, Wear, Electrical insulation, Thermal properties, and Availability and cost. This includes; Selection of the proper manufacturing process or processes, All sums to what is called The Technological Root 2- Proper choice (selecting) of substitute (alternative) materials when needed, 3- Contributing and Evaluating Materials tests results, 4- Studying and Composing Materials Data sheets before placing an order, 5- Doing Research Activities to enhance materials performance. # What are the other contributions of the Materials Engineer?
Dr. Saad B. H. Farid
Materials Selection Lecture otes Budinski Classification of Materials: -
These otes is by oway a Replacement of Classroom Attendence !!!
Materials
Engineering materials: Basically, they are of four types:A. Metals: Elements with a valence of 1, 2 or 3. They are crystalline solids composed of atoms held together by a matrix of electrons. The Electron Gas that surrounds the Lattice of atomic nuclei is responsible for most of the properties. 1. General properties: High electrical conductivity, high thermal conductivity, ductile and relatively high stiffness, toughness and strength. They are ready to machining, casting, forming, stamping and welding. Nevertheless, they are susceptible to corrosion.
Dr. Saad B. H. Farid
Materials Selection Lecture otes
These otes is by oway a Replacement of Classroom Attendence !!!
2. Further description: Engineering metals are generally Alloys. Alloys are metallic materials formed by mixing two or more elements, e.g. i. Mild steel Fe + C Fe + C + Cr + Mn etc.
ii. Stainless steel iii. C ote iv. Cr
improves Strength improves the corrosion resistance etc.
3. Classification: of metals and alloys: i. Ferrous: Plain carbon steel, Alloy steel, Cast iron, ii. Nonferrous: Light Alloys (Al, Mg, Ti, Zn), Heavy Alloys (Cu, Pb, Ni), Refractory Metals (Mo, Ta, W), Precious metals (Au, Ag, Pt) 4. Applications: i. Electrical wiring ii. Structures: buildings, bridges, etc. iii. Automobiles: body, chassis, springs, engine block, etc. iv. Airplanes: engine components, fuselage, landing gear assembly, etc. v. Trains: rails, engine components, body, wheels vi. Machine tools: drill bits, hammers, screwdrivers, saw blades, etc. vii. Magnets viii. Catalysts 5. Examples: i. Pure metal elements (Cu, Fe, Zn, Ag, etc.) ii. Alloys (Cu-Sn=bronze, Cu-Zn=brass, Fe-C=steel, Pb-Sn=solder) iii. Intermetallic compounds (e.g. Ni3Al)
Dr. Saad B. H. Farid
Materials Selection Lecture otes
These otes is by oway a Replacement of Classroom Attendence !!!
B. Ceramics: Inorganic, non-metallic crystalline compounds, usually oxides (SiO2, Al2O3, MgO, TiO2, BaO), Carbides (SiC), Nitrides (Si3N4), Borides (TiB2), Silicides (WSi2, MoSi2). Some literature includes glasses in the same category, however; glasses are amorphous (non-crystalline) compounds i.e. they possess short range order of atoms. 1. General properties: Light weight, Hard, High strength, stronger in compression than tension, tend to be brittle, low electrical conductivity, High temperature resistance and corrosion resistance. 2. Further description: Ceramics also includes ferrites (ZnFe2O4), semiconductors (ZnO, TiO2, CuO, SiC, AlN, BN, C, Si, Ge, SiGe), piezoelectric and ferroelectric ceramic (BaTiO3, PZT=PbZrTiO3) and superconducting ceramics (YBa2Cu3O7). 3. Classification: of ceramics: i. Traditional Ceramics: Includes pottery, china, porcelain productsetc, these products utilizes natural ceramic ores. ii. Advanced Ceramics: Alumina, magnesia, Carbides, Nitrides, Borides, Silicides etc, they are synthetic materials, usually of better mechanical properties. Electronic ceramics falls in the same category. iii. Glass, Glass Ceramic and Vitro Ceramic: Glasses are essentially vitreous (amorphous, non crystalline), Glass ceramics are mostly recrystallized from glassy medium and, Vitro
Dr. Saad B. H. Farid
Materials Selection Lecture otes
These otes is by oway a Replacement of Classroom Attendence !!!
Ceramics have crystalline microstructure which are partially vitreous at the grain boundaries. 4. Applications: i. Electrical insulators ii. Abrasives iii. Thermal insulation and coatings iv. Windows, television screens, optical fibers (glass) v. Corrosion resistant applications vi. Electrical devices: capacitors, varistors, transducers, etc. vii. Highways and roads (concrete) viii. Biocompatible coatings (fusion to bone) ix. Self-lubricating bearings x. Magnetic materials (audio/video tapes, hard disks, etc.) xi. Optical wave guides xii. Night-vision 5. Examples of technical ceramics i. Barium titanate (often mixed with strontium titanate) displays ferroelectricity, meaning that its mechanical, electrical, and thermal responses are coupled to one another and also history-dependent. It is widely used in electromechanical transducers, ceramic capacitors, and data storage elements. ii. Bismuth strontium calcium copper oxide, a hightemperature superconductor iii. Boron carbide (B4C), which is used in ceramic plates in some personnel, helicopter and tank armor.
Dr. Saad B. H. Farid
Materials Selection Lecture otes
These otes is by oway a Replacement of Classroom Attendence !!!
iv. Boron nitride is structurally isoelectronic to carbon and takes on similar physical forms: a graphite-like one used as a lubricant, and a diamond-like one used as an abrasive. v. Ferrite (Fe3O4), which is ferrimagnetic and is used in the magnetic cores of electrical transformers and magnetic core memory. vi. Lead zirconate titanate is another ferroelectric material. vii. Magnesium diboride (MgB2), which is an unconventional superconductor. viii. Sialons / Silicon Aluminium Oxynitrides, high strength, high thermal shock / chemical / wear resistance, low density ceramics used in nonferrous molten metal handling, weld pins and the chemical industry. ix. Silicon carbide (SiC), which is used as a susceptor in microwave furnaces, a commonly used abrasive, and as a refractory material. x. Silicon nitride (Si3N4), which is used as an abrasive powder. xi. Steatite (MgSiO3), used as an electrical insulator. xii. Uranium oxide (UO2), used as fuel in nuclear reactors. xiii. Yttrium barium copper oxide (YBa2Cu3O7-x), another high temperature superconductor. xiv. Zinc oxide (ZnO), which is a semiconductor, and used in the construction of varistors.
Dr. Saad B. H. Farid
Materials Selection Lecture otes
These otes is by oway a Replacement of Classroom Attendence !!!
xv. Zirconium dioxide (zirconia), its high oxygen ion conductivity recommends it for use in fuel cells. In another variant, metastable structures can impart transformation toughening for mechanical applications; most ceramic knife blades are made of this material. 6. Semiconductors Applications and Examples i. Computer CPUs ii. Electrical components (transistors, diodes, etc.) iii. Solid-state lasers iv. Light-emitting diodes (LEDs) v. Flat panel displays vi. Solar cells vii. Radiation detectors viii. Microelectromechanical devices (MEMS) ix. Examples: Si, Ge, GaAs, and InSb Lecture 02 C. Polymers: High molecular weight organic substance made up of a large number of repeat (monomer) units. Their properties are linked directly to their structure, which is dictated mostly by intermolecular bonds. 1. General properties: compared with metals, polymers have lower density, lower stiffness and tend to creep. They have higher thermal expansion and corrosion resistance. Furthermore, polymers have low electrical conductivity and low thermal conductivity. The prime weakness is that polymers do not withstand high temperatures.
Dr. Saad B. H. Farid
Materials Selection Lecture otes
These otes is by oway a Replacement of Classroom Attendence !!!
2. Further description: Polymers generally formed via a Polymerization Process, in which the polymer chain builds up from monomers with the aid of heat and/or chemical agents. The C-C bonds form the backbone of the polymer chain; when the chains grow very long, they get tangled (twisted) and loose their lattice order, thus, changing increasingly to the amorphous state. Consequently, polymers are semi-crystalline to some degree of crystallinity that can be measured by X-ray Diffraction. 3. Classification: according to their properties: i. Plastics: (Hard), they can be semi-crystalline or amorphous (glassy). 1. Thermoplastics: Such as Polyethylene (PE) and Polymethylmethacrylate (Acrylic and PMMA) are composed of linear polymer chains. They flow under shear when heated. They can be compression- or injection- molded. 2. Thermosets: Such as Polystyrene (PS) and Polyvinylchloride (PVC) are composed of branched polymer chains. They not flow when heated. The monomers are cured in a mold (RIM). ii. Elastomers: (Soft) Rubbery cross-linked solids that will deform elastically under stress, e.g. natural rubber Thermoplastic elastomers are a special type of
Dr. Saad B. H. Farid
Materials Selection Lecture otes
These otes is by oway a Replacement of Classroom Attendence !!!
elastomer in which the cross-linking becomes reversible upon heating. iii. Solutions: Viscosity modifiers, polymeric surfactants, lubricants. 4. Applications and Examples i. Adhesives and glues ii. Containers iii. Moldable products (computer casings, telephone handsets, disposable razors) iv. Clothing and upholstery material (vinyls, polyesters, nylon) v. Water-resistant coatings (latex) vi. Biodegradable products (corn-starch packing peanuts) vii. Biomaterials (organic/inorganic interfaces) viii. Liquid crystals ix. Low-friction materials (Teflon) x. Synthetic oils and greases xi. Gaskets and O-rings (rubber) xii. Soaps and surfactants D. Composite: A combination of two or more materials to achieve better properties than that of the original materials. These materials are usually composed of a Matrix and one or more of Filler material. Wood is a natural composite of cellulose fibers in a matrix of polymer called lignin. The primary objective of engineering composites is to increase strength to weight ratio. Composite material properties are not necessarily isotropic, i.e., directional properties can be synthesized
Dr. Saad B. H. Farid
Materials Selection Lecture otes
These otes is by oway a Replacement of Classroom Attendence !!!
according to the type of filler materials and the method of fabrication. 1. General properties: Low weight, high stiffness, brittle, low thermal conductivity and high fatigue resistance. Their properties can be tailored according to the component materials. 2. Further description:
3. Classification: i. Particulate composites (small particles embedded in a different material): e.g. Cermets (Ceramic particle embedded in metal matrix) and Filled polymers. ii. Laminate composites (golf club shafts, tennis rackets, Shield Glass) iii. Fiber reinforced composites: e.g. Fiber glass (GFRP) and Carbon-fiber reinforced polymers (CFRP) 4. Applications: i. Sports equipment (golf club shafts, tennis rackets, bicycle frames) ii. Aerospace materials iii. Thermal insulation
Dr. Saad B. H. Farid
10
Materials Selection Lecture otes iv. Concrete
These otes is by oway a Replacement of Classroom Attendence !!!
v. "Smart" materials (sensing and responding) vi. Brake materials 5. Examples: i. Fiberglass (glass fibers in a polymer) ii. Space shuttle heat shields (interwoven ceramic fibers) iii. Paints (ceramic particles in latex) iv. Tank armor (ceramic particles in metal)
Other Types of Engineering Materials 1. Classes: Some literatures allocate a unique category for it. 2. Biomaterials: (really using previous 5): Including Bone substitution, Wide variety of Dental materials and else. 3. Liquids and Gases: play a major role in thermal, hydraulic and pneumatic systems. Used in heat transfer, materials flow, power/pressure transmission and lubrication. Have low electrical and thermal conductivity. 4. atural materials: e.g. Wood, Leather, Cotton/wool/silk, Bone. Sample Questions 1. What are the responsibilities of Materials engineer? 2. What are the special properties of ceramics and glasses? 3. What are they used for? 4. What are the industrially important glasses? 5. What kind of different ceramics are there? 6. What is a high-performance ceramic? 7. What is the drawback of ceramics compared to metals? 8. Why are metals and metal alloys used? 9. What is so special about high performance metals?
Dr. Saad B. H. Farid
11
Materials Selection Lecture otes
These otes is by oway a Replacement of Classroom Attendence !!!
10. In the space-age, what features in a material or material structure would be important in the future? 11. What kind of metals is used in space equipment, and why are they used? 12. Explain the expression thermosetting and thermoplastic. 13. What is the glass transition temperature? 14. What happens to a thermoplastic when it is cooled down below melting temperature? 15. How do thermosetting polymers respond to subsequent heating? 16. What are some attractive engineering properties of plastics? 17. What is the unique mechanical property of elastomeric materials? 18. What is rubber? 19. Why are the thermosetting polymers so different from thermoplastic polymers? 20. What decides if a polymer will be thermosetting or thermoplastic? 21. What are three important, common thermosetting polymers? 22. What are three important, common thermoplastic polymers? 23. What is a composite material? 24. What is the greatest advantage of composite materials? 25. What does Fiber Reinforced Plastics (FRP) mean? 26. What kind of reinforcement can composites have? Illustrate them. 27. What is so special about spider silk, can you find out the prospect for commercialization of spider silk? 28. What is the primary function of the reinforcement and the matrix in the composite material? 29. How do we classify composites? 30. What is the strength of the composite primarily depending on? 31. Define Intermetallic compounds, give examples. 32. What material you choose for acid resistance container?
Dr. Saad B. H. Farid
12
Materials Selection Lecture otes
These otes is by oway a Replacement of Classroom Attendence !!!
Fracture
Basic modes of materials Failure: 1. Fracture, 2. Fatigue, 3. Creep. Lecture 03
For metals: Ductile Fracture: when a material yields as a result of excessive deformation, Brittle Fracture, Fatigue, Creep. For Polymers can also fail as a result of yielding and excessive deformation. Ceramic Materials tend to fail by brittle fracture rather than excessive yielding. 20.2.1 Ductile Fracture When a ductile material has a gradually increasing tensile stress applied, it behaves elastically up to a limiting stress and then beyond that stress plastic deformation occurs. As the stress is increased the crosssectional area of the material is reduced and a necked region is produced. A ductile material there is a considerable amount of plastic deformation before failure occurs in the necked region as a result of excessive yielding. When it occurs, the fracture shows a typical cone and cup formation with the surfaces of the fractured material dull or fibrous. This is because, under the action of the increasing stress, small material cracks form which gradually grow in size until there is an internal, almost horizontal, crack. The final fracture occurs when the material shears at an angle of 45 to the aids of the direct stress. This type of failure is known as ductile failure.
Dr. Saad B. H. Farid
13
Materials Selection Lecture otes
These otes is by oway a Replacement of Classroom Attendence !!!
With ductile failure the sequence of events is considered to be: 1. Following elastic strain the material becomes plastically deformed and a neck forms (Figure 20.3(a)). 2. Within the neck, small cavities or voids are formed (Figure 20.3(b)). These develop as a result of the stress causing small particles of impurities or other discontinuities in the material to either fracture or separate from the metal matrix. The more such nuclei there are available to trigger the development of these cavities, the less the material will extend before fracture and so the less ductile the material. Thus increasing the purity of a material increases its ductility 3. These cavities then link up to form an internal crack which spreads across the material in a direction at right angles to applied tensile stress (Figure 20.3(c)). 4. The crack finally propagates to the material surface by shearing in a direction which is approximately at 45o to the applied stress to give a fracture in the typical form of a cup and cone (Figure 20.3(d)). 20.2.2 Brittle fracture If you drop a china cup and it breaks, it is possible to pick up the pieces and stick them back together again and have something which still looks like a cup. The china cup has failed by what is termed brittle fracture. Brittle fracture is the main mode of failure for glass and ceramics. With a brittle fracture the material fractures before plastic deformation has occurred. Figure 20.4 shows possible forms of brittle tensile failure for metals. The surfaces of the fractured material appear bright and granular due to the reflection of light from individual crystal surfaces. This is because the fracture has grains within the material cleaving along planes of atoms.
Dr. Saad B. H. Farid
14
Materials Selection Lecture otes
These otes is by oway a Replacement of Classroom Attendence !!!
We can consider the sequence of events (Stages) leading to brittle fracture to be: 1. When stress is applied, the bonds between atoms and between grains in the material are elastically strained. 2. At some critical stress the bonds break, remember the material is brittle and there is no plastic deformation and hence small-scale sup, and a crack propagates through the material to give fracture. 20.2.3 Polymer Brittle failure with polymeric materials is a common form of failure with materials below their glass transition temperature, i.e. amorphous polymers. The resulting fracture surfaces show a mirror-like region, where the crack has grown slowly, surrounded by a region which is rough and coarse where the crack has propagated at speed (Figure 20.5). In an amorphous polymer the chains are arranged randomly with no orientation. when stress is applied, it can cause localized chain slippage and an orientation of molecule chains (Figure 20 6) with the result that the applied stress causes small voids to form between the aligned molecules and fine cracks, termed crazing, are formed. This is what constitutes the mirror-like region. Because of the inherent weakness of the material in the crazed region it serves as a place for cracks to propagate from and cause the material to fracture. Initially the crack grows by the growth of the voids along the midpoint of the craze. These then coalesce to produce a crack which then travels through the material by the growth of voids ahead of the advancing crack tip. This part of the fracture surface shows as the rougher region. With crystalline polymers, the application of stress results in the folded molecular chains becoming unfolded and aligned (see Section 7.5). The result is then considerable, permanent deformation and necking. Prior to the material yielding and necking starting, the material is quite likely to begin to show a cloudy appearance. This is due to small voids being produced within the material. Further stress causes these voids to coalesce to produce a crack which then travels through the material by the growth of voids ahead of the advancing crack tip. Figure 20.7 shows typical forms of stress-strain graphs for polymers showing this form of failure.
Dr. Saad B. H. Farid
15
Materials Selection Lecture otes
These otes is by oway a Replacement of Classroom Attendence !!!
20.2.4 Ceramics Ceramics are brittle materials, whether glassy or crystalline. Typically fractured ceramic shows around the origin of the crack a mirror-like region bordered by a misty region containing numerous micro cracks (Figure 20.8). In some cases, the mirror-like region may extend over the entire surface. 20.2.5 Composites The fracture surface appearances and mechanisms for composites depend on the fracture characteristics of the matrix and reinforcement materials and on the effectiveness of the bonding between the two. Thus, for example, for a glass-fiber reinforced polymer, depending on the strength of the bonds between fibers and polymer, the fibers may break first and then a crack propagate in shear along the fibermatrix interface. Eventually the load which had been mainly carried by the fibers is transferred to the matrix which then fails;
Dr. Saad B. H. Farid
16
Materials Selection Lecture otes
These otes is by oway a Replacement of Classroom Attendence !!!
