Académique Documents
Professionnel Documents
Culture Documents
TMP 847 E
Transféré par
FrontiersDescription originale:
Titre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
TMP 847 E
Transféré par
FrontiersDroits d'auteur :
Formats disponibles
OPINION ARTICLE
published: 08 May 2013
doi: 10.3389/fnins.2013.00064
The anterior cingulate cortex: an integrative hub for human
socially-driven interactions
Claudio Lavin1,2,3 , Camilo Melis 3 , Ezequiel Mikulan 4 , Carlos Gelormini 4 , David Huepe 2 and
Agustin Ibañez 2,4*
1
Center of Argumentation and Reasoning Studies, Universidad Diego Portales, Santiago, Chile
2
Laboratory of cognitive and social neuroscience, Universidad Diego Portales, Santiago, Chile
3
Facultad de Economía y Empresa, Centro de Neuroeconomía, Universidad Diego Portales, Santiago, Chile
4
Laboratory of Experimental Psychology and Neuroscience, Institute of Cognitive Neurology (INECO), Favaloro University, Buenos Aires, Argentina
*Correspondence: aibanez@ineco.org.ar
Edited by:
Steve W. Chang, Duke University, USA
Masaki Isoda, Kansai Medical University, Japan
Reviewed by:
Steve W. Chang, Duke University, USA
The activity of the anterior cingulate cor- for instance, a robust affectation of ERN (Etkin et al., 2011). The ACC receives
tex (ACC) has been related to decision- has been found (Stemmer et al., 2004; inputs from these structures relative to the
making (Gehring and Willoughby, 2002; Hogan et al., 2006). Intracranial mea- differences between expected and actual
Sanfey et al., 2003; Mulert et al., 2008), surements confirmed ACC involvement in outcomes of a given decision, and pro-
socially-driven interactions (Sanfey et al., ERN (Brazdil et al., 2005; Jung et al., vides outputs to coordinate dorsolateral
2003; Rigoni et al., 2010; Etkin et al., 2011), 2010), and the same evidence has been prefrontal structures in order to organize
and empathy-related responses (van Veen found with source localization (Dehaene behavioral responses (Cohen et al., 2005;
and Carter, 2002; Gu et al., 2010; Lamm et al., 1994; Holroyd et al., 1998; van Veen Mansouri et al., 2009; Shackman et al.,
et al., 2011). We present a perspec- and Carter, 2002; Donamayor et al., 2011; 2011; see Figure 1).
tive of how to interpret the evidence of Bediou et al., 2012; Ibáñez et al., 2012) and Furthermore, several studies show
ACC involvement in these three processes, magneto-encephalography (Miltner et al., ACC activation indexing empathy-related
propose an ACC integrative function, 2003). These findings are supported by response in pain/no-pain paradigms. The
and provide a methodological pathway fMRI studies that indicate the activation ACC is a core component of the pain net-
to study decision making, empathy, and of the dorsal and rostral areas of the work which is active when subjects receive
social interaction in a combined experi- ACC when subjects receive feedback after pain stimuli and can also be activated
mental approach. losses associated with errors in decision- when observing others in such situa-
Error detection and outcome moni- making tasks (Bush et al., 2002; Marsh tions (see Figure 1). This pain network
toring are two important decision pro- et al., 2007). There is also animal evi- involves activity in the bilateral anterior
cesses related to ACC activation (Bush dence that shows specific anterior cingu- insula (AI), rostral ACC, brainstem, and
et al., 2000; Gehring and Willoughby, late sulcus activation with respect to one’s cerebellum when observing a loved one
2002; Hewig et al., 2011). Although the foregone rewards, and of the anterior cin- experiencing pain, and activity in the pos-
ACC was previously associated with basic gulate gyrus (ACCg) with respect to self, terior insular/secondary somatosensory
error detection processes (Carter et al., others’ or both players’ rewards (Chang cortex, the sensorimotor cortex (SI/MI),
1998; van Veen et al., 2001), evidence et al., 2013). This evidence shows that the and caudal ACC when experiencing pain
from electroencephalographic (EEG) and ACC is a part of the decision-making net- (Singer et al., 2004, 2006; Jackson et al.,
functional magnetic resonance imaging work that involves activity in prefrontal 2005, 2006; Decety and Jackson, 2006;
(fMRI) during the last decade has sug- and parietal areas related to the observa- Lamm et al., 2011). Moreover, the acti-
gested the involvement of the ACC in tion of alternatives (Platt and Glimcher, vation of the ACC in observational-pain
high-level processing (in outcome/error 1999; Westendorff et al., 2010), and activ- paradigms is modulated by contextual
monitoring and action planning; Bush ity in the orbitofrontal (OFC) and ven- information about the one observed.