Alternatively the matrix may fracture first and the entire load is then transferred to the fibers which carry the increasing load until they break. The result is a fractured surface with lengths of fiber sticking out from it, rather like bristles out from a brush. Factors affecting the fracture of a material include: 1. The presence of notches of sudden changes in section which may produce stress concentration. 2. The speed with which the load is applied. 3. The temperature of a material determining whether it behaves as a brittle or ductile material. 4. Temperature changes producing thermal shock loading. 20.3.1 Stress concentration. If you want to break a small piece of material, one way is to make a small notch in the surface of the material and then apply a force. The presence of a notch, or any sudden change in section of a piece of material, can very significantly change the stress at which fracture occurs. The notch or sudden change in section produces what are called stress concentrations. They disturb the normal stress distribution and produce local cogenerations of stress. The amount by which the stress is raised depends on the depth of the notch, or change in section, and the radius of the tip of the notch. The greater the depth of the notch the greater the amount by which the stress is increased. The smaller the radius of the tip of the notch the greater the amount by which the stress is increased. This increase in stress is termed the stress concentration factor. A crack in a brittle material will have quite a pointed tip and hence a small radius. Such a crack thus produces a large increase in stress at its tip. One way of arresting the progress of such a crack is to drill a hole at the end of the crack to increase its radius and so reduce the stress concentration. A crack in a ductile material is less likely to lead to failure than in a brittle material because a high stress concentration at the end of a notch leads to plastic flow and so an increase in the radius of the tip of the notch. The result is then a decrease in the stress concentration. 20.3.2 Speed of loading Another factor which can affect the behavior of a material is the speed of loading. A sharp blow to the material may lead to fracture where the same stress applied more slowly would not. With a very high rate of application of stress there may be insufficient time for plastic deformation of a material to occur and so what was, under normal conditions, a ductile material behaves as though it were brittle.
Dr. Saad B. H. Farid
17
Materials Selection Lecture otes
These otes is by oway a Replacement of Classroom Attendence !!!
The Charpy and Izod tests give a measure of the behavior of a notched sample of material when subject to a sudden impact load. The results are expressed in terms of the energy needed to break a standard size test piece; the smaller the energy needed the easier it is for failure to occur with shock loads in service. The smaller energies are associated with materials which are termed brittle; ductile materials needing higher energies for fracture to occur. 20.3.3 Temperature The temperature of a material can affect its behavior when subject to stress. Many metals which are ductile at high temperatures are brittle at low temperatures. For example, steel may behave as a ductile material above, say, 0C but below that temperature it becomes brittle. Figure 20.9 shows how the impact test results might vary with the temperature at which a test piece was tested for such material. The ductilebrittle transition temperature is thus of importance in determining how a material will behave in service. The transition temperature with steel is affected by the alloying elements in the steel. Manganese and nickel reduce the transition temperature. Thus for low-temperature work, a steel with these alloying elements is to be preferred. Carbon, nitrogen and phosphorus increase the transition temperature. 20.3.4 Thermal shocks Pouring hot water into a cold glass can cause the glass to crack. This is a case of thermal shock loading. The layer of glass in contact with the hot water tends to expand but is restrained by the colder outer layers of the glass, these layers not heating up quickly because of the poor thermal conductivity of glass. The result is the setting up of stresses which can be sufficiently high to cause failure of the brittle glass. Thermal shock was discussed in more detail in Section 13.6 in connection with ceramics. 20.4 Griffith crack theory: In 1920, A. A. Griffith advanced the theory that all materials contain small cracks but that a crack will not propagate until a particular stress is reached, the value of this stress depending on the length of the crack.
Dr. Saad B. H. Farid
18
Materials Selection Lecture otes
These otes is by oway a Replacement of Classroom Attendence !!!
For the materials engineer any defect (chemical, inhomogeneity, crack, dislocation, and residual stress) is considered as Griffith crack, i.e. an in-homogeneity that can cause stress concentration which can be developed to failure at particular value of stress. 20.5 Fracture toughness Gc Fracture toughness can be defined as being a measure of the resistance of a material to fracture, i.e. a measure of the ability of a material to resist crack propagation. 20.5.1 Stress intensity factor Another way of considering the toughness of a material is in terms of the intensity factor at the tip of a crack that is required for it to propagate. Note that the stress concentration factor (see Section 20.3.1) and the stress intensity factor are not the same quantity. The stress concentration factor is the ratio of the maximum stress in the vicinity of a notch, crack or change in section to the remotely applied stress. The stress intensity factor K is used for the quantity (c) and is usually quoted in the units of MN m-3/2. Thus, equation [8] gives for the value of the critical stress intensity factor Kc, often termed the fracture toughness, when crack propagation can occur: Kc2 = Gc E E is the Modulus of elasticity, Hence the stress for crack propagation to occur is: = Kc/(c) The smaller the value of Kc means the less tough the material. The critical stress intensity factor Kc is a function of the material and plate thickness concerned. The thickness factor is because the form of crack propagation is influenced by the thickness of the plate. In thin plates, failure is by shear on planes at 45o to the tensile forces across the crack (Figure 20.12). Thicker plates show a central flat fracture with 45o shear fractures at the sides; the thicker the plate the greater the amount of central flat fracture. The effect of this on the value of the critical stress intensity factor is shown by the graph in Figure 20.13. High values of Kc occur with thin sheets and decreases as the sheet thickness is increased to become almost constant at large thicknesses. At such large thicknesses, the portion of the fracture area which has sheared is very small, most of the fracture being flat and at right angles to the tensile forces. This lower limiting value of the critical stress intensity factor is called the plane strain fracture toughness and is denoted by K1c. This factor is solely a property of the material. It is the value commonly used in design for all but the very thin sheets; it being the lowest value of the critical stress
Dr. Saad B. H. Farid
19
Materials Selection Lecture otes
These otes is by oway a Replacement of Classroom Attendence !!!
intensity factor and hence the safest value to use. The lower the value of K1c means the less tough the material is assumed to be. Table 20.2 gives some typical values.
20.5.2 Factors affecting fracture toughness 1- Composition of the material Different alloy systems have different fracture toughness. Thus, for example, many aluminium alloys have lower values of plane strain toughness than steels. Within each alloy system there are, however, some alloying elements which markedly reduce toughness e.g. phosphorus and sulphur in steels. 2- Heat treatment
Dr. Saad B. H. Farid
20
Materials Selection Lecture otes
These otes is by oway a Replacement of Classroom Attendence !!!
Heat treatment can markedly affect the fracture toughness of a material. Thus, for example, the toughness of steel is markedly affected by changes in tempering temperature. 3- Material thickness See Figure 20.13. 4- Service conditions Service conditions such as temperature, corrosive environment and fluctuating loads can all affect fracture toughness. 20.6 Failure case studies In considering the failure of a material there are a number of key questions that need to be addressed: 1. Was the material to specification? 2. Was the right material chosen for the task? 3. Was the material correctly heat treated? 4. Was the situation in which the material was used, and hence the properties thought necessary for the material, wrongly diagnosed? 5. Were factors such as stress, stress concentrations, potential flaw sizes considered in the design? 6. Did an abnormal situation occur, perhaps as a result of human error, and was the material therefore subject to unforeseen (unexpected) conditions? 7. Has the assembly of the structure, e.g. welding, been correctly carried out? The main types of failure are: 1. Failure by fracture due to static overload, the fracture being either brittle or ductile. 2. Buckling in columns due to compressive overloading. 3. Yield under static loading which then leads to misalignment or overloading on other components.. 4. Failure due to impact loading or thermal shock. 5. Failure by fatigue fracture (see Chapter 21). 6. Creep failure (see Chapter 22). 7. Failure due to the combined effects of stress and corrosion (see Chp. 23). 8. Failure due to excessive wear. Questions: Explain what is meant by fracture toughness. Explain the terms stress intensity factor K, critical stress intensity factor Kc and plane strain fracture toughness K1c. What factors can affect the values of the plane strain fracture toughness?
Dr. Saad B. H. Farid
21
Materials Selection Lecture otes
These otes is by oway a Replacement of Classroom Attendence !!!
Fatigue
Lecture 04
In service many components undergo thousands, often millions, of changes of stress. Some arc repeatedly stressed and unstressed, while some undergo alternating stresses of compression and tension. For others the stress may fluctuate about some value. Many materials subject to such conditions fail, even though the maximum stress in any individual stress change is less than the fracture stress determined by a simple tensile test. Such a failure, as a result of repeated stressing, is called Fatigue failure. It has been said that fatigue causes at least 80% of the failures in modern engineering components. 21.2 Fatigue failure A macroscopic examination of the surfaces of components that have failed by cyclic loading shows distinct surface markings which are characteristic of fatigue failure. Figure 21.1 shows the various stages involved in fatigue failure. 1. A fatigue crack often starts at some point of stress concentration (Figure 21.1(a)). This point of origin of the failure can be seen on the failed material as a smooth, flat, semicircular or elliptical region, and is often referred to as the nucleus. 2. Surrounding the nucleus is a burnished zone with ribbed markings resembling seashell markings or the marks left on a beach by the tide (Figure 21.1(b)). These markings are produced by the crack propagating relatively slowly through the material and the resulting fractured surfaces rubbing together during the alternating stressing of the component. 3. When the crack is long enough, it spontaneously propagates to give a sudden abrupt fracture of the remaining material (Figure 21.1(c)).
Dr. Saad B. H. Farid
22
Materials Selection Lecture otes
These otes is by oway a Replacement of Classroom Attendence !!!
We can explain fatigue failure as being the result of slip, the direction of which reverses with the stress cycle. Thus slip may occur in one direction on one slip plane and the reverse way on an adjacent slip plane during the reverse stress cycle. The above stages in the growth of fatigue cracks are thus: 1- The nucleus and initial crack This is the initiation zone of the fatigue crack. It can be some initial surface defect of the material or arise from the slip causing surface disturbances at the free surface termination of bands of slip (Figure 21.2). Further application of stress results in the nucleus turning into a crack. The repeated slips that take place results in an increase in dislocation density as a result of the plastic deformation and cause the material to work harden. 2- Crack propagation The fatigue crack slowly propagates with the metal extruded from the slip bands forming the ridges in the burnished zone. 3- Failure The stage 2 crack continues growing until, when the critical crack length is reached, there is a complete brittle or ductile failure of the material. 21.3 Fatigue tests: Fatigue tests can be carried out in a number of ways; the way used being the one needed to simulate the type of stress changes that will occur to the material of the component when in service. For example there are bending-stress machines which bend a test piece of the material alternatively one way and then the other (Figure 21.3(a)), torsionalfatigue machines which twist the test piece alternatively one way and then the other (Figure 21.3(b)) and another type which produces alternating tension and compression by direct stressing (Figure 20.3(c)).
Dr. Saad B. H. Farid
23
Materials Selection Lecture otes The tests can be carried out with stresses which alternate about zero stress (Figure 21.4(a)), apply a repeated stress which varies from zero to some maximum stress (Figure 21.4(b)) or apply a stress which varies about some stress value and does not necessarily reach zero at all (Figure 21.4(c)). With (a), the stress varies between + and -, tensile stress being denoted by a positive sign and compressive by a negative sign. The stress range is thus 2 and the mean stress zero. With (b), the mean stress is half the stress range. With (c), the mean stress is more than half the range. The following are standard definitions used to describe the variables:
These otes is by oway a Replacement of Classroom Attendence !!!
Stress range = maximum stress - minimum stress [1] Stress amplitude S = (maximum stress-minimum stress) [2] Mean stress = (maximum stress + minimum stress) [3] Load ratio = maximum stress/minimum stress [4]
Figure 21.4 Alternating Stress (a) about zero stress (b) from zero to some maximum, (c) about some stress value.
Dr. Saad B. H. Farid
24
Materials Selection Lecture otes
These otes is by oway a Replacement of Classroom Attendence !!!
21.3.1 S- graphs The number of cycles of stress reversal that a specimen can continue before failure occurs depends on the stress amplitude, the bigger the stress amplitude the smaller the number of cycles that can be continued. The fatigue limit is the stress amplitude at a particular number of cycles which will result in failure. For Figure 21.5(a), there is a stress amplitude SD, called the endurance limit, for which the material well endure an infinite number of stress cycles with smaller stress amplitudes. For any stress amplitude greater than the endurance limit, failure will occur if the material undergoes a sufficient number of stress cycles. For Figure 21.5(b), there is no stress amplitude at which failure cannot occur. For such materials a fatigue limit SN may be quoted for a particular number of cycles N.
Figure 21.5 Typical S-N graphs for: (a) a steel (b) an aluminium alloy. 21.4 Factors affecting fatigue properties The main factors affecting the fatigue properties of a component are: 1- Stress concentrations; Stress concentrations are caused by such design features as sudden changes in cross-section, keyways, holes and sharp corners. Figure 21.11 shows the effect of the stress concentration produced by a small hole on the S-N graph for a steel. Stress concentrations are also caused by surface scratches, dents, machining marks and corrosion which have left the surface in a roughened form. Generally, Stress concentrations reduce the fatigue lifetime for a component. 2- Residual stresses; Residual stresses can be produced by many fabrication and finishing processes. If the stresses produced are such that the surfaces have compressive residual stresses then the fatigue properties are
Dr. Saad B. H. Farid
25
Materials Selection Lecture otes
These otes is by oway a Replacement of Classroom Attendence !!!
improved, but if tensile surface residual stresses are produced then poorer fatigue properties result. The case hardening of steels by carburizing results in compressive surface residual stresses and so improves the fatigue resistance (Figure 21.13). Many machining processes result in the production of tensile surface residual stresses and so result in poorer fatigue resistance.
3- Temperature; An increase in temperature can lead to a reduction in fatigue properties as a consequence of oxidation or corrosion of the metal surface is increasing. For example, the nickel-chromium alloy Nimonic90 undergoes surface degradation at temperatures of about 700 to 800oC and, as a consequence, there is poorer fatigue performance at these temperatures. 4- Microstructure of alloy; The microstructure of an alloy is a factor in determining the fatigue properties. This is because the origins of fatigue failure are extremely localized, involving slip at crystal planes. Because of this, the composition of an alloy and its grain size can affect its fatigue properties. Inclusions, such as lead in steel, can act as nuclei for fatigue failure and so impair fatigue properties.
Dr. Saad B. H. Farid
26
Materials Selection Lecture otes
These otes is by oway a Replacement of Classroom Attendence !!!
21.4.1 Metals and fatigue resistance: Steels typically have an endurance limit which is generally about 0.4 to 0.5 times the tensile strength of the material. Inclusions in steels can impair the fatigue properties, thus steels with lead or sulphur present to enhance machinability are to be avoided if poor fatigue properties are required. The optimum structure for good fatigue properties for steels is tempered martensite. Cast steels and cast irons tend so have relatively low endurance limits. 21.4.2 The Fatigue properties of polymers: Fatigue tests can be carried out on polymers in the same way as on metals. A factor not present with metals is that when a polymer is subject to an alternating stress it becomes significantly warmer. The faster the stress is alternated, i.e. the higher the frequency of the alternating stress the grater the temperature rise. Under very high-frequency alternating stresses, the temperature rise may be large enough to melt the polymer. Thus with polymers fatigue failure may be either as a consequence of fatigue crack initiation and propagation or by polymer softening which occurs to such an extent that the polymer component can no longer support the load. 21.4.3 The fatigue properties of composites: The fatigue properties of composite materials depend on such factors as the interaction between the mechanical properties of the matrix and the reinforcement, the strength of the bond between the two, the volume fractions of the two, the direction and type of loading, the loading frequency and the temperature. For a random discontinuous glass fiberreinforced polymer composite, the various stages of failure might be: 1- Development of micro cracks as a result of de-bonding of reinforcement from matrix. 2- The micro cracks propagate in the matrix and result in the matrix-resin cracking. 3- Finally the cracks may have propagated sufficiently for separation of the reinforcement from the matrix. Example Questions: Explain what is meant by fatigue failure Describe the various stages in the failure of a component by failure Explain the terms fatigue limit and endurance limit Explain S-N graph for an aluminium alloy, steel, what is the difference?
Dr. Saad B. H. Farid
27
Materials Selection Lecture otes
These otes is by oway a Replacement of Classroom Attendence !!!
Creep
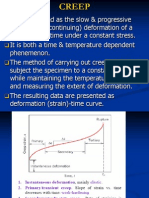
Creep: is the deformation of a material with the passage of time when the material is subject to a constant stress. There are many situations where a piece of material is exposed to a stress for a prolonged period of time where the creep takes place.
Lecture 05
22.2 Creep Data Figure 22.1 shows the essential features of a creep test. A constant stress is applied to the test piece, sometimes by the simple method of suspending loads from it. Because creep tests with metals arc usually performed at high temperatures a thermostatically controlled heater surrounds the test piece the temperature of the test piece is generally measured by a thermocouple Figure 22.2 shows the general form of results from a creep test. Following an instantaneous elastic strain region, the curve generally has three parts. During the primary creep period the strain is changing but the rate at which it is changing with time decreases. During the secondary creep period the strain increases steadily with time at a constant rate. During the tertiary creep period the rate at which the strain is changing increases and eventually causes failure.
Dr. Saad B. H. Farid
28
Materials Selection Lecture otes
These otes is by oway a Replacement of Classroom Attendence !!!
A family of graphs can be produced that show the creep for different initial stresses at a particular temperature (Figure 22.3(a)) and for different temperatures for a particular initial stress (Figure 22.3(b)). 22.2.2 Stress to rupture Because of creep, an initial stress, which did not produce early failure, can result in failure after some period of time. Such an initial stress is referred to as the stress to rupture in some particular time. Thus, an acrylic plastic may have a rupture stress of 50 MPa at room temperature for failure in one week. The value of the stress to rupture depends, for a particular material, on the temperature and the time. Table 22.1 shows the stress to rupture data that might be quoted for a 0.2% plain carbon steel. The data means that at 400C the carbon steel will rupture after 1000 hours if the stress is 295 MPa. If the steel at 400C is required to last for 10 000 hours then the stress must be below 147 MPa. 1f however, the temperature is 500C, then to last 100 000 hours the stress must be below 30 MPa. The stress to rupture a material in a particular time depends on the temperature.
Dr. Saad B. H. Farid
29
Materials Selection Lecture otes
These otes is by oway a Replacement of Classroom Attendence !!!
22.2.4 Stress relaxation So far in this chapter, in the discussion of creep we have assumed that the stress remained constant and the strain varied with time. However, there arc situations where the strain is kept constant, e.g. a bolt clamping two plates together. If a bolt is tightened up then a consequence of creep is that the bolt will gradually become less tight with time. Creep causes the stress applied by the bolt to relax with time. Consider a bolt which is tightened onto a rigid component. The total strain for a strained bolt will be constant and not vary with time since the length of the stretched shank remains constant. The total strain at any time is the sum of the elastic strain el and the creep strain cr. Total strain= el + cr As the creep strain increases with time so the elastic strain decreases. Figure 22.7 shows the general picture. The elastic strain is /E, where the stress is applied to the bolt and E its modulus of elasticity. Thus as the elastic strain decreases the stress decreases. 22.3 Creep with metals/stages Following the initial instantaneous strain, primary creep occurs as a result of the movements of dislocations. Initially the dislocations can move easily and quickly, hence the rapid increase in strain with time. However, dislocation pile-ups start to occur at grain boundaries, i.e. work hardening occurs, and this slows down the rate of creep. Work hardening occurs more rapidly than recovery processes. The term recovery is used to describe the effects of diffusion resulting in the unlocking of dislocations tied up with obstructions and the mutual annihilation of dislocations on meeting each other. The amount of recovery that occurs depends on the temperature; the higher the temperature the greater is the amount of recovery. Therefore, at low temperatures when there is little recovery the work hardening quite rapidly reduces the rate of creep and we get the logarithmic creep described by figure 22.4. At higher temperatures the work hardening effect is reduced by recovery and so the creep rate is less reduced. As a result we obtain the power-law creep described by fig22.4.
Dr. Saad B. H. Farid
30
Materials Selection Lecture otes
These otes is by oway a Replacement of Classroom Attendence !!!
During the secondary creep stage, the rate of recovery is sufficiently fast to balance the rate of work hardening. As a result, the material creeps at a steady rate. In addition to dislocation movements there is also, during this stage of creep, some sliding of grains past each. This is referred to as grain boundary slide. This mechanism becomes more significant the higher the temperature. Fine-grained materials contain more grain boundaries per unit volume of a material than coarsegrained materials and thus it is more difficult for significant grain boundary slide to occur with fine-grained materials. Thus fine-grained materials tend to be more creep resistant than coarse-grained ones. With tertiary creep, the rate of strain increases rapidly with time and finally results in fracture. This accelerating creep rate occurs when voids or micro cracks occur at grain boundaries. These are the result of vacancies migrating to such areas and grain boundary slide. The voids grow and link up so that finally the material fails at the grain boundaries. 22.3.1 Creep-resistant metals The movement of dislocations is an essential feature of creep; hence creep can be reduced by reducing such movement. One mechanism is the use of alloying elements which give rise to dispersion hardening. Finely dispersed precipitates are effective barriers to the movement of dislocations. The Nimonic series of alloys has excellent creep resistance and is based on an 80% nickel20% chromium alloy with the addition of small amounts of titanium, aluminium, carbon or other elements to form fine precipitates. Table 22.2 shows the effects of these alloying additions on the creep properties. The Nimonic alloys also have excellent resistance to corrosion at high temperatures and so this, combined with their high creep resistance, makes them useful for high-temperature applications such as gas turbine blades.
Dr. Saad B. H. Farid
31
Materials Selection Lecture otes
These otes is by oway a Replacement of Classroom Attendence !!!
22.3.2 Factors affecting creep with metals The main factors affecting creep behavior with metals are: 1- Temperature The higher the temperature; is the greater the creep at a particular stress (Figure 20.3(a)). 2- Stress The higher the stress at a particular temperature, is the greater the creep (Figure 20.3(b)). 3- Metal Figure 22.8 shows how the stress to rupture different materials in 1000 hours varies with temperature. Al alloys fail at quite low stresses when the temperature rises above 200C. Titanium alloys can be used at higher temperatures before the stress to rupture drops to very low values, while stainless steel and nickel-chromium alloys offer better resistance to creep. 4- Alloy composition The creep behavior of an alloy can be affected by the addition of quite small amounts of precipitation forming elements (see Section 22.3.1). 5- Grain size Grain size can have an effect on the creep behavior of a material (see Section 22.3).
22.4 Creep with Polymers While creep is only generally significant for metals at high temperatures it can be significant with polymers at normal temperatures. The creep behavior of a polymer depends on the temperature and stress level, just like metals. It also depends on the type of plastic involved; flexible plastics show more creep than stiff ones.
Dr. Saad B. H. Farid
32
Materials Selection Lecture otes
These otes is by oway a Replacement of Classroom Attendence !!!
Figure 22.9 shows how the strain on a sample of polyacetal at 20C varies with time for different stresses. The higher the stress causes the greater the creep. As can be seen from the graph, the polymer creeps quite significantly in a period of just over a week, even at relatively low stresses. Often such graphs have a log scale for both the strain and the time.
Fig. 22.9: Creep behavior of polyacetal at different stresses at constant temp. 22.4.2 Recovery from creep On removing the load from a polymer, the material can recover most, or even all, of the strain given sufficient time. This is different from metals where the strain produced by creep is not recoverable. A consequence of creep and recovery with plastics is that where a component is subject to an intermittent load, under load the component creeps but when the load is removed the component recovers. The time taken to recover depends on the initial strain and the time for which the material was creeping under the load.
A fractional recovery strain value of 1 means that; the material has completely recovered and is back to its original size.
Dr. Saad B. H. Farid
33
Materials Selection Lecture otes
These otes is by oway a Replacement of Classroom Attendence !!!
A reduced time value of 1 means that the recovery time is the same as the time it was under load and during which creep occurred. Figure 22.14 shows a graph for the recovery from creep of nylon 66. 22.4.3 Viscoelastic behavior of polymers Metals at room temperature tend to behave like elastic materials so that when a load is applied the metal almost instantly becomes extended arid maintains the same extension regardless of time. When the load is removed the metal almost instantly recovers its original dimensions (assuming it was not loaded beyond its elastic limit). Polymers behave as Viscoelastic Materials because their behavior is part way between that of a viscous fluid and that of an elastic solid. Unlike metals, there is a comparatively slow response to loading and unloading. The response is to some extent like the flow of a very viscous liquid. With such a liquid, the application of forces causes the liquid to flow and it continues to flow as long as the forces are applied. Figure 22.15 shows the typical form of a straintime graph for a polymer when loaded and then unloaded. Example Problems: 1. Explain what is meant by creep. 2. Describe the form of a typical strain-time graph resulting from a creep test and the various stages involved in the creep. 3. Describe the effects of (a) increased stress and (b) increased temperature on the creep behavior of materials. 4. Figure 22.21 shows how the strain changes with time for two different polymers when subject to a constant stress. Which material creeps most?
Dr. Saad B. H. Farid
34
Materials Selection Lecture otes
These otes is by oway a Replacement of Classroom Attendence !!!
Exam1, sample questions
Lecture 06 1, 2 - Introduction Materials types, Their Properties, Classification and Applications, The 32 sample questions are placed at the end of the lecture. 3- Fracture, failure In considering the failure of a material, what are the key questions that need to be addressed? State the main types of failure of materials. Given a fractured metal specimen, how to determine whether the fracture was ductile or brittle? How does the presence of a notch or an abrupt change in section have an effect on the failure behavior? Draw the stages in ductile fracture. Describe the stages in ductile fracture. When the ductile fracture taking place? Describe its appearance. When the brittle fracture taking place? Describe its appearance. Describe fracture in amorphous polymers. Describe fracture in almost crystalline polymers. Describe the appearance of the fracture surface for brittle fracture a polymer. Describe fracture in ceramics. Describe fracture in composites. State factors that affecting the fracture of a material. State Griffith crack theory. What is the crack in Griffith crack theory? State the equation of the fracture toughness Kc. State the equation of the stress necessary for crack propagation .
Dr. Saad B. H. Farid
35
Materials Selection Lecture otes
These otes is by oway a Replacement of Classroom Attendence !!!
Draw the effect of thickness on crack propagation. Draw the effect of plate thickness on the critical stress intensity factor K1c. Define fracture toughness. Describe factors affecting fracture toughness. 4- Fatigue As a Materials Engineer, write an assay on the Fatigue phenomena, guided by the following issues: 1- The definition of the Fatigue, 2- The conditions at which the Fatigue takes place, 3- The stages of the Fatigue failure, 4- The difference between Fatigue of metals and that of polymers, 5- Describe S- graphs for: (a) Steel, (b) an Aluminium alloy, 6- State fatigue; a. stress range= b. stress amplitude S= c. mean stress= d. load ratio= ------------------------7- The three test modes of fatigue. 5- Creep As a Materials Engineer, write an assay on the creep phenomena, guided by the following issues: 2- The definition of the creep, 3- The conditions at which the creep takes place, 4- The stages of the creep phenomena, 5- The difference between creep of metals and that of polymers, 6- Improving the materials resistance to Creep,
Dr. Saad B. H. Farid
36
Materials Selection Lecture otes 7- Recovery from Creep, Draw typical creep graph for a metal.
These otes is by oway a Replacement of Classroom Attendence !!!
Draw creep graphs showing the effect of: a- Different ambient temperatures, b- Different applied stresses. Describe factors affecting creep properties. Describe the stages of the growth of fatigue crack. Draw the stages of the growth of fatigue crack. 6- Exam 1 ext: Lecture 07
Dr. Saad B. H. Farid
37
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
Selection for Properties
Lecture 07-08
24.1 Introduction A number of questions need to be answered before a decision can be made as to the specification required of a material and hence a decision as to the optimum material for a particular task. The questions can be grouped under four general headings: 1. What properties are required? 2. What are the processing requirements and their implications for the choice of material? 3. What is the availability of materials? 4. What is the cost? The following indicate the type of questions that are likely to-be considered in trying to arrive at answers to the above general questions. Properties: 1. What mechanical properties are required? This means consideration of such properties as strength, stiffness, hardness, ductility, toughness, fatigue resistance, wear properties, etc. Coupled with this question is another one: Will the properties be required at low temperatures, about room temperature or high temperatures? 2. What chemical properties are required? This means considering the environment to which the material will be exposed and the possibility of corrosion. 3. What thermal properties are required? This means consideration of such properties as specific heat capacity, linear coefficient of expansion and thermal conductivity. 4. What electrical properties are required? For example, does the material need to be a good conductor of electricity or perhaps an insulator? 5. What magnetic properties are required? Does the material need to have soft or hard magnetic properties or perhaps be essentially non-magnetic? 6. What dimensional conditions are required? For example, does the material need to be capable of a good surface finish, have dimensional stability, be flat, have a particular size, etc.
Dr. Saad B. H. Farid
38
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
Processing Parameters: 1. Are there any special processing requirements which will limit the choice of material? For example, does the material have to be cast or perhaps extruded? 2. Are there any material treatment requirements? For example, does the material have to be annealed or perhaps solution hardened? 3. Are there any special tooling requirements? For example, does the hardness required of a material mean special cutting tools are required? Availability: 1. Is the material readily available? Is it, for example, already in store, or perhaps quickly obtainable from normal suppliers? 2. Are there any ordering problems for that material? Is the material only available from special suppliers? Is there a minimum order quantity? 3. What form is the material usually supplied in? For example, is the material usually supplied in bars or perhaps sheet? This can affect the processes that can be used. Cost: 1. What is the cast of the raw material? Could a cheaper material be used? 2. What quantity is required? What quantity of product is to be produced per week, per month, per year? What stocking policy should be adopted for the material? 3. What are the cost implications of the process requirements? Does the process require high initial expenditure? Are the running costs high or low? Will expensive skilled labor be required? 4. What are the cost penalties for over specification? If the material is, for example stronger than is required, will this significantly increase the cost? If the product is manufactured to higher quality than is required, what will be the cost implications?
Dr. Saad B. H. Farid
39
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
1.3.2 Mechanical properties: stress and strain When a material is subject to external forces which stretch it and make it extend, then it is said to be in tension (Figure 1.1(a)). When a material is subject to forces which squeeze it and make it contract, then it is said to be in compression (Figure 1.1(b)). An object, in some situations, can be subject to both tension and compression, e.g. a beam (Figure 1.2) which is being bent, the bending causing the upper surface to contract and so be in compression and the lower surface to extend and be in tension. In discussing the application of forces to materials, an important aspect is often not so much the size of the force itself as the size of the force applied per unit area. If we stretch a strip of material by a force F applied over its cross-sectional area A, then the force applied per unit area is F/A (Figure 1.3), this being termed the stress: Stress = force/area [2] Stress has the units of pascal (Pa), with 1 Pa = 1 N/m2. Stresses are often rather large and so prefixes are used with the unit. Thus 1 MPa (mega pascal) = 1 000 000 Pa. The area used in calculations of stress is generally the original area that existed before the application of the forces, not the area after the force has been applied. This stress is thus sometimes referred to as the engineering stress, the term true stress being used for the force divided by the actual area existing in the stressed state. When a material is subject to tensile or compressive forces, it changes in length, the term strain, (Figure 1.4), being used for the fractional change in length: Strain= change in length/original length [3] Since strain is a ratio of two lengths it has no units. Strain is frequently expressed as a percentage. Strain as a %= change in length/original length 100 [4]
Dr. Saad B. H. Farid
40
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
Thus the strain of 0.01 as a percentage is 1%, i.e. the change in length is 1% of the original length. The behavior of materials subject to tensile and compressive forces can be described in terms of their stress-strain behavior. If gradually increasing tensile forces are applied to, say, a strip of mild steel, then initially when the forces are released the material springs back to its original shape. The material is said to be elastic. If measurements are made of the extension at different forces and a graph plotted, then the force needed to produce a given extension is found to be proportional to the extension and the material is said to obey Hookes law. Figure 1.5(a) shows a graph when Hookes law is obeyed. Such a graph applies to only one particular length and cross-sectional area of a particular material. We can make the graph more general so that it can be applied to other lengths and cross-sectional areas of the material by dividing the extension by the original length to give the strain and the force by the cross-sectional area to give the stress. Then we have, for a material that obeys Hookes law: Stress Strain [5] The stress-strain graph (Figure 15(b)) is just a scaled version of the forceextension graph in Figure 15(a). Figure 1.6 shows the type of stress-strain graph which would be given by a sample of mild steel. Initially the graph is straight line and the material obeys Hooks law. The point at which the straight line behavior is not followed is called the limit of proportionality. With low stresses the material springs back completely to its original shape when the stresses are removed, the material being said to be elastic. At higher forces this does not occur and the material is then said to show some plastic behavior. The term plastic is used for that part of the behavior which results in permanent deformation. This point often coincides with the point on a stress-strain graph at which the graph stops being a straight line, i.e. the limit of proportionality. The stress at which the material
Dr. Saad B. H. Farid
41
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
starts to behave in a non-elastic manner is called the elastic limit. The term tensile strength is used for the maximum value of the stress that the material can withstand without breaking, the compressive strength being the maximum compressive stress the material can withstand without becoming crushed. With some materials, e.g. mild steel, there is a noticeable dip in the stressstrain graph at some stress beyond the elastic limit. The strain increases without any increase in load. The material is said to have yielded and the point at which this occurs is the yield point. Some materials, such as aluminium alloys (Figure 1.7), do not show a noticeable yield point and it is usual here to specify proof stress. The 0.2% proof stress is obtained by drawing a line parallel to the straight line part of the graph but starting at a strain of 02%. The point where this line cuts the stress-strain graph is termed the 0.2% yield stress. A similar line can be drawn for the 0.1% proof stress. The stiffness of a material is the ability of a material to resist bending. When a strip of material is bent, one surface is stretched and the opposite face is compressed, as was illustrated in Figure 12. The mort a material bends, the greater is the amount by which the stretched surface extends and the compressed surface contracts. Thus, a stiff material would be one that gave a small change in length when subject to tensile or compressive forces. This means a small strain when subject to tensile or compressive stress and so a large value of stress/strain and hence a steep initial gradient of the stressstrain graph (Figure 1.8). This gradient is called the modulus of elasticity (or Young's modulus), symbol E: Modulus of elasticity E= stress/strain [6] The units of the modulus are the same as those of stress, since strain has no units. With 1 GPa =109 Pa, typical values are about 200 GPa for steels and 70 GPa for aluminium alloys. For most engineering materials, the modulus of elasticity is the same in tension as in compression. Figure 1.9 shows the stress-strain graphs for a number of materials.
Dr. Saad B. H. Farid
42
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
Figure 1.9: The stress-strain graphs for a number of materials. a: Cast Iron The stress-strain graph for cast iron (Figure 1.9(a)) is virtually just a straight line with virtually all elastic behavior and little plastic deformation. The slight curved part at the top of the graph indicates a small departure from straight-line behavior and a small amount of plastic behavior. The graph gives the limit of proportionality as about 280 MPa, the tensile strength about 300 MPa and the modulus of elasticity about 200 GPa. b: Glass The stress-grain graph for glass (Figure 1.9(b)) has a similar shape to that for cast iron, with virtually all elastic behavior and little plastic deformation. The graph indicates the limit of proportionality is about 250 MPa, the tensile strength about 260 MPa and the modulus of elasticity about 70 GPa. c: Mild steel
Dr. Saad B. H. Farid
43
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
The stress-strain graph for mild steel (Figure 1.9(c)) shows a straight-line portion followed by a considerable amount of plastic deformation. Much higher strains are possible than with cast iron or glass, i.e. mild steel stretches much more. The limit or proportionality is about 240 MPa, the tensile strength about 400 MPa and the modulus of elasticity about 200 GPa. d: Polyethylene The stress-strain graph for polyethylene (Figure 1.9(d)) shows only a small region where elastic behavior occurs and a very large amount of plastic deformation possible. Very large strains are possible a length of such material being capable of being stretched to almost four times its initial length. The limit of proportionality is about 8 MPa, the tensile strength about 11 MPa and the modulus of elasticity about 0.1 GPa. e: Rubber The tensile strength is about 25 MPa. Very large strains are possible and the material shows an elastic behavior to very high strains. The modulus of elasticity is not so useful a quantity for elastomers as it refers to only a very small portion of the stress-strain graph. A typical modulus would be about 30 MPa. For some materials, the difference between the elastic limit stress and the stress at which failure occurs is very small, as with the cast iron in Figure 1.9(a) with very little plastic deformation occurring. Thus, the length of a piece of cast iron after breaking is not much different from the initial length. Such materials are said to be brittle, in contrast to a material which suffers a considerable amount of plastic strain before breaking, and as said to be ductile, e.g. the mild steel in Figure 1.9(c). If you drop a glass, a brittle material, and it breaks, then it is possible to stick all the pieces together again and restore the glass to its original shape. If a car is involved in a collision, the bodywork of mild steel is less likely to shatter like the glass but more likely to dent and show permanent deformation, i.e. the material has shown plastic deformation. Ductile materials permit manufacturing methods which involve bending them to the required shapes or using a press to squash the material into the required shape. Brittle materials cannot be formed to shape in this way. The percentage of elongation of a test piece after breaking is used as a measure of ductility: Percentage elongation = final length-initial length 100% initial length
Dr. Saad B. H. Farid
44
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
A reasonably ductile material, such as mild steel, will have a percentage elongation of about 20%, a brittle material such as a cast iron less than 1%. Thermoplastics tend to have percentage elongations of the order of 50 to 500%, thermosets of the order of 0.1 to 1%. Thermosets are brittle materials, thermoplastics generally not. The stress-strain properties of plastics depend on the rate at which the strain is applied, unlike metals where the strain rate is not usually a significant factor, and the properties change significantly when there is a change in temperature (Figure 1.10) with both the modulus of elasticity and the tensile strength decreasing with an increase in temperature. Because of that for many polymeric materials, as with the rubber in Figure 1.9(e), there as no initial straight line part of the stress-strain graph and a value for the modulus of elasticity cannot be arrived at, the secant modulus is sometimes quoted, this being the stress/strain value at on, strain (Figure 1.11). Table 1.1 gives typical values of yield stress or 0.2 % proof stress, tensile strength and modulus of elasticity for a range of materials. If you repeatedly flex a strip of material back and forth it is possible to break it without the stresses ever reaching the tensile or compression strength values. This method of breaking materials is termed fatigue and discussed in Chapter 21. Example A bar of material with a cross-sectional area of 50 mm2 is subject to tensile forces of 100 N. What is the tensile stress? The tensile stress is the force divided by the area and is thus = 2MPa Example A strip of material has a length of 50 mm. When it is subject to tensile forces it increases in length by 0.020 mm. What is the strain? The strain is the change in length divided by the original length and is thus = 0.0004, Expressed as a percentage, the strain is 004%.
Dr. Saad B. H. Farid
45
Materials Selection Lecture otes Example
These otes is by o way a Replacement of Classroom Attendance!!!
A material has a yield stress of 200 MPa. What tensile forces will be needed to cause yielding with a bar of the material with a cross-sectional area of 100 mm2? Since stress is force/area, then: Yield force = yield stress area = 20 kN Example A sample of an aluminium alloy has a tensile strength of 140 MPa, What will be the maximum force that can be withstood by a rod of that alloy with a cross-sectional area of 1 cm2? Since the tensile strength is maximum force/area: = 14.0 kN
Dr. Saad B. H. Farid
46
Materials Selection Lecture otes Example
These otes is by o way a Replacement of Classroom Attendance!!!
For a material with a tensile modulus of elasticity of 200 GPa, what strain will be produced by a stress of 4 MPa? Provided the stress does not exceed the limit of proportionality, since the modulus of elasticity is stress/strain; strain = 0.000 02, Expressed as a percentage, the strain is 0.002%. Example Which of the following plastics is the stiffest? ABS Polycarbonate Polypropylene PVC tensile modulus 2.5 GPa tensile modulus 2.8 GPa tensile modulus 1.3 GPa tensile modulus 3.1 GPa
The stiffest plastic is the one with the highest tensile modulus and so is the PVC. Example A 200 mm length of a material has a percentage elongation of 100%, by how much longer will a strip of the material be when it breaks? Using equation [7]: Change in length = % elongation original length = 20 mm 100 Example Which of the following materials is the most ductile? 80-20 brass, 70-30 brass, 60-40 brass, percentage elongation 50% percentage elongation 70% percentage elongation 40%
The most ductile material is the one with the largest percentage elongation, i.e. the 70-30 brass. Example A sample of carbon steel has a tensile strength of 400 MPa and a percentage elongation of 35%. A sample of an aluminium-manganese alloy has a tensile strength of 140 MPa and a percentage elongation of 100%. What does this data tell you about the mechanical behavior of the materials?
Dr. Saad B. H. Farid
47
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
The higher value of the tensile strength of the carbon steel indicates that the material is stronger and, for the same cross-sectional area, a bar of carbon steel could withstand higher tensile forces than a corresponding bar of the aluminium alloy. The higher percentage elongation of the carbon steel indicates that the material has a greater ductility than the aluminium alloy. Indeed the value is such as to indicate that the carbon steel is very ductile. The steel is thus stronger and more ductile. 24.2 Selection for static strength Static strength can be defined as the ability to resist a short-term steady load at moderate temperatures without breaking or crushing or suffering excessive deformations. If a component is subject to a uni-axial stress the yield stress is commonly taken as a measure of the strength if the material is ductile and the tensile strength if it is brittle. Measures of static strength are thus yield strength, proof stress, tensile strength, compressive strength and hardness, the hardness of a material being related to the tensile strength of a material. If the component is subject to biaxial or triaxial stresses, e.g. a shell subject to internal pressure, then there are a number of theories which can be used to predict material failure. The maximum principal stress theory, which tends to be used with brittle materials, predicts failure as occurring when the maximum principal stress reaches the tensile strength value, or the elastic limit stress value, that occurs for the material when subject to simple tension. The maximum shear stress theory, used with ductile materials, considers failure to occur when the maximum shear stress in the biaxial or triaxial stress situation reaches the value of the maximum shear stress that occurs for the material at the elastic limit in simple tension. With biaxial stress, this occurs when the difference between the two principal stresses is equal to the elastic limit stress. Another theory that is used with ductile materials is that failure occurs when the strain energy per unit volume is equal to the strain energy at the elastic limit in simple tension. It should be recognized that a requirement for strength in a component requires not only a consideration of the static strength of the material but also the design. Thus for bending, an I-beam is more efficient than a rectangular cross-section beam because the material in the beam is concentrated at the top and bottom surfaces where the stresses are high and is not wasted in regions where the stresses are low. A thin shell or skin can be strengthened by adding ribs or corrugations.
Dr. Saad B. H. Farid
48
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
For most ductile-wrought materials, the mechanical properties in compression are sufficiently dose to those in tension, that was for the more readily available tensile properties to be used as an indicator of strength in both tension and compression. Metals in the cast condition, however, may be stronger in compression than in tension. Brittle materials, such as ceramics, are generally stronger in compression than in tension. There are some materials where there is significant anisotropy, i.e. the properties depend on the direction in which it is measured. This can occur with, for example wrought materials where there are elongated inclusions and the processing results in them becoming orientated in the same direction, or in composite materials containing unidirectional fibers. The mechanical properties of metals are very much affected by the treatment they undergo, whether it be heat treatment or working. Thus it is not possible to give anything other than a crude comparison of alloys in terms of tensile strengths. The properties of polymeric materials are very much affected by the additives mixed in with them in their formulation and thus only a crude comparison of mechanical properties of different polymers is possible. There is also the problem with thermoplastics in that, even at 20C they can show quite significant creep and this is more marked as the temperature increases. Thus their strengths are very much time dependent. Unreinforced thermoplastics have low strengths when compared with most metals; however, their low density means they have a favorable strength to weight ratio. Table 24.1 gives a general comparison of tensile strengths of a range of materials, all the data referring to temperatures around about 20C. Table 24.2 gives a general comparison of typical specific strengths, i.e. tensile strength divided by the density, to give a measure of strength per unit mass. Table 24.3 gives commonly used steels for different levels of tensile strength, the relevant limiting ruling sections being quoted.
Dr. Saad B. H. Farid
49
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
The limiting ruling section is the maximum diameter of round bar at the centre of which the specified properties may be obtained. The reason for this is that during heat treatment, different rates of cooling occur at the centers of bars, or indeed any cross-section, due purely to differences in sizes and this affects the microstructure produced by the treatment.
Dr. Saad B. H. Farid
50
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
1.3.3 Mechanical properties: toughness
Lecture 09-10
The materials in many products may contain cracks or sharp corners or other changes in shape that can readily generate cracks. A tough material can be considered to be one that, though it may contain a crack, resists breaking as a result of the crack growing and running through the material. Think of trying to tear a sheet of paper or a sheet of some cloth. If there is an initial crack then the material is much more easily torn. In the case of the paper, the initial cracks may be perforations put there to enable the paper to be torn easily. In the case of a sheet of cloth, it may be the initial nick cut in the edge by a dressmaker to enable it to be torn easily. In the case of, say, the skin of an aircraft where there may be holes, such as windows or their fastenings, which are equivalent to cracks, there is a need for cracks not to propagate A tough material is required. Toughness can be defined in terms of the work that has to be done to propagate a crack through a material, a tough material requiring more energy than a less tough one. Consider a length of material being stretched by tensile forces. When a length of material is stretched by an amount x, as a result of a constant force F1 then the work done is the force x distance moved by point of application of force and thus work = F1 x1 Thus if a force-extension graph is considered (Figure 1.12), the work done, when we consider a very small extension, is the area of that strip under the graph. The total work done in stretching a material to an extension x, i.e. through an extension which we can consider to be made up of a number of small extensions with x=x1 +x2+x3+..., is thus: work = Fx1 + F2x2 + F3x3 + and so is the area under the graph up to x. If we divide both sides of this equation by the volume, i.e. the product of the cross-sectional area A of the strip and its length L, we have:
But the term in each bracket is just the product of the stress and strain. Thus the work done per unit volume of material is the area under the stress-strain
Dr. Saad B. H. Farid
51
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
graph up to the strain corresponding to extension x, The area under the stress-strain graph up to some strain is the energy required per unit volume of material to produce that strain. For a crack to propagate, a material must fail. Thus, the area under the stress-strain graph up to the breaking point is a measure of the energy required to break unit volume of the material and so for a crack to propagate. A large area is given by a material with a large yield stress and high ductility. Such materials can this be considered to be tough. An alternative way of considering toughness is the ability of a material to withstand shock loads. A measure of this ability to withstand suddenly applied forces is obtained by Impact tests, such as the Charpy and Izod tests (see Chapter 3). In these tests, a test piece is struck a sudden blow and the energy needed to break it is measured. A brittle material will require less energy than a ductile material. The results of such tests are often used as a measure of the brittleness of materials. 24.3 Selection for stiffness Stiffness can be considered to be the ability of a material to resist deflection when loaded. Thus if we consider a cantilever of length L subject to a point load F at its free end (Figure 24.1), then the deflection y at the free end is given by:
with E is the tensile modulus and I the second moment of area of the beam cross-section with respect to the neutral axis. Thus, for a given shape and length cantilever, the greater the tensile modulus results in the smaller the deflection. Similar relationships exist for other forms of beam. Hence we can state that the greater the tensile modulus the greater the stiffness. The deflection of a beam is a function of both E and I. This, for a given material, a beam can be made stiffer by increasing its second moment of area. The second moment of area of a section is increased by placing as much as possible of the material as far as possible from the axis of bending. Thus an I-section is a particularly efficient way of achieving stiffness. Similarly a tube is more efficient than a solid rod. Another situation which is related to the value of EI is the buckling of columns when subject to compressive loads. The standard equation used for
Dr. Saad B. H. Farid
52
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
buckling has it occurring for a column of length L when the load F reaches the value
This is Eulers equation. The bigger the value of EI the higher the load required to cause buckling. Hence we can say that the column is stiffer the higher the value of EI. Note that a short and stubby column is more likely to fail by crushing when the yield stress is exceeded rather than buckling. Buckling is, however, more likely to be the failure mode if the column is slender. The tensile modulus of a metal is little affected by changes in its composition or heat treatment. However, the tensile modulus of composite materials is very much affected by changes in the orientation of the fillers and the relative amounts. Table 24.4 shows typical tensile modulus values for materials at 20oC. 24.4 Selection for fatigue resistance The failure of a component when subject to fluctuating loads is as a result of cracks which tend to start at some discontinuity in the material and grow until failure occurs. The main factors affecting fatigue properties are stress concentrations caused by component design, corrosion, residual stresses, surface finish/treatment, temperature, the microstructure of the alloy and its heat treatment. Only to a limited extent does the choice of material determine the fatigue resistance of a component. In general, for metals the endurance limit or fatigue limit at about 107 to 108 cycles lies between about a third and a half of the static tensile strength. For steels the fatigue limit is typically between 0.4 and 0.5 that of the static strength Inclusions in the steel, such as sulphur or lead to improve machinability, can, however, reduce the fatigue limit. For grey cast iron the fatigue limit is about 0.4 that of the static strength for nodular and malleable irons, in the range 0.5 for ferritic grades, to 0.3 for the higher strength pearlitic malleable irons, for blackheart, whiteheart and the lower strength pearlitic malleable irons about 0.4. With aluminium alloys the end. limit is about 0.3 to 0.4 that of the static strength, for copper alloys about 0.4 to 0.5.
53
Dr. Saad B. H. Farid
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
Fatigue effects with polymers are complicated by the fact that the alternating loading results in the polymer becoming heated. This causes the elastic modulus to decrease and at high enough frequencies this may be to such an extent that failure occurs. Thus, fatigue in polymers is very much frequency dependent. 24.5 Selection for toughness Toughness can be defined as the resistance offered by a material to fracture. A tough material is resistant to crack propagation. A measure of toughness is given by two main measurements: 1 The resistance of a material to impact Loading which is measured in the Charpy or Izod tests by the amount of energy needed to fracture a test piece, the higher the energy the more ductile a material is. 2 The resistance of a material to the propagation of an existing crack in a fracture toughness test, this being specified by the plain strain fracture toughness K1C. The lower its value is the less tough the material. Table 24.5 gives typical values of the plane strain fracture toughness at 20C. Within a given type of metal alloy there is an inverse relationship between yield stress and toughness, the higher the yield stress the lower the toughness. Thus if, for instance, the yield strength of low alloy, quenched and tempered steels is pushed up by metallurgical means, then, the toughness declines. Steels become less tough with increasing carbon content and larger grain size. The toughness of plastics is improved by incorporating rubber or another tougher polymer, copolymerization, or incorporating tough fibers. For example, styrene-acrylonitrile (SAN) is brittle and far from tough. It can, however, be toughened with the rubber polybutadiene to give the tougher acrylonitrile-butadiene-styrene (ABS). 24.6 Selection for creep and temperature resistance The creep resistance of a metal can be improved by incorporating a fine dispersion of particles to impede the movement of dislocations. The
Dr. Saad B. H. Farid
54
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
Nimonic series of alloys, based on an 80/20 nickel-chromium alloy, have good creep resistance as a consequence of fine precipitates formed by the inclusion of small amounts of titanium, aluminium, carbon or other elements. Creep increases as the temperature increases and is thus a major factor in determining the temperature at which materials can be used. Another factor is due to the effect on the material of the surrounding atmosphere. This can result in surface attack and scaling which gradually reduces the cross-sectional area of the component and so its ability to carry loads. Such effects increase as the temperature increases. The Nimonic series of alloys have good resistance to such attack. Typically they can be used up to temperatures of the order of 900C. For most metals creep is essentially a high-temperature effect; however, this is not the case with plastics. Here creep can be significant at room temperatures. Generally thermosets have higher temperature resistance than thermoplastics; however, the addition of suitable fillers and fibers can improve the temperature properties of thermoplastics. Table 24.6 indicates typical temperature limitations for a range of materials.
Dr. Saad B. H. Farid
55
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
Lecture 11-15 1.3.4 Mechanical properties: hardness and wear The hardness of a material is a measure of the resistance of a material to abrasion or indentation A number of scales are used for hardness, depending on the method that has been used to measure it. The tensile strength for a particular material is roughly proportional to the hardness. Thus the higher the Hardness of a material, the higher is likely to be the tensile strength. Wear is the progressive loss of material from surfaces as a result of sliding or rolling contact between surfaces or from the movement of fluids containing particles over surfaces. Because wear is a surface effect, surface treatments and coatings play an important role in improving wear resistance. Lubrication can be considered to be a way of keeping surfaces apart and so reducing wear. A number of different mechanisms for wear have been identified: 1- Adhesive wear On an atomic scale, even smooth surfaces appear rough and thus when two surfaces are brought together; contact is made at only a few points (Figure 1.13). As a consequence, the forces holding the surfaces together can result in very high stresses at the few very small area of contact. Surface projections thus become plastically deformed by the pressure and can weld together. Sliding thus involves breaking these welded bonds, the breaks resulting in cavities being produced on one surface, projections on the other and frequently tiny abrasive particles. The term adhesive wear or scoring, galling or seizing, is used for this type of wear when two solid surfaces slide over one another under pressure. If the harnesses of the two surfaces are high, the wear rate can be reduced. Also, high strength, high toughness and ductility all contribute to reducing such wear, preventing the tearing of material from the surfaces.
Dr. Saad B. H. Farid 56
Materials Selection Lecture otes 2- Abrasive wear
These otes is by o way a Replacement of Classroom Attendance!!!
The term abrasive wear is used when material is removed from a surface by contact with hard particles; sliding resulting in the pushing out of the softer material by the harder material (figure 1.14). Such wear is common in machinery used to handle abrasive materials. Materials with a high hardness, high toughness and high strength are most resistant to such wear. 3- Corrosive wear When rubbing between surfaces takes place in a corrosive environment, surface reactions can take place and reaction products formed on the surfaces. These generally poorly adhere to the surfaces and the rubbing removes them. The process thus involves the repeated forming of reaction products and their removal by the rubbing. Lubricants can be used to separate surfaces and protect the surfaces from the corrosive environment, 4- Surface fatigue Adhesive and abrasive wear depends on direct contact between surfaces and can be prevented by separating the surfaces with lubricant film. However, with rolling with bearings wear can still occur though the surfaces are separated. This is because, although direct contact between the surfaces does not occur, the opposing surfaces experience large stresses transmitted through the lubricant film. As rolling proceeds, the stresses can become alternating and fatigue failure thus becomes possible for surface protrusions. Hence wear can occur. 1.3.5 Electrical properties: conductivity The electrical resistivity is a measure of the electrical resistance of a material, being defined by: ; where R is the resistance of a length L of a material of crosssectional area A (Figure 1 15) The unit of resistivity is the ohm meter (m). An e1ectrical insulator such as a ceramic will have a very high resistivity, typically of the order of 1010 m or higher. An electrical conductor such as copper will have a very low resistivity, typically of the order of l04 nm. The term semiconductor is used for those materials which have resistivities roughly halfway between conductors and insulators, i.e., of the order of 102 m.
Dr. Saad B. H. Farid 57
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
The electrical conductance G of a length of material is the reciprocal of its resistance and has the unit of -1. This unit is given a special name, the siemen (S). The electrical conductivity is the reciprocal of the resistivity:
The unit of conductivity is thus -1m-1 or S/m. Since conductivity is the reciprocal of the resistivity, an electrical insulator will have a very low conductivity; of the order of 10-10 S/m. while an electrical conductor will have very high conductivity, of the order of 108 S/m. Semiconductors have conductivities of the order of 10-1 S/m.
Table 1.2 shows typical values of resistivity and conductivity for insulators, semiconductors and conductors. Pure metals and many metal alloys have resistivities that increase when the temperature increases; some metal alloys do, however, Show increases in resistivities when the temperature increases. For semiconductors and insulators, the resistivity increases with an increase in temperature. Example Using the value of electrical conductivity given in Table 1.2, determine the electrical conductance of a 2m length of Nichrome wire at 200oC if it has a cross-sectional area of 1 mm2.
Dr. Saad B. H. Farid 58
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
Example Suggest a material that could be used for the heating element of an electric fire? The heating element must be a conductor of electricity. The power dissipated by the element is V2/R, thus the lower the resistance R the greater the power produced by a given voltage V. The material must also be able to withstand high temperatures without melting or oxidizing. Nichrome wire is commonly used. The wire is wound on a spiral around an insulating ceramic support. 1.3.6 Electrical properties: dielectrics When a pair of parallel conducting plates is connected to a d.c. supply (Figure 1.16), charge flows onto one of the plates and off the other plate. One of the plates becomes positively charged and the other negatively charged. The amount of charge Q on a plate, whether it is negative or positive, is proportional to the potential difference V between the plates. Hence: Q=CV where C is the constant of proportionality, called the capacitance. The unit of capacitance is the farad (F) when V is in volts and Q in coulombs. The factors determining the value of the capacitance are the plate area A, the separation d of the plates and the medium between them:
where is the factor, called the absolute permittivity, which is related to the medium between the plates. A more usual way of writing the equation is, however, in terms of how the permittivity of a material compares with that of a vacuum. Thus:
Dr. Saad B. H. Farid
59
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
where = ro. o is called the permittivity of free space and has a value of 8.85 x 10-12 F/m. r is called the relative permittivity. It has no units, merely stating the factor that must be used to multiply the permittivity of free space in order to obtain the permittivity of some material. For a vacuum the relative permittivity is 1, for plastics it is between about 2 and 3, for glass between 5 and 10. The relative permittivity is often termed the dielectric constant and the material between the conducting plates the dielectric. The relative permittivity, or dielectric constant, is the term used to describe the property of a material to store charge. The higher it is, the greater the amount of charge stored for a particular potential difference. If the potential difference between two plates separated by a dielectric is too high or the thickness of the dielectric is too small, the dielectric breaks down and the electrical charge can move through it between the two plates. The dielectric strength is a measure of the highest voltage that an insulating material can withstand without electrical breakdown. It is defined as:
The units of dielectric strength are volts per meter. Polyethylene has a dielectric strength of about 4 x 107 V/m. This means that a 1 mm thickness of polyethylene will require a voltage of about 40 000 V across it before it will break down. When an alternating current is applied to two plates separated by dielectric, a fraction of the energy is lost each time the current alternates. With a perfect dielectric, the current leads the voltage by 90o. However, a useful model we can adopt is of the capacitor with the lossy dielectric as being represented as a capacitor with a perfect dielectric in parallel with a resistor giving the power dissipation (Figure 1.17). The current now leads the voltage by 90o - , where is termed the dielectric loss angle. From the phasor diagram:
where is the angular frequency. The power loss in the parallel resistor is V2/R and thus the power loss per cycle of alternating current is (V2/R)T= (V2/R)(2/), where T is the periodic time. The maximum energy stored by the capacitor is CVmax2, with Vmax = 2V for a sinusoidal
Dr. Saad B. H. Farid 60
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
waveform. The fraction of the maximum energy lost each cycle divided by 2 is termed the loss factor and is thus given by: Table 1.3 shows some typical values of dielectric constant, dielectric strength and loss factor tan .
Example An electrical capacitor is to be made with a sheet of polythene of thickness 0.1 mm between the capacitor plates. What is the greatest voltage that can be connected between the capacitor plates if there is not to be electrical breakdown? The dielectric strength is 4 107 V/rn. The dielectric strength is defined as the breakdown voltage divided by the insulator thickness, hence: breakdown voltage = dielectric strength thickness = 4 107 0.1 103 = 4000V Example A 0.1 F capacitor has a dielectric with a loss factor of 0.003. What will be the power loss when an alternating voltage of 240 V, 50 Hz is connected across it? The power loss in the parallel resistor, resulting from the dielectric being lossy, is given by, since tan = 1/RC: power loss = V2/R = V2C tan =2402 2 50 0.l 10-6 0.003 =5.4 x 10-3 W
Dr. Saad B. H. Farid
61
Materials Selection Lecture otes 1.3.7 Thermal properties
These otes is by o way a Replacement of Classroom Attendance!!!
Thermal properties that are generally of interest in the selection of materials include how much a material will expand for a particular change in temperature; how much the temperature of a piece of material will change when there is a heat input into it, and how good a conductor of heat it is. The linear expansivity or coefficient of linear expansion is a measure of the amount by which a length of material expands when the temperature increases. It is defined as: and has the unit of K-1 The term heat capacity is used for the amount of heat needed to raise the temperature of an object by l K. Thus if 300 J is needed to raise the temperature of a block of material by 1 K, then its heat capacity is 300 J/K. The specific heat capacity c is the amount of heat needed per kilogram of material to raise the temperature by 1 K, hence:
It has the unit of J kg-1 K-1. Because metals have smaller specific heat capacities than plastics, weight-for-weight metals require less heat to reach a particular temperature than plastics, e.g. copper has a specific heat capacity of about 340 J kg-1 K-1 while polythene is about 1800 J kg-1 K-1. The thermal conductivity of a material is a measure of the ability of a material to conduct heat. There will only be a net flow of heat energy through a length of material when there is a difference in temperature between the ends of the material. Thus, the thermal conductivity is defined in terms of the quantity of heat that will flow per second divided by the temperature gradient (Figure 1.18), i.e.:
and has the unit of Wm-1K-1. A high thermal conductivity means a good conductor of heat. It means a small temperature gradient for a particular rate of heat flux. Metals tend to be good conductors, e.g. copper has a thermal conductivity of about 400 Wm-1K-1. Materials that are bad conductors of
Dr. Saad B. H. Farid 62
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
heat have low thermal conductivities, e.g. plastics have thermal conductivities of the order 0.3 Wm-1K-1 or less. Very low thermal conductivities occur with foamed plastics, i.e. those containing bubbles of air. For example, foamed polymer polystyrene, known as expanded polystyrene and widely used for thermal insulation, has a thermal conductivity of about 0.02 to 0.03 Wm-1K-1. Table 1.4 gives typical values of the linear expansivity, the specific heat capacity and the thermal conductivity for metals, polymers and ceramics. Example By how much will a 10 cm strip of (a) copper, (b) PVC expand when the temperature changes from 20 to 30C? Use the data given in Table 1.4. (a) For copper: expansion = 18 10-6 0.10 10= 19 10-6 m = 0.018 mm (b) For the PVC: expansion = 75 10-6 0.10 10 = 75 10-6m = 0.075 mm The expansion of the PVC is some four times greater than that of the copper.
Example The heating element for an electric fire is wound on an electrical insulator. What thermal considerations will affect the choice of insulator material?
Dr. Saad B. H. Farid
63
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
The insulator will need to have a low heat capacity so that little heat is used to raise the material to temperature. This means using a material with as low a density, and hence low mass, and low specific heat capacity as possible. It also will need to be able to withstand the high temperatures without deformation or melting. A ceramic is indicated. 1.3.8 Optical properties An important optical property of a material is its refractive index. When a ray of light passes from one medium to another, e.g. air into glass; reflection and refraction occur; at the interface (Figure 1.19). With reflection the angle of incidence equals the angle of reflection. With refraction the ray of light bends from its straightline path in passing across the interface. The refractive index in going from medium A to B, AnB, is given by:
where i is the angle of incidence, i.e. the angle between the incident ray in medium A and the normal, and r the angle of refraction, i.e. the angle between the refracted ray in medium B and the normal. This is known as Snells law. In Figure l.19 the ray of light is shown as starting in medium A and moving into medium B, bending towards the normal. This occurs because the velocity of light in medium A is greater than that in medium B. Suppose we reverse the path and have the ray of light passing from medium B into medium A (Figure 1.20). The same path is followed, but in the reverse direction, the ray now bending away from the normal. This is because the light is passing from a medium where the speed of light is lower to one where it is higher. Thus we have, when using the same notation for the angles, a refractive index in this case of:
Dr. Saad B. H. Farid
64
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
When a ray of light travels from a material into one, in which it has a lower speed, it bends towards the normal. When a ray of light travels from a material into one in which it has a greater speed it bends away from the normal. When we have this condition of the ray bending away from the normal then we can have a particular incident angle which results in the refracted ray of light bending through 90o and thus not being transmitted across interface (Figure 1.21). The angle of incidence in such a case is termed the critical angle C. We then have:
For angles of incidence greater than the critical angle, the ray of light is totally reflected at the interface, there being no refracted ray. As an illustration of the significance of the critical angle in the choice of optical materials, consider the material used for fiber optics. The basic optical fiber consists of a central core of material in which the velocity of light is higher than in the surrounding cladding. Light for which the angle of incidence is greater than the critical angle is transmitted along such a fiber by total internal reflection, none of such light being lost from the fiber by being refracted through the cladding (Figure 1.22). The refractive index used above is that for light travelling from one material to another and is referred to as the relative refractive index. For example, we thus have AnB for light travelling from medium A to medium B. The refractive index is in fact the ratio of the velocities of light in the two media:
It is convenient to define an absolute refractive index of a medium as beig that given when light travels from a vacuum into that medium, i.e.:
Dr. Saad B. H. Farid
65
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
To arrive at the relationship between the absolute refractive indices of two media A and B and the relative refractive index in going from A to B, consider the situation shown in Figure 1.23 when light travels from a vacuum into medium A and then into B. At the interface between the vacuum and medium A we can write:
where c is the velocity of light in a vacuum and cA that in medium A. For the interface between medium A and medium B we can write: This can be rewritten as:
Thus, knowing the absolute refractive indices for two media enables us to calculate the relative refractive index for the two media. But we have AnB = sinA/sinB, thus we can write Snell's law as: Table 1.5 shows values of the absolute refractive index for light of wavelength 589 nm (yellow light), this being taken as the value of refractive index which is typically used for white light.
Light when incident on a material can be reflected, absorbed and transmitted. The transparency of a material, such as a plastic, depends on its light-absorbing and light-scattering properties. The term total transmission factor is used for the ratio of the total transmitted light intensity to the incident light intensity, assuming it is concentrated in a parallel beam perpendicular to the surface of the sample. For comparison purposes the values are usually quoted for a thickness of 1 mm. The reflection factor is the ratio of the light intensity reflected at an angle equal to the angle of incidence and the intensity of the incident beam, assuming it is concentrated in a parallel beam. The clarity with which detail in an object can be seen
Dr. Saad B. H. Farid 66
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
when viewed through a sample of the material depends on the amount of light scattered in the material. It is perfect only when no light is scattered. Polyethylene typically has a refractive index of about 1.52 and a direct transmission factor, for low density polyethylene, of about 40-45%. Polyvinyl chloride has a refractive index of about 1.54 and a direct transmission factor of about 90%. This high transparency means it is frequently used as a glass substitute, it having the advantage of not breaking so readily. Example Determine the critical angle for a glass-air interface if the glass has a refractive index of 1.5. The refractive index of the glass is for light going from air to glass. Thus:
Hence the critical angle C is 41.8. Example An optical fiber consists of a glass core clad with another material. The core has an absolute refractive index of 1.40 and the cladding an absolute refractive index of 1.42. What is the critical angle for light incident on the glass-cladding interface? The refractive index for Light passing from glass to the cladding is: Thus, the critical angle is 81.9. 13.9 Chemical properties Attack of materials by the environment in which they are situated can be a major problem. The rusting of iron in air is an obvious example of such an attack. Tables are available giving the comparative resistance to attack of materials in various environments, e.g. in aerated water, in salt water, too strong acids, strong alkalis, organic solvents and ultraviolet radiation. While some polymers are highly resistant to chemical attack, others are liable to stain, craze, soften, swell or dissolve completely. For example, nylon shows little degradation with weak acids but is attacked by strong acids; it is resistant to alkalis and organic solvents. Polymers have generally high resistance to attack in water and thus are widely used for containers and
Dr. Saad B. H. Farid 67
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
pipes. Polymers are generally affected by exposure to sunlight. Ultraviolet light, present in sunlight, can cause a breakdown of the bonds in the polymer molecular chains and result in surface cracking. For this reason, plastics often have an ultraviolet inhibitor mixed with the polymer when the material is produced. 1.3.10 Magnetic properties In the vicinity of permanent magnets and current-carrying conductors, a magnetic field is said to exist. The magnetic field pattern can be plotted using a compass needle or demonstrated by scattering iron filings in the vicinity. The term magnetic line of force is used for a line traced out by such plotting or the iron filings. A useful way of considering magnetic fields is in terms of magnetic flux, this being something that is considered to flow along these lines of force like water through pipes. The term magnetic flux density B is used for the amount of flux passing through unit area. Thus if flux passes through an area A, then the flux density is:
Magnetic flux is produced within magnetic materials when electrical currents pass through coils of wire wrapped round cores of such materials. The magnetizing field strength H is NI/L, where N is the number of turns on the coil, I the current and L the length of the coil. The flux density produced for a given magnetizing field depends on the magnetic material used for the core. With a vacuum for the core, the flux density Bo is oH, where o is a constant called the permeability of free space. The term relative permeabilityr is used for the unit-less factor by which the flux density in a material B compares with that which would have been produced with a vacuum as the core Bo:
1. Diamagnetic materials These have relative permeabilities slightly below 1. Copper is an example of such a material. 2. Paramagnetic materials These have relative permeabilities slightly greater than 1. Aluminium is an example of such a material. 3. Ferromagnetic and ferrimagnetic materials These have relative permeabilities considerably greater than 1, ferromagnetic materials being metals and ferrimagnetic materials being
Dr. Saad B. H. Farid 68
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
ceramics. Iron, cobalt and nickel are examples of ferromagnetic materials, iron oxide Fe3O4 and nickel ferrite NiFe2O3 examples of ferrimagnetic materials. For iron the relative permeability is typically about 2000 to 10 000, though special steels can have values of the order of 60 000 to 90 000. The relative permeability for a ferromagnetic or ferrimagnetic material is not constant, depending on the size of magnetizing field used. When an initially unmagnetized material is placed in an increasing magnetizing field, the flux density within the material increases. This is shown in Figure (1.24). The gradient of the graph, i.e. B/H, is not constant and so the relative permeability B/oH = B/Bo is not a constant.
After a particular magnetizing field is reached, the magnetic flux reaches a constant value, this being termed saturation. If the magnetic field is then reduced back to zero, the material may not simply just retrace its path back down the same graph line and may retain some magnetism when the applied magnetic field is zero. The retained flux density is termed the remanent flux density or remanence. To demagnetize the material, i.e. bring B to zero, a reverse field called the coercive field or coercivity must be applied. Figure 1.25 shows how the flux density B within the material might vary when the magnetizing field is increased to saturation, then decreased to
Dr. Saad B. H. Farid
69
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
zero, then increased to saturation in the opposite direction, then decreased to zero, etc. The resulting graph is called a hysteresis loop. In Figure 1.25 the hysteresis loops are shown for two materials, termed hard and soft magnetic materials. Compared with a soft magnetic material, hard magnetic material has high remanence so that a high degree 01 magnetism is retained in the absence of a magnetic field, a high coercivity so that it is difficult to demagnetize and a large area enclosed by the hysteresis loop. The area of the loop is related to the energy dissipated in the material during each cycle of magnetization. A soft material is very easily demagnetized, having low coercivity and the hysteresis loop only enclosing a small area. Hard magnetic materials are used for such applications as permanent magnets while soft magnetic materials are used for transformers where the magnetic material needs to be easily demagnetized and little energy dissipated in magnetizing it. A typical soft magnetic material used for a transformer core is an iron-3% silicon alloy. The main materials used for permanent magnets are the iron-cobalt-nickelaluminium alloys, ferrites and rare earth alloys. Table 1.6 gives properties of typical soft magnetic materials and Table 1.7 gives details for hard magnetic materials. For hard magnetic materials an important parameter is the demagnetization quadrant (Figure 1.26) of the hysteresis loop, it indicating how well a permanent magnet is able to retain its magnetism. The bigger the area the greater the amount of energy needed to demagnetize the material. A measure of this area is given by the largest rectangle which can be drawn in the area, this being the maximum value of the product BH.
Dr. Saad B. H. Farid
70
Materials Selection Lecture otes Example Which of the following applications (a) a compass needle, (b) the core of an electromagnet, requires a soft and which a hard magnetic material? (a) A hard magnetic material is required since the compass needle is required to be a permanent magnet. (b) A soft magnetic material is required since the electromagnet is required to loose its magnetism when the energizing current is switched off. 1.4 comparing materials
These otes is by o way a Replacement of Classroom Attendance!!!
Table 1.8 summarizes the properties of metals, polymers and ceramics indicating the typical range of values likely to be encountered at about 20C. Example Which type of material, metal, polymer or ceramic would be the most likely to give materials with each of the following properties: (a) High density. (b) High melting point. (c) High electrical conductivity. (d) Low specific heat capacity. (e) Low tensile modulus of elasticity. (a) Metals contain the materials with the highest densities. (b) The highest melting points are given by the ceramics. (c) The highest electrical conductivities are given by the metals. (d) The lowest specific heat capacities are given by the metals. (e) Polymers give the materials with the lowest tensile modulus of elasticity.
71
Dr. Saad B. H. Farid
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
1.4.1 Costs Costs can be considered in relation to the basic costs of the raw materials, the costs of manufacturing products, and the life and maintenance costs of the finished product. In comparing the basic costs of materials, the comparison is often on the basis of the cost per unit weight or cost per unit volume. Table 1.9 shows the relative costs of some materials. However, often a more important comparison is on the basis of the cost per unit strength or cost per unit stiffness for the same volume of material. This enables the cost of, say, a beam to be considered in terms of what it will cost to have a beam of a certain strength or stiffness. Hence if, for comparison purposes, we consider a beam of volume 1m3 then, if the tensile strength of the material is 500 MPa and the cost per cubic meter 800, the cost per MPa of strength will be 800/500 1.6. The costs of manufacturing will depend on the processes used. Some processes require a large capital outlay and then can be used to produce large numbers of the product at a relatively low cost per item. Other processes may have little in the way of setting-up costs but a large cost per unit product.
Dr. Saad B. H. Farid
72
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
The cost of maintaining a material during its life can often be a significant factor in the selection of materials. A feature common to many metals is the need for a surface coating to protect them from corrosion by the atmosphere. The rusting of steels is an obvious example of this and dictates the need for such activities as the continuous repainting of, e.g., the Forth Railway Bridge.
Problems
Dr. Saad B. H. Farid
73
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
24.7 Selection for corrosion resistance For metals subject to atmospheric corrosion the most significant factor in determining the chance of corrosive attack is whether there is an aqueous electrolyte present. This could be provided by condensation of moisture occurring as a result of the climatic conditions. The amount of pollution in the atmosphere can also affect the corrosion rate. Corrosion can often be much reduced by the selection of appropriate materials. For metals immersed in water, the corrosion depends on the substances that are dissolved or suspended in the water. Carbon steels and low-alloy steels are not particularly corrosion resistant, rust being the evidence of such corrosion. In an industrial atmosphere, in fresh and sea water, plain carbon steels and low-alloy steel have poor resistance. Painting, by providing a protective coating of the surface, can reduce such corrosion. The addition of chromium to steel can markedly improve its corrosion resistance. Steels with 4-6% chromium have good resistance in an industrial atmosphere, in fresh and sea water, while stainless steels have an excellent resistance in an industrial atmosphere and fresh water but can suffer some corrosion in sea water. The corrosion
Dr. Saad B. H. Farid
74
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
resistance of grey cast iron is good in an Industrial atmosphere but not so good in fresh or sea water, though still better than that of plain carbon steels. Aluminium when exposed to air develops an oxide layer on its surface which then protects the substrate from further attack. Wrought alloys are often clad with thin sheets of pure aluminium or an aluminium alloy to enhance the corrosion resistance of such alloys. Thus in air, aluminium and its alloys have good corrosion resistance. When immersed in fresh or sea water, most aluminium alloys offer good corrosion resistance, though there are some exceptions which must be clad in order to have good corrosion resistance. Copper in air forms a protective green layer which protects it from further attack and thus gives good corrosion resistance. Copper has also good corrosion resistance in fresh and sea water, hence the widespread use of copper piping for water distribution systems and central heating systems. Copper alloys likewise have good corrosion resistance in industrial atmospheres, fresh and sea water through demetallification can occur with some alloys, e.g. dezincification of brass with more than 15% zinc. Nickel and its alloys have excellent resistance to corrosion in industrial air, fresh and sea water, Titanium and its alloys have excellent resistance, probably the best resistance of all metals, in industrial air, fresh and sea water and is thus widely used where corrosion could be a problem. Plastics do not corrode in the same way as metals and thus, in general, have excellent corrosion resistance. Hence, for example, the increasing use of plastic pipes for the transmission of water and other chemicals. Polymers can deteriorate as a result of exposure to ultraviolet radiation, e.g. that in the rays from the sun, heat and mechanical stress. To reduce such effects, specific additives are used as fillers in the formulation of a plastic. Most ceramic materials show excellent corrosion resistance. Glasses are exceedingly stable and resistant to attack, hence the widespread use of glass containers. Enamels, made of silicate and borosilicate glasses, are widely used as coatings to protect steels and cast irons from corrosive attack. Table 24.7 gives a rough indication of the corrosion resistance of materials to different environments.
Dr. Saad B. H. Farid
75
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
24.7.1 Dissimilar metal corrosion Table 24.8 shows the galvanic series of metals in sea water. The series will differ if the environment is freshwater or industrial atmosphere, though the same rough sequence tends to occur but the potentials are likely to vary. The list is in order of corrosion tendency, giving the free corrosion potentials, and enables the prediction of the corrosion resistance of a combination of dissimilar metals. The bigger the separation of any two metals in the series, the more severe the corrosion of the more active of them when a junction between the pair of them is exposed to sea water. The more negative potential metal acts as the anode and the less negative or positive as the cathode in an electrochemical cell.
Dr. Saad B. H. Farid
76
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
24.8 Selection for wear resistance Wear is the progressive loss of material from surfaces as a result of contact with other surfaces. It can occur as a result of sliding or rolling contact between surfaces or from the movement of fluids containing particles over surfaces. Because wear is a surface effect, surface treatments and coatings play an important role in improving wear resistance. Lubrication can be considered to be a way of keeping surfaces apart and so reducing wear. Mild steels have poor wear resistance. However, increasing the carbon content increases the wear resistance. Surface harden-able carbon or lowalloy steels enable wear resistance to be improved as a result of surface treatments such as carburizing, cyaniding or carbonitriding. Even better wear resistance is provided by nitriding medium-carbon chromium or chromiumaluminium steels, or by surface hardening high-carbon high- chromium steels. Grey cast iron has good wear resistance for many applications. Better wear resistance is, however, provided by white irons. Among non-ferrous alloys, beryllium coppers and cobalt-base alloys, such as Stellite, offer particularly good wear resistance. 24.8.1 Bearing materials Metallic materials for use as bearing surfaces need to be hard and wear resistant, with a low coefficient of friction, but at the same time sufficiently tough. Generally these requirements are met by the use of a soft, but tough, alloy in which hard particles are embedded. When one surface slides over another, the frictional force is proportional to the normal force and is independent of the apparent area of contact between the sliding surfaces. These are termed the laws of friction. The term apparent area
Dr. Saad B. H. Farid
77
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
has been used because no matter how smooth a surface, on an atomic scale it is irregular, and contact between two sliding surfaces only occurs at a limited number of discrete points. The real area of contact is thus only a small fraction of the apparent area of contact. It is these small, real, contact areas that have to carry the load between surfaces. Because the real areas are so small, the pressure at the contact points will be very high, even under light loading. With metals, the pressure will generally be high enough to cause appreciable plastic deformation and adhesion between the two surfaces at these points. This is termed cold welding for metals. These junctions are sheared when surfaces slide over each other. The frictional force thus arises from the force to shear junctions and the force required to plough the asperities of one surface through those of the other surface. Bearing materials can be classified, in the main, into four categories: 1- Whitemetals These are tin-base or lead-base alloys with the addition of mainly antimony or copper. They have a microstructure of hard intermetallic compounds of tin and antimony embedded in a soft matrix. The hard particles support the load, since the asperities penetrate the softer material, but the greater area of contact between the surfaces is with the soft material. Thus sliding takes place within a thin smeared film of the softer material. Whitemetals have relatively low fatigue strength and this can limit their use to low-load conditions. Reducing the thickness of the bearing material can improve the fatigue properties but does require care because of the size of the hard intermetallic particles. Tin-base alloys resist corrosion better, have higher thermal conductivity, have higher modulus of elasticity and higher yield stress but are significantly more expensive than lead-base alloys. Both forms of alloy are relatively soft. Bearings are usually manufactured by casting onto prepared steel strip and then forming the resulting strip. Table 24.9 shows typical properties. 2- Copper-base alloys These offer a wider range of strength and hardness than whitemetals. They include tin bronzes with between 10 and 18% tin, leaded tin- bronzes containing 1 or 2% lead, phosphor bronzes and copper-lead alloys containing about 25 to 30% lead. The properties of the copper-lead alloys depend on the lead content, the higher the amount of lead the lower the fatigue strength but the better the sliding properties. They have poorer corrosion resistance than whitemetals but better wear resistance, a higher modulus of elasticity and better fatigue resistance. Bearings are manufactured by casting onto steel strip or sintering copper and lead on the
Dr. Saad B. H. Farid 78
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
strip. The bronzes have higher strengths, hardness, modulus of elasticity and better fatigue resistance than the copper-lead alloys and the whitemetals. They tend to be used for high load-bearing loads. Table 24.9 shows typical properties. 3- Aluminium-base alloys Aluminium-tin alloys with about 5 to 7% tin, 1% copper, 1% nickel and small amounts of other elements give bearing materials with a high fatigue strength, hardness and strength which makes them suitable for high-load bearings. However, they have the disadvantage of a high thermal expansivity which can lead to loose bushes or even seizure against other surfaces. Steelbacked aluminium bearings can be manufactured by hot rolling the aluminium alloy onto the steel to permit solid-state welding. Bearings can also be sand or die cast. Table 24.9 shows typical properties.
4- on-metallic bearing materials Polymers suitable for bearing materials include phenolics, nylon, acetal and PTFE. For some applications the polymers have fillers, e.g. graphite-filled nylon, PTFE with a silicon lubricant, acetal with PTFE filler. In addition to the fillers used to decrease the coefficient of friction, other fillers such as glass fibers are added to increase strength and dimensional stability. Polymers have the advantage of a very low coefficient of friction but the disadvantage of a low thermal conductivity. Polymer rubbing against polymer can lead to high rates of wear, but polymer against steel gives a very low wear rate. Polymers have thermal expansivities much greater than metals and so can present problems, e.g. a higher running clearance between surfaces is needed. They tend to be used under low load conditions where they have the advantage of being cheap. Table 24.10 shows the properties of
Dr. Saad B. H. Farid 79
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
commonly used polymeric bearing materials. Typical applications are: PTFE bearings in car steering linkages and food processing equipment; phenolics for marine propeller shafts; acetals for electrical appliances. 5- Metal-non-metallic bearing materials Graphite-impregnated metals and PTFE-impregnated metals are widely used bearing materials. Such materials are able to utilize the load-bearing and temperature advantages of metals with the low coefficient of friction and soft properties of the non-metals. Thus graphite-impregnated metals rubbing against a steel mating surface can be used with load pressures up to about 40 MPa and operating temperatures up to 500C, while PTFE impregnated metals can be used with loads up to 100 MPa and temperatures of 250C. A bearing material is inevitably a compromise between the opposing requirements of softness and high strength. One way of achieving strength with a relatively soft bearing material is to use the soft material as a lining on a steel backing, e.g. whitemetals, aluminium or copper-base alloys as thin layer on a steel backing. Plastics when bonded to a steel backing can be used at higher speeds than otherwise would be possible, because the steel is able to dissipate heat better than the plastic alone and also the thinner the layer of plastic the smaller the amount by which it will expand. 24.9 Selection for thermal properties The thermal conductivity of a material is a measure of the rate at which heat is transferred through the material. In general, metals have high thermal conductivities while polymers and ceramics have low conductivities. The specific heat capacity of a material is the energy required to raise the temperature of 1 kg of that material by 1C. In general, metals have low specific heats with polymers having higher values. Materials expand when heated and the problems of differential expansion between different materials in a component can often be an important concern. In general, ceramics have low coefficients of expansion, metals higher values and polymers even higher. Table 24.12 gives typical values for 20C.
Dr. Saad B. H. Farid 80
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
Dr. Saad B. H. Farid
81
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
24.10 Selection for electrical properties In general, metals are good electrical conductors with low resistivities. The metals in common use in engineering which have the highest electrical conductivities are silver, copper and aluminium. In each the conductivity is highest when the material is of the highest purity and in the fully annealed condition. Often, however, a compromise has to be reached in that the high purity, fully annealed, metals do not have sufficient strength to enable them to be, for example, strung as wire between posts. Polymers and ceramics have, in general, very low electrical conductivities and are classified as being electrical insulators. Table 24.13 shows the resistivities and conductivities of a range of commonly used solid metals and alloys at about 20C. Note that conductance is the reciprocal of resistance and has the unit Siemens (S) and that conductivity is the reciprocal of the resistivity and has the unit S/rn. In engineering, conductivity is often expressed as a percentage of the conductivity that annealed copper has at 20C. Such values are said to be IACS values. Table 2413 shows resistivities for insulators.
Dr. Saad B. H. Farid
82
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
24.11 Selection for magnetic properties In considering the selection of a magnetic material, the questions to be posed are as to whether soft or hard, and often in the case of soft as to whether the material is a good electrical conductor. Table 24.15 gives the properties of some commonly encountered soft magnetic materials and Table 24.16 those for hard magnetic materials. The Curie temperature is the temperature at which thermal energy results in the loss of ferromagnetism.
Dr. Saad B. H. Farid
83
Materials Selection Lecture otes 24.12 Available forms of materials
These otes is by o way a Replacement of Classroom Attendance!!!
A major factor affecting the choice of material is the form and size it can be supplied in. Thus if the design indicates, for example, an I-girder with specified dimensions in a particular steel and that is not a size in which the material is normally supplied, then there may well have to be a change in the material used since the cost of obtaining the non-standard size of the material may rule it out. The as supplied form and size can determine whether further processing is required and, if so, to what extent. The surface conditions of the supplied material may also be important, particularly if the material is to be used without further processing. Thus, for example, hot rolled steel could have a loose, flaky scale on its surface and thus have to be machined. A particular manufacturer might supply steel as rounds, squares, flats, tees, channels, circular hollow sections, square hollow sections, rectangular hollow sections, rolled steel joists, universal beams and columns, sheets, galvanized sheets, plates, etc. with a variety of sizes and surface finishes. Polymeric materials are generally supplied as granules ready for, processing. However, the composition of the granules, for what is the same polymeric material, can vary depending in what other materials, e.g. fibers, have been added. 24.13 Cost of materials The costs of materials change with time, and to eliminate the need to specify any particular unit of currency, relative costs are used for the purpose of materials selection when we only require to determine the optimum material. The relative costs tend to be per unit mass defined in relationship to that of mild steel, often mild steel bar. Thus:
Table 24.17 gives some typical values (also see Table 1.9). The relative cost per kg can be converted into relative cost per m3 by multiplying it by the density.
Dr. Saad B. H. Farid
84
Materials Selection Lecture otes
These otes is by o way a Replacement of Classroom Attendance!!!
Problem Suggest the key property or properties required of a material and the types of materials that might be used in the following situations: (a) Pipes used in the distribution of hot water. (b) A component where strength is required at temperatures in the region of 700C. (c) A component which is required to be stiff. (d) A component where strength is required in a marine environment. (a) A light-load bearing material. (f) A container to hold acids. (g) A component to be used with direct stresses of about 1000 MPa. (h) A component subject to impact loading. (1) A component subject to cyclic loading. (j) A transformer core.
Dr. Saad B. H. Farid
85
Materials Selection Lecture otes, 2010
Ass. Prof. Dr. Saad B. H. Farid
Part III- Selection of Processes
* dont confuse with the "processing parameters" page 16 2.1 Introduction This section is a consideration of the characteristics of the various types of processes which determine the types of products that can be produced. In making a decision about the manufacturing process to be used for a product there are a number of questions that have to be answered: 1. What is the material? The type of material to be used influences the choice of processing method. For example, for casting of high melting point material, the process must be either sand casting or investment casting. 2. What is the shape? The shape of the product is generally a very important factor in determining which type of process. For example, a product in the form of a tube could be produced by centrifugal casting, drawing or extrusion but not generally by other methods 3. What is the kind of detail is involved? Is the product to have holes, threads, inserts, hollow sections, fine detail, etc.? Thus, forging could not be used if there was a requirement for hollow sections. 4. What dimensional accuracy and tolerances are required? High accuracy would rule out sand casting, though investment casting might well be suitable. 5. Are any finishing processes to be used? Is the process used to give the product its final finished state or will there have to be an extra finishing process? For example, planing will not produce as smooth a surface as grinding. 6. What quantities are involved? Some processes are economic for small quantities; others are economic for large quantities or continuous production. For example, open die forging could be economic for small numbers where, closed die forging are economic for large numbers.
44
Materials Selection Lecture otes, 2010
Ass. Prof. Dr. Saad B. H. Farid
2.2 Surface finish 1. Roughness is defined as the irregularities in the surface texture which are inherent in the production process but excluding waviness and errors of form; see figure (8-a).
B B center line
Roughness
Waviness
Error of form
Area between surfaces above center line equals area below
-a-bFigure 8: a- the terms roughness, waviness and error of form. b- measuring roughness
2. A sand cast product will have a surface finish which is much rougher than one which has been die cast. 3. Roughness takes the form of a series of peaks and valleys which may vary in both height and spacing and is a characteristic of the process used. 4. Waviness may arise from such factors as machine or work deflections, vibrations, heat treatment or warping strains. Roughness and waviness may be cause departures of the surface from the true geometrical form. One measure of roughness is the arithmetical mean deviation, denoted by the symbol Ra defined as the arithmetical average of the variation of the profile above and below a reference line throughout the prescribed sampling length. The reference line may be the centre line, this being a line chosen so that the sums of the areas contained between it and those parts of the surface profile which lie on either side of it are equal (Figure 8-b). Thus; for a sample length in millimeters and areas in square millimeters Ra is defined as:
Ra =
sum of areas A + sum of areas B 1000 sample length
Table 17 indicates the significance of Ra values in terms of the surface texture. The degree of roughness that can be tolerated for a
45
Materials Selection Lecture otes, 2010
Ass. Prof. Dr. Saad B. H. Farid
component depends on its use. Thus, for example, precision sliding surfaces will require Ra values of the order of 0.2 to 0.8 m with more general sliding surfaces 0.8 to 3 m. Gear teeth are likely to require Ra values of 0.4 to 1.6 m, friction surfaces such as clutch plates 0.4 to 1.5 m, mating surfaces 1.5 to 3 m. Table 18 shows what is typically achievable with different processes. Thus, sand casting produces a much rougher surface than die casting; hot rolling produces a rougher surface than cold rolling; sawing produces a much rougher surface than milling. Table 17: Relation of Ra values to surface texture Surface texture Roughness Ra m Very rough 50 Rough 25 Semi-rough 12.5 Medium 6.3 Semi-fine 3.2 Fine 1.6 Coarse-ground 0.8 Medium-ground 0.4 Fine-ground 0.2 Super-fine 0.1 Table 18: Roughness values for different processes Process Roughness Ra m Sand casting 25 - 12.5 Hot rolling 25 - 12.5 Sawing 25 - 3.2 Planing, shaping 25 - 0.8 Forging 12.5 - 3.2 Milling 6.3 - 0.8 Boring, turning 6.3 - 0.4 Investment casting 3.2 - 1.6 Extruding 3.2 - 0.8 Cold rolling 3.2 - 0.8 Drawing 3.2 - 0.8 Die casting 1.6 - 0.8 Grinding 1.6 - 0.1 Honing 0.8 - 0.1
46
Materials Selection Lecture otes, 2010
Ass. Prof. Dr. Saad B. H. Farid
2.3 Metal-forming processes The following is a discussion of the characteristics of the various processes used for metal forming and the types of products that can be obtained from them. 2.3.1 Casting of Metals Casting can be used for components from masses of about 10-3 kg to I04 kg with wall thicknesses from about 0.5 mm to 1 m. Castings need to have rounded corners, no abrupt changes in section and gradual sloping surfaces. Casting is likely to be the optimum method in the circumstances listed below but not for components that are simple enough to be extruded or deep drawn. The casting process is selected when: 1. The part has a large internal cavity There would be a considerable amount of metal to be removed if machining was used. 2. The part has a complex internal cavity Machining might be impossible; by casing, however, very complex internal cavities can be produced. 3. The part is made of a material which is difficult to machine The hardness of a material may make machining very difficult, e.g. white cast iron. 4. The metal used is expensive and so there is to be little waste Machining is likely to produce more waste than occurs with casting. 5. The directional properties of a material are to be minimized Metals subject to a manipulative process often have properties which differ in different directions. 6. The component has a complex shape Casting may be more economical than assembling a number of individual parts. 7. The tooling cost for making the moulds When many identical castings are required; the mould cost (used many times) will be spread over many items and make the process economic. 8. The mould type and cost for single product Where just a single product is required, the mould used must be as cheap as possible.
47
Materials Selection Lecture otes, 2010
Ass. Prof. Dr. Saad B. H. Farid
Each casting method has important characteristics which determine its appropriateness in a particular situation. Table 19 illustrates some of the key differences between the casting methods. The following factors determine the type of casting process used: 1. Large heavy casting Sand casting can be used for very large castings. 2. Complex designs Sand casting is the most flexible method and can be used for very complex castings. 3. Thin walls Investment casting or pressure die casting can cope with walls as thin as 1mm. Sand casting cannot cope with such thin walls. 4. Good reproduction of detail Pressure die casting or investment casting gives good reproduction of detail), sand casting is being the worst. 5. Good surface finishes Pressure die casting or investment casting gives the best finish, sand casting being the worst. 6. High melting point alloys Sand casting or investment casting can be used. 7. 10. Tooling cost a. This is highest with pressure die casting. b. Sand casting is cheapest. c. With large number production, the tooling costs for metal moulds can be paid over a large number of castings, d. Whereas, the cost of the mould for sand casting is the same no matter how many castings are made since a new mould is required for each casting. Table 19: Casting processes
Process Sand casting Gravity die casting Investment casting Centrifugal casting Usual materials Section thickness mm Most >4 Non-ferrous 3 to 50 All L - 75 Most 3 - 100 Size kg 0.1 200 000 0.1 - 200 0.005 - 700 25mm 1.8m diameter 0.0001 - 5 0.1 -200 Production Roughness rate, items Ra m per hour 25 - 12.5 1 - 60 3.2 - 1.6 5 - 100 3.2 1.6 Up to 1000 25 - 12.5 1.6 - 0.8 1.6 0.8 Up to 50 Up to 200 Up to 200
Pressure die casting: Non-ferrous 1 - 8 high pressure Pressure die casting: Non-ferrous 2 - 10 low pressure
48
Materials Selection Lecture otes, 2010
Ass. Prof. Dr. Saad B. H. Farid
2.3.2 Manipulation of Metals Manipulative methods involve the shaping of a material by means of plastic deformation methods. Such methods include forging, extruding, rolling, and drawing. Depending on the method, components can be produced from as small as about 10-5 kg to 100 kg, with wall thicknesses from about 0.1 mm to 1m. Table 20 shows some of the characteristics of the different processes Table 20: Manipulative processes
Process Closeddie forging Roll forming Drawing Usual materials Steels, Al, Cu, Mg alloys Any ductile material Any ductile material Section Minimum Maximum Production Roughness size size rate, items thickness Ra m mm per hour 3 upwards 0.2 6 0.1 25 0.1 20 3 mm diameter 6 mm diameter 6 mm diameter 0.15 m diameter 3.2 12.5 0.8 3.2 0.8 3.2 0.8 3.2 Up to 3000 Up to 2000 Up to 300
Impact Any ductile extrusion material
Hot Most ductile 8 mm 500 mm 1 100 0.8 3.2 Up to 720 extrusion materials diameter diameter Cold Most ductile 8 mm 1 100 4 m long 0.8 3.2 Up to 720 extrusion materials diameter Note: ductile materials are commonly aluminium copper and magnesium alloys and to a lesser extent carbon steels and titanium alloys.
Compared with casting, wrought products tend to have a greater degree of uniformity and reliability of mechanical properties. The manipulative processes do, however, tend to give a directionality of properties which is not the case with casting. Manipulative processes are likely to be the optimum method for product production when: 1. The part is to be formed from sheet metal Depending on the form required, shearing, bending or drawing may be appropriate if the components are not too large. 2. Long lengths of constant cross-section are required Extrusion or rolling is the optimum methods for long lengths of quite complex cross-section. It can be produced without any need for machining.
49
Materials Selection Lecture otes, 2010
Ass. Prof. Dr. Saad B. H. Farid
3. The part has no internal cavities i.Forging can be used when there are no internal cavities, particularly if better toughness and impact strength is required than are obtainable with casting. ii.Directional properties can be obtained to the material to improve its performance in service. 4. Seamless cup-shaped objects or cans are required Deep drawing or impact extrusion would be optimum methods. 5. The component is to be made from material in wire or bar form Bending or upsetting can be used. 2.3.3 Metal Powder Processes (Metallurgy) Include the production of "green" parts by compaction followed by sintering. Powder processes enables: 1. Large numbers of small items (components) to be made at high rates of production; with little, if any, finishing machining required. 2. It enables components to be made with all metals and in particular with those which otherwise cannot easily processed, e.g. the high melting point metals of molybdenum, tantalum and tungsten. 3. Production of parts with specific degree of porosity, e.g. porous bearings to be oil filled. 4. The mechanical compaction of powders only, however, permits two-dimensional shapes to be produced, unlike casting and forging. 5. The shapes are restricted to those that are capable of being ejected from the die. Thus, for example, reverse tapers, undercuts and holes at right angles to the pressing direction have to be avoided. 6. Powdered metals are more expensive than metals for use in manipulative or casting processes; however, this higher cost may be compensate by the absence of scrap, the elimination of finishing machining and the high rates of production. 2.3.4 Machining of Metals Is a cutting process, like planing, shaping, boring and turning. The following factors are relevant in determining the optimum process or processes: 1- Operations should be designed so that the minimum amount of material is removed. This reduces material costs, energy costs involved in the machining and costs due to tool wear.
50
Materials Selection Lecture otes, 2010
Ass. Prof. Dr. Saad B. H. Farid
2- The time spent on the operation should be kept to a minimum to keep labor costs low. 3- The skills required affect the labor costs. 4- The properties of the material being machined should be considered; in particular the hardness. i. In general, the harder a material the longer it will take to cut. The hardness also, however, affects the choice of tool material that can be used, ii. In the case of very hard materials, grinding is a process that can be used because the tool material and the abrasive particles can be very hard. iii. Where a considerable amount of machining occurs, the use of free machining grades of materials (machinable materials) should be considered as a means of minimizing cutting times. 5- The process, or processes, chosen should take into account the quantity of products required and the required rate of production. 6- The geomantic form of the product should be considered in choosing the most appropriate process or processes. 7- The required surface finish and dimensional accuracy also affect the choice of process or processes. Machining operations vary quite significantly in cost, especially when a particular tolerances to be achieved. For example, to achieve a tolerance of 0.10mm, the rank order of the processes is: Shaping Planing Horizontal boring Milling Turret (capstan) Most expensive
Least expensive
The cost of all processes increases as the required tolerance is decreased. At high tolerances, grinding is one of the cheapest processes. Different machining operations produces different surface finishes, see Table 21.
51
Materials Selection Lecture otes, 2010
Ass. Prof. Dr. Saad B. H. Farid
Table 21: Surface finishes Ra m Machining process Planing and shaping 25 - 0.8 Least smooth
Drilling Milling Turning Grinding 8 - 1.6 6.3 - 0.8 6.3 - 0.4 1.6 - 0.l
Most smooth
The choice of process will also depend on the geometric form required for the product. Table 22 indicates the processes that can be used for different geometric forms. Table 22: Machining processes for particular geometric forms
Type of surface Plane surface Externally cylindrical surface Internally cylindrical surface Flat and contoured surfaces and slots Shaping, planing, face milling, surface grinding Turning, grinding Drilling, boring, grinding Milling, grinding
In general, Machining is a relatively expensive process when compared with many other methods of forming materials. The machining process is, however, a very flexible process which allows the generation of a wide variety of forms. A significant part of the total machining cost of a product is due to setting-up times when there is a change from one machining step to another. By reducing the number of machining steps and hence the number of setting-up times a significant saving becomes possible. Thus, the careful sequencing of machining operations and the choice of machine to be used is important. 2.3.5 Joining processes with Metals 1- Fabrication involves the joining of materials and enables very large structures to be assembled, much larger than can be obtained by other methods such as casting or forging. 2- The main joining processes are essentially adhesive bonding, soldering and brazing, welding and fastening systems. 3- The factors that determine the joining process are the materials involved, the shape of the components being joined, whether the joint is to be permanent or temporary, limitations imposed by the environment and cost. 4- Welded, brazed and adhesive joints, and some fastening joints, e.g. riveted, are generally meant to be permanent joints, while soldered joints and bolted joints are readily taken apart and rejoined.
52
Materials Selection Lecture otes, 2010
Ass. Prof. Dr. Saad B. H. Farid
2.4 Polymer forming processes
Injection molding and extrusion are the most widely used processes. Injection molding is generally used for the mass production of small items, often with intricate (complex) shapes. Extrusion is used for products which are required in continuous lengths or which are fabricated from materials of constant cross-section. The following are some of the factors involved in selecting a process: 1- Rate of production Cycle times are typically: Injection molding and Blow 10-60 s, Compression molding 20-600 s, Rotational molding 70- 1200 s, Thermo-forming 10-60 s 2- Capital investments required a. Injection molding requires the highest capital investment with extrusion and blow molding requiring less capital. b. Rotational molding, compression molding, transfer molding, thermoforming and casting require the least capital investment 3- Most economic production rate a. Injection molding, extrusion and blow molding are economic only with large production runs. b. Thermoforming, rotational molding and machining are used with small production runs. Table 23 indicates the minimum output that is likely to be required to make the processes economic. Table 23: Minimum output
Process Machining Rotational molding Sheet forming Extrusion Blow molding Injection molding Economic output number 1 100 items 100 1000 items 100 1000 items 300 3000 m length 1000 10 000 items 10 000 100 000 items
4- Surface finishes A- Injection, blow and rotational molding, thermoforming, transfer and compression molding, and casting all give very good surface finishes. B- Extrusion gives only a fairly good surface finish. 5- Metals inserts during the process These are possible with injection molding, rotational molding, transfer molding and casting. 6- Dimensional accuracy Injection molding and transfer molding are very good. Compression molding and casting are good, extrusion is fairly poor.
53
Materials Selection Lecture otes, 2010
Ass. Prof. Dr. Saad B. H. Farid
7- Item size Injection molding and machining are the best for very small items. Section thicknesses of the order of 1mm can be obtained with injection molding, forming and extrusion. 8- Enclosed hollow shapes Blow molding and rotational molding can be used 9- Intricate, complex shapes Injection molding, blow molding, transfer molding and casting can be used. 10- Threads Threads can be produced with injection molding, blow molding, casting and machining. 11- Large formed sheets Thermoforming can be used. 12- The assembly processes that can be used with plastics are welding, adhesive bonding, riveting, press and snap-fits, and thread systems. Table 24 shows the processing methods that are used for some commonly used thermoplastics and Table 25for thermosets. Table 24: Processing methods for thermoplastics
Polymer ABS Acrylic Cellulosics Polyacetal Polyamide Polycarbonate Polyester Polyethylene HD Polyethylene LD Polyethylene Terephthalate Polypropylene Polystyrene Polysulphone PYFE PVC Extrusion x x x x x x x x x x x x x x x Injection moulding x x x x x x x x x x x x x x Extrusion Bending Rotational Thermoblow and As film Casting moulding forming moulding joining x x x x x x x x x x x x x x x x x x x x x x x x x x x
x x x x
x x
Table 25: Processing methods for thermosets
Polymer Epoxy Melamine formaldehyde Phenol formaldehyde Polyester Urea formaldehyde Compression moulding x x x x Transfer moulding x x x x Casting x x x Laminate x x x x x Foam x x x Film
54
Materials Selection Lecture otes, 2010
Ass. Prof. Dr. Saad B. H. Farid
Powder Technology used for
metals, alloys, ceramic and polymers.
Powder Technology = Powder Processes + Heat Treatments (e.g. Sintering, pre-Sinteringetc), The selection of a collection of Powder Processes + Heat Treatments = The Powder Technological Root
Powder Technology Processes
1. Crushing, Grinding and Milling Crushers and grinders (or millers) are chosen according to the type of the starting materials and the desired final particle size. The significance of the process in that its increase the surface area of the powder particles; this provides more homogeneity and more contact area between the particles which improves Forming and Sintering. 2. Classification of Powders The starting powders are classified utilizing standard sieve set. The resultant powder batches are differing in average particle size and distribution according to the sieve number. In addition, the powder batches can be mixed with designed average particle size and distribution. The classification step has a significant effect on the properties of the final product. Like physical, mechanical and thermal properties. 3. Mixing of powders Homogeneity in chemical composition and particle size distribution is essential in powder technology. Homogeneity can be achieved through good mixing of powder batches; utilizing particular equipments like: Mixers: like Paddle mixers, Blade mixers and Tube mixers. Millers: like Ball millers, Rod millersetc. Usually, the miller is a metallic or ceramic cylinder partially filled with hard balls or rods. The cylinder rotates via rollers with constant speed. The rotating speed can be chosen as desired. The impacts between the powder particle and the balls or rods assist grinding and homogenizing. 4. Forming Forming is the manufacturing of green bodies that have enough strength to handle, i.e. for transportation to the furnace. Forming is achieved via different procedures which depend on the type of the powder. The processed powder can be dry powder, have some sort of plasticity, a paste or slurry.
55
Materials Selection Lecture otes, 2010
Ass. Prof. Dr. Saad B. H. Farid
Usually, these powders are formed via compaction with different types of dies. The compaction of powders aimed to pack the powder particle and the green body have its shape and strength. The main types of compaction of powders are the Dry, Semi Dry and Isostatic Compression. Where, the main type of compaction of powder pastes is Extrusion, Injection Molding. The forming of Slurry is via special technique called Slip Casting. a. Die Compaction A technique used extensively for forming of ceramic powder. Where, the powder is filled in a hard and tough die. Then the powder is compressed with particular pressure utilizing special press systems. The resultant green compact should have enough strength to handle. Lubricants and plasticizers can be used with this process. Lubricants are oily (waxy) liquids which reduces the "die-wall friction" and the "powder compact - wall" friction. Plasticizers are special oils (slippery) liquids which reduces the inter-particle friction.
Top Punch
Die Body Powder Sample Bottom Punch Shims
The die compaction can be categorized into two types: (see figure) Single Die Compaction; where the pressing is carried out via the upper (top) punch only, and Double Die Compaction; where the pressing is carried out via both upper and lower (bottom) punch. The double die compaction produces green compacts with more uniform density compared with single die compaction. b. Isostatic Press Also called Cold Isostatic Press (CIP) in which a balanced hydraulic pressure is applied on the power located in flexible, tightly closed envelope. The pressing fluid could be liquid or at most compressed air. High degree of homogeneity of green density
56
Materials Selection Lecture otes, 2010
Ass. Prof. Dr. Saad B. H. Farid
is achieved via this technique; the following figure illustrates the principle.
c. Hot Isostatic Press (HIP) As in CIP, a balanced hydraulic pressure is applied on the power located in flexible, tightly closed envelope. The fluid conveys both hydraulic pressure and heat. Thus, densification takes due to the application of pressure and heat. This technique is the best known so far that produce high and uniform sintered products. d. Hydro-plastic Forming In which clay minerals are processed making use of the plate-like microstructure of these minerals. An adequate (sufficient) amount of water is added, and then the paste is manipulated to the required form. The formed green bodies are dried carefully before firing (sintering). e. Extrusion Extrusion of powder pastes can be done when a sufficient amount of moisture content and plasticizers are present. Mixes contains clay minerals need less amount of plasticizers. The "extruder" presses the powder paste from one side to longitudinal channel through which air bubbles should be withdrawn. A die is attached at the other end of the extruder. The die is of special shape (circular opening etc) that controls the final form of the paste. Special attention should be paid for the moisture content of the starting paste and to the drying step. f. Slip Casting This technique is used for materials which can produce suspension in liquids like water. The suspension is poured in sponge like (rich of porosity) cast, e.g. pre- synthesized gypsum cast. The liquid is partially absorbed by the cast and the extra slip is poured off. The resultant "shell" (green body) is removed from the
57
Materials Selection Lecture otes, 2010
Ass. Prof. Dr. Saad B. H. Farid
cast after it gained sufficient strength. The following figure illustrates these steps.
5. Sintering Sintering is critical step in fabricating and developing ceramic and metallic materials. It is a high-temperature process during which a powder compact generally shrinks, decreasing its pore volume, and increases its bulk density. The solid state sintering process during which shrinkage occurs can be divided into initial, intermediate, and final stages as shown in figure below. Sintering is a process of consolidation of particles under the temperature below the melting point and caused, therefore, mostly by solid state reactions. Sintering forms solid bonds reducing the free surface. Thus, the total interfacial free energy of an assembly of particles is reduced.
1. Final stage Relative Density
Intermediate stage
Initial stage 0. Sintering Time Typical densification curve
58
Materials Selection Lecture otes, 2010
Ass. Prof. Dr. Saad B. H. Farid
In this process, the grain grows and the pore volume is reduced, leading to a compact mass. The temperature necessary to induce such bonding depends upon the characteristics of material and particle size distribution. Many theories describing various stages and transport phenomena have been proposed to describe the sintering phenomenon. The sintering phenomena can be categorized as follows: Solid State Sintering Liquid Phase Sintering Activated Sintering a. Solid State Sintering (S.S.S.) This type of sintering takes place between particles of single or multiple phases without the existence of liquid (melt) phase, where homogenization occurs during the sintering of mixed phase that form a single-phase product. Some powders such as Alumina and Magnesia can be sintered in the pure state by solid state sintering. The initial stage of sintering is identified by formation of particle contacts and no grain growth. The intermediate stage is identified by the presence of continuous open-pore channels and grain growth. The final stage is identified as the closed-pore stage and continuous grain growth. b. Liquid Phase Sintering (L.Ph.S.) The liquid phase sintering process is usually divided into three different partly overlapping stages. The first stage involves melt formation, spreading over the solid particle surfaces and some shrinkage due to rearrangement. The second stage involves partial solution of the solid into the liquid and re-precipitation of the solid from the liquid. The major part of the densification and shape adjustments of the solid particles takes place at this stage. The third stage has a more rigid structure due to a solid skeleton and thus shows noticeably slow densification. c. Activated Sintering (A.S.) In this case, reaction takes place during sintering process which enhances sintering. For example; Sialons are generally produced by a version of activated sintering. This is because the material is usually formed from a powder mixture of Si3N4, AIN and Al2O3 by chemical process in which the powders are dissolved in a liquid phase and reprecipitated in the form of sialon
59
Materials Selection Lecture otes, 2010
Ass. Prof. Dr. Saad B. H. Farid
2.6 The cost aspects of process selection
The manufacturing cost, for any process, can be considered to be made up of two elements; initial costs and running costs; for example; (a) Low initial cost but high running cost per item, e.g. sand casting, (b) High initial cost but low running cost per item, e.g. die casting. The Cost elements are made up as follows: 1- Initial costs Capital costs for installations, e.g. the cost of a machine or a foundryetc. However, in any one year there will be depreciation (decrease) of the assessment and this is the capital cost that is paid against the output of the product in that year. Another element of initial costs is the cost of dies or tools needed specifically for the product concerned. Other factors we could include in the initial costs are plant maintenance and tool, die repair, or refurbish. 2- Running costs The running costs are the material costs, the labor costs, the power costs, and any finishing costs required. Professional Materials Engineering Code:
Selection of Materials + Selection of Process = Selection of Technological Root
Problems:
1- Suggest a casting process for the following situation. A small one-off casting is required using aluminium, there is a lot of fine detail which has to be reproduced and a good surface finish is required. 2- Suggest processes that might be used to make the following metal products: (a) A toothpaste tube from a very soft alloy. (b) Mass production of grooved pulley wheels. (c) An aluminium can for drink storage. (d) A hollow hexagonal length of brass rod. (e) Railway lines. (f) A kitchen pan from aluminium. 3- Suggest processes that might be used to make the following polymer products: (a) A small toy at high production rates in a thermoplastic material. (b) A1liter bottle for a soft drink at high production rates, using a thermoplastic material. (c) A switch cover at high production rates in a thermosetting material. (d) Milk bottle about 340 mm diameter and 760 mm high from polyethylene. (e) A thermop1astic strip for use as a draught excluder with windows, long lengths being required. (f) Polyethylene bags with high production rates. (g) The body of a camera with reasonably high production rates. (h) The bodywork of an electric drill, threaded holes and high production rates being required.
60
Materials Selection Lecture otes, 2010
Ass. Prof. Dr. Saad B. H. Farid
Example Flow Chart for Selection of Technological Root
61
Materials Selection Lecture otes, 2010
Ass. Prof. Dr. Saad B. H. Farid
Bibliography for extended study
Materials Selection and DesignGeneral Budinski, K., Engineering Materials, Properties and Selection, Prentice-Hall, Englewood Cliffs, NJ, 1979. Charles, J. A., F. A. A. Crane, and J. A. G. Furness, Selection and Use of Engineering Materials, 3rd ed., Butterworth, Oxford, 1987. Dieter, G. E., Engineering Design, A Materials and Processing Approach, 2nd ed., McGraw-Hill, NewYork, 1991. Ertas, A., and J. C. Jones, The Engineering Design Process, John Wiley & Sons, New York, 1993. Farag, M. M., Selection of Materials and Manufacturing Processes for Engineering Design, Prentice-Hall, Englewood Cliffs, NJ, 1989. French, M. J., Conceptual Design for Engineers, The Design Council, London and Springer, Berlin, 1985. Howell, S. K., Engineering Design and Problem Solving, 2nd ed., Prentice-Hall, Upper Saddle River, NJ, 2002. Lewis, G., Selection of Engineering Materials, Prentice-Hall, Englewood Cliffs, NJ, 1990. Ullman, D. G., The Mechanical Design Process, McGraw-Hill, New York, 1992. Materials Selection in Designing with Metals ASM Metals Handbook, 10th ed., ASM International, Metals Park, OH, 1990. Aluminum and Aluminum Alloys, A. L. Phillips, ed., American Welding Society, New York, 1967. Materials Selection in Designing with Ceramics and Glasses Wachtman, J. B., Mechanical Properties of Ceramics, John Wiley & Sons, New York, 1996. Materials Selection in Designing with Polymers Powell, P. C., and A. J. Ingen Housz, Engineering with Polymers, 2nd ed., Stanley Thornes, Cheltenham, UK, 1998. Materials Selection in Designing with Composites Mallick, P. K., Fiber-Reinforced Composites: Materials, Manufacturing, and Design, Marcel Dekker, NewYork, 1988. Materials Selection in Designing with Biologics Hill, D., Design Engineering of Biomaterials for Medical Devices, John Wiley & Sons, New York, 1998.
62
Vous aimerez peut-être aussi
- Material Selection Notes 2Document206 pagesMaterial Selection Notes 2hschoi12Pas encore d'évaluation
- Composites TestingDocument29 pagesComposites TestingThiru MoorthyPas encore d'évaluation
- What Are PlasticsDocument84 pagesWhat Are PlasticsBhuvanesh PonnanPas encore d'évaluation
- Materials Selection Project PDFDocument14 pagesMaterials Selection Project PDFeddunbarPas encore d'évaluation
- Lesson 13 - Material Selection Process - Rev. 0Document21 pagesLesson 13 - Material Selection Process - Rev. 0Naufal RafifPas encore d'évaluation
- Selection of Materials To Resist Failure: 1 Materials and Process Selection For Engineering Design: Mahmoud FaragDocument44 pagesSelection of Materials To Resist Failure: 1 Materials and Process Selection For Engineering Design: Mahmoud FaragSesmaroBermanPas encore d'évaluation
- Hypermesh TopicsDocument9 pagesHypermesh TopicsARUN PATILPas encore d'évaluation
- Corrosion Btech PDFDocument29 pagesCorrosion Btech PDFKeshav ShyamPas encore d'évaluation
- Engineering MaterialsDocument53 pagesEngineering MaterialsRAGINI PASUPULETIPas encore d'évaluation
- Mechanical Properties PDFDocument57 pagesMechanical Properties PDFvardhaPas encore d'évaluation
- 06 Sparger PDFDocument17 pages06 Sparger PDFHafidho Ilham MPas encore d'évaluation
- Non-Traditional Machining Processes ExplainedDocument33 pagesNon-Traditional Machining Processes ExplainedRavichandran GPas encore d'évaluation
- Composite Materials: Dr. S.M.K. HosseiniDocument61 pagesComposite Materials: Dr. S.M.K. HosseiniJainam ShahPas encore d'évaluation
- Solid Modeling AssignmentDocument18 pagesSolid Modeling AssignmentKarthick SanPas encore d'évaluation
- Analysis of A Failed Pipe Elbow in Geothermal Production Facility PDFDocument7 pagesAnalysis of A Failed Pipe Elbow in Geothermal Production Facility PDFAz ArPas encore d'évaluation
- Drying Processes: Mr. Pankaj Kusum Ramdas KhuspeDocument9 pagesDrying Processes: Mr. Pankaj Kusum Ramdas KhuspedadaPas encore d'évaluation
- 228 TOP Gas Turbines and Jet Engines MCQsDocument34 pages228 TOP Gas Turbines and Jet Engines MCQsTobaPas encore d'évaluation
- Mebc Q BankDocument8 pagesMebc Q BankSamir LohiyaPas encore d'évaluation
- TUTORIAL Material SelectionDocument5 pagesTUTORIAL Material SelectionbbaskaranPas encore d'évaluation
- Surface Chemistry MCQs Questions: Paper - 1 and 2Document4 pagesSurface Chemistry MCQs Questions: Paper - 1 and 2singamroopaPas encore d'évaluation
- INVESTMENT CASTING DESIGN GUIDEDocument27 pagesINVESTMENT CASTING DESIGN GUIDENisha Singh100% (1)
- Selection of Materials For Engineering ApplicationsDocument151 pagesSelection of Materials For Engineering ApplicationsCharitha Ranwala100% (1)
- Guide Material Selection Process for Engineering DesignDocument35 pagesGuide Material Selection Process for Engineering DesignAlok KumarPas encore d'évaluation
- Mechanics of Materials Lecture Notes PDFDocument130 pagesMechanics of Materials Lecture Notes PDFNaveed HassanPas encore d'évaluation
- Introduction-2: Ahmet ErkliğDocument37 pagesIntroduction-2: Ahmet ErkliğSelva KumarPas encore d'évaluation
- The Nernst Equation and Pourbaix DiagramsDocument16 pagesThe Nernst Equation and Pourbaix DiagramsRSL0% (1)
- 2 Thermodynamics & KineticsDocument71 pages2 Thermodynamics & KineticssmrutiPas encore d'évaluation
- Understanding Creep and Fatigue Deformation in MaterialsDocument20 pagesUnderstanding Creep and Fatigue Deformation in Materialsgaspardo123Pas encore d'évaluation
- Basic On K-E ModelDocument7 pagesBasic On K-E ModelSayeem ZamanPas encore d'évaluation
- Lecture Notes For CO 1 Part 2 - FailureDynL (Chapter 6)Document115 pagesLecture Notes For CO 1 Part 2 - FailureDynL (Chapter 6)Firdaus Ilias100% (1)
- Carbon Carbon CompositesDocument4 pagesCarbon Carbon CompositesHuzaifa ShabbirPas encore d'évaluation
- Aerospace Interview QuestionsDocument9 pagesAerospace Interview QuestionsrsugarmanPas encore d'évaluation
- Composites Structural Weight OptimizationDocument146 pagesComposites Structural Weight OptimizationViswanathan Srk100% (2)
- Differentiation Between Packed and Plate Distillation ColumnDocument10 pagesDifferentiation Between Packed and Plate Distillation ColumnAhmed Garoot100% (2)
- Strength of Materials - Mechanical Engineering Multiple Choice Questions and Answers For Competitive ExamsDocument212 pagesStrength of Materials - Mechanical Engineering Multiple Choice Questions and Answers For Competitive Examsahmish kabbaxePas encore d'évaluation
- Modelling Composite Materials PDFDocument22 pagesModelling Composite Materials PDFchetan_thakur4278Pas encore d'évaluation
- CFD Methodology 01Document14 pagesCFD Methodology 01fffPas encore d'évaluation
- Polymer Processing Course DescriptionDocument3 pagesPolymer Processing Course DescriptionarobaidiPas encore d'évaluation
- Introduction To Composite MaterialsDocument74 pagesIntroduction To Composite Materialsswordprinces100% (1)
- Tool Wear and Tool LifeDocument16 pagesTool Wear and Tool Lifedraco555Pas encore d'évaluation
- ProjectDocument24 pagesProjectSantosh Kumar HottaPas encore d'évaluation
- LECTURE ON MATERIALS FOR UREA PLANTSDocument19 pagesLECTURE ON MATERIALS FOR UREA PLANTSdeepankar kumarPas encore d'évaluation
- Scanning probe microscopy techniques and principlesDocument4 pagesScanning probe microscopy techniques and principlesbabakPas encore d'évaluation
- General Introduction Introduction of Composites: Historical Development / Historical Overview: PastDocument514 pagesGeneral Introduction Introduction of Composites: Historical Development / Historical Overview: PastMuthuvel MPas encore d'évaluation
- Material Selection TutorialDocument18 pagesMaterial Selection TutorialArShAdPas encore d'évaluation
- Aluminium Titanate: Chemical FormulaDocument6 pagesAluminium Titanate: Chemical FormulaaadhanPas encore d'évaluation
- 7 BondingCompositesDocument63 pages7 BondingCompositesVisan NaiduPas encore d'évaluation
- Tutorial 21 - Seal RelaxationDocument11 pagesTutorial 21 - Seal RelaxationalperenacarPas encore d'évaluation
- Basics of ThermodynamicsDocument32 pagesBasics of Thermodynamicsramshad ekPas encore d'évaluation
- Mechanics of FluidsDocument144 pagesMechanics of Fluidsanon_104522736100% (1)
- Air Refrigeration SystemDocument29 pagesAir Refrigeration SystemSumit KumarPas encore d'évaluation
- Carbon Carbon CompositeDocument22 pagesCarbon Carbon Compositeyogeshkmr01100% (1)
- SK Mondal PDFDocument9 pagesSK Mondal PDFramPas encore d'évaluation
- Metal Casting TechniquesDocument81 pagesMetal Casting TechniquesAshok PradhanPas encore d'évaluation
- Week 1 - IPE 2203-LecturesDocument51 pagesWeek 1 - IPE 2203-LecturesMD Al-AminPas encore d'évaluation
- Introduction to Material Science and EngineeringDocument5 pagesIntroduction to Material Science and EngineeringJohnrey ParchamentoPas encore d'évaluation
- Materials EngineeringDocument61 pagesMaterials EngineeringAshwit KumarPas encore d'évaluation
- Lecture 1 IntroductionDocument21 pagesLecture 1 IntroductionKelvin Ken WitsPas encore d'évaluation
- Me 323-A1m - Tade0, Clarisse F.Document11 pagesMe 323-A1m - Tade0, Clarisse F.Tadeo, Clarisse F.Pas encore d'évaluation
- Ch01 Introduction v2plDocument13 pagesCh01 Introduction v2plRoyce DicaprioPas encore d'évaluation
- Higher Education Department Webinar 30.05.2021Document1 pageHigher Education Department Webinar 30.05.2021Karthick NPas encore d'évaluation
- EEE Alumni Interaction 01.06.2021Document1 pageEEE Alumni Interaction 01.06.2021Karthick NPas encore d'évaluation
- "Smart City Infrastructures": Online Alumni InteractionDocument1 page"Smart City Infrastructures": Online Alumni InteractionKarthick NPas encore d'évaluation
- ME8501 MM - Generalized Measurement SystemDocument1 pageME8501 MM - Generalized Measurement SystemKarthick NPas encore d'évaluation
- ME8501 MM - Types of MetrologyDocument1 pageME8501 MM - Types of MetrologyKarthick NPas encore d'évaluation
- International Conference On Radiation Biology (ICRB 2016)Document12 pagesInternational Conference On Radiation Biology (ICRB 2016)Karthick NPas encore d'évaluation
- VTMT - Aicte FDP - Bigdata BrochureDocument2 pagesVTMT - Aicte FDP - Bigdata BrochureKarthick NPas encore d'évaluation
- Me8491 emDocument1 pageMe8491 emKarthick NPas encore d'évaluation
- (SESBT 2020) : Sustainable Energy Solutions For A Better TomorrowDocument8 pages(SESBT 2020) : Sustainable Energy Solutions For A Better TomorrowKarthick NPas encore d'évaluation
- Srmasc Admission Brochure 2020Document1 pageSrmasc Admission Brochure 2020Karthick NPas encore d'évaluation
- VIT Chennai hosts 5-day online workshop on MetallurgyDocument2 pagesVIT Chennai hosts 5-day online workshop on MetallurgyKarthick NPas encore d'évaluation
- ME8501 MM - Elements of Measuring System (SWIPE)Document1 pageME8501 MM - Elements of Measuring System (SWIPE)Karthick NPas encore d'évaluation
- ME8501 MM - Need For Metrology PDFDocument1 pageME8501 MM - Need For Metrology PDFKarthick NPas encore d'évaluation
- ME8501 MM - Definition of SI Units PDFDocument1 pageME8501 MM - Definition of SI Units PDFKarthick NPas encore d'évaluation
- ME8501 MM - Accuracy Vs PrecisionDocument1 pageME8501 MM - Accuracy Vs PrecisionKarthick NPas encore d'évaluation
- Functional Management: National Level E-Quiz SeriesDocument1 pageFunctional Management: National Level E-Quiz SeriesKarthick NPas encore d'évaluation
- ME8501 MM - Traceability & CalibrationDocument1 pageME8501 MM - Traceability & CalibrationKarthick NPas encore d'évaluation
- ME8501 MM - Unit Systems PDFDocument1 pageME8501 MM - Unit Systems PDFKarthick NPas encore d'évaluation
- MATHS QuizDocument1 pageMATHS QuizKarthick NPas encore d'évaluation
- ME8501 MM - What Is Metrology PDFDocument1 pageME8501 MM - What Is Metrology PDFKarthick NPas encore d'évaluation
- Webinar - ResearchDocument1 pageWebinar - ResearchKarthick NPas encore d'évaluation
- ME8501 MM - SI Units PDFDocument1 pageME8501 MM - SI Units PDFKarthick NPas encore d'évaluation
- Webinar - SpaceDocument1 pageWebinar - SpaceKarthick NPas encore d'évaluation
- Online Quiz: Department of Information TechnologyDocument1 pageOnline Quiz: Department of Information TechnologyKarthick NPas encore d'évaluation
- Webinar - Materials PDFDocument1 pageWebinar - Materials PDFKarthick NPas encore d'évaluation
- Co Po For Me6504 MMDocument3 pagesCo Po For Me6504 MMKarthick NPas encore d'évaluation
- Webinar - Electric VehicleDocument1 pageWebinar - Electric VehicleKarthick NPas encore d'évaluation
- Heat TreatmentDocument4 pagesHeat TreatmentAshwani DograPas encore d'évaluation
- ME8491 Engineering Metallurgy COURSE PLANDocument4 pagesME8491 Engineering Metallurgy COURSE PLANKarthick NPas encore d'évaluation
- 10th Standard Tamilnadu State Board Science (English Medium)Document283 pages10th Standard Tamilnadu State Board Science (English Medium)Karthick NPas encore d'évaluation
- Stress Strain DiagramDocument5 pagesStress Strain DiagramkalaivananmekPas encore d'évaluation
- Hollow Sections in Structural Applications 2nd EditionDocument240 pagesHollow Sections in Structural Applications 2nd Edition_at_to_100% (1)
- Análisis de La Formabilidad de Láminas de Acero AISI 304 Con Diferentes Espesores Mediante Sus Propiedades de TracciónDocument9 pagesAnálisis de La Formabilidad de Láminas de Acero AISI 304 Con Diferentes Espesores Mediante Sus Propiedades de TracciónLuis Carlos Moscote AtencioPas encore d'évaluation
- Astm A193/a 193MDocument13 pagesAstm A193/a 193MCLEMENTPas encore d'évaluation
- AmDocument68 pagesAmegdggffddPas encore d'évaluation
- Utp Af A7Document1 pageUtp Af A7VIRPOPPas encore d'évaluation
- Ch17 Metal FormingDocument37 pagesCh17 Metal FormingSaman BrookhimPas encore d'évaluation
- Materials Properties Guide: Alloys, Heat Treatments & MoreDocument49 pagesMaterials Properties Guide: Alloys, Heat Treatments & Moreenchong091Pas encore d'évaluation
- 2 Bulk Deformation ProcessesDocument67 pages2 Bulk Deformation ProcessesAsadPas encore d'évaluation
- Physics of Climbing Ropes - Part 4Document17 pagesPhysics of Climbing Ropes - Part 4Jagrawecya FE unsoedPas encore d'évaluation
- ME414 Section 1Document12 pagesME414 Section 1OvaizOwaisPas encore d'évaluation
- EML3500 CH 2 SlidesDocument71 pagesEML3500 CH 2 SlideshaysamPas encore d'évaluation
- Manufacturing Processes: by Premchand Kumar Deoghar (Jharkhand)Document49 pagesManufacturing Processes: by Premchand Kumar Deoghar (Jharkhand)PremKumarPas encore d'évaluation
- 18 Fundamentals of Metal Forming Review Questions and Multiple Choice QuizDocument2 pages18 Fundamentals of Metal Forming Review Questions and Multiple Choice Quizhzeem50% (2)
- SS304 Hardening BehaviourDocument4 pagesSS304 Hardening BehaviourrbagriPas encore d'évaluation
- Fundementals of Metal FormingDocument23 pagesFundementals of Metal FormingMasa FuadPas encore d'évaluation
- CH 2 Slides 10th Ed SIDocument72 pagesCH 2 Slides 10th Ed SIRezende JulioPas encore d'évaluation
- Lecture 07 Mechanical Properties 2Document20 pagesLecture 07 Mechanical Properties 2antoine demeirePas encore d'évaluation
- Non-Ferrous Metals Manufacturing ProcessesDocument10 pagesNon-Ferrous Metals Manufacturing ProcessesKarthik SubramaniPas encore d'évaluation
- Uddeholm Pocket Book E2Document76 pagesUddeholm Pocket Book E2sunilPas encore d'évaluation
- "Machinability Studies Using Hot Machining Technique": A Case Study OnDocument21 pages"Machinability Studies Using Hot Machining Technique": A Case Study OnKarthik B KamathPas encore d'évaluation
- Specification For The Use of Steel Tanks in The Water Industry PDFDocument15 pagesSpecification For The Use of Steel Tanks in The Water Industry PDFJorge Alberto Martinez OrtizPas encore d'évaluation
- 04 0002 PDFDocument122 pages04 0002 PDFjbPas encore d'évaluation
- Engineering Properties of Nickel and Nickel AlloysDocument240 pagesEngineering Properties of Nickel and Nickel AlloysOR Premium FreePas encore d'évaluation
- CuZn 39 PB 3Document2 pagesCuZn 39 PB 3lomas34Pas encore d'évaluation
- Analysis of Fracture and Cracks of Oldham S Couplings - 2013 - Engineering FailDocument7 pagesAnalysis of Fracture and Cracks of Oldham S Couplings - 2013 - Engineering FailArimateia SoaresPas encore d'évaluation
- Fundamental of Metal FormingDocument5 pagesFundamental of Metal FormingAnuj BhardwajPas encore d'évaluation
- EMM 315 Materials Forming Processes - METAL FORMINGDocument91 pagesEMM 315 Materials Forming Processes - METAL FORMINGKimani JohnPas encore d'évaluation
- Low-Cycle Fatigue Properties of Steel 42crmo4: R. Kunc, I. PrebilDocument8 pagesLow-Cycle Fatigue Properties of Steel 42crmo4: R. Kunc, I. PrebilVijayakumar SamyPas encore d'évaluation
- Steel: Stones Bricks Cement ConcreteDocument46 pagesSteel: Stones Bricks Cement ConcreteKaushik RPas encore d'évaluation