et al., 2000). The error-related negativ- tromedial prefrontal cortex related to the For instance, observing a prosocial sub-
ity (ERN) and feedback-related negativity representation of option values (Buckley ject receiving pain stimulation triggers
(FRN), two event-related potentials (ERP) et al., 2009; Mullette-Gillman et al., 2011). empathy responses reflected in increased
that consistently follow action errors and There is also evidence of connections of bilateral activity of the AI and the ACC,
negative outcomes, respectively (e.g., San the ACC to the insula, related to interocep- compared to observing an antisocial sub-
Martin et al., 2010), are associated with tive markers of negative emotions (Ibanez ject (Singer et al., 2006). This evidence
activity in the ACC. The evidence of et al., 2010b; Jones et al., 2011; Kunz et al., suggests the involvement of the ACC
the ACC involvement in the ERN and 2011; Couto et al., 2013). In addition, there in high-level cognitive processing when
FRN is consistent across different types is evidence that central-rostral areas of the observing others and its modulation by
of studies. In patients with ACC lesions, ACC are connected to the limbic system critical contextual cues.
www.frontiersin.org May 2013 | Volume 7 | Article 64 | 1
Lavin et al. ACC and social interaction
responses modulate cooperative behav-
ior, outcome processing, and decision-
making. In brief, most of the evidence
provided focuses on just one variable (e.g.,
outcome monitoring or empathy) and
there is no theoretical approach that has
been able to integrate all variables together.
Furthermore, ERP studies on the contex-
tual cues involved in error or outcome pro-
cessing tend to associate unpleasant social
contexts with negative economic feedback
(Boksem and De Cremer, 2010). For this
reason, it is hard to evaluate the influence
of contextual social cues on the processes
of decision-making. Also, traditional fMRI
studies, which focused on empathy, tended
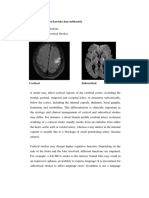
FIGURE 1 | Brain areas commonly active during empathy-related responses and decision
to put aside variables associated with out-
making tasks. (A) Axial view of the bilateral insula. (B) Sagittal view of the anterior cingulate cortex
(ACC), medial prefrontal cortex (MPFC), and orbitofrontal cortex (OFC). come processing.
A further approach for studying the
role of the ACC in the integration of
This high-level contextual processing of grative role of the ACC, the specificity social information, empathy and decision-
the ACC has also been studied regarding of the ACC activation in decision-making making, should involve the confrontation
socio-affective variables within traditional paradigms when there are contextual of these factors in a single paradigm.
decision-making paradigms. ACC is active cues, together with the role of the This would allow us to observe the
when people observe others’ action errors, ACC in empathy-related responses with- influence of contextual information on
but this activation is modulated by group out outcome feedback give support to this empathy responses, and, in turn, to
membership of social stimuli (Newman- interpretation. evaluate whether these responses modu-
Norlund et al., 2009; Hein et al., 2010). There is consistent evidence of the late the monitoring of wins and losses.
ERP studies have also provided evidence in active role that the ACC plays in the For instance, fairness/unfairness consider-
this line, showing FRN modulation asso- processing of multimodal of context- ations about others’ behavior may trig-
ciated with (1) unfairness considerations dependent events, compared to non- ger different levels of empathy-related
in socio-economical interactions (Boksem contextual stimuli (Downar et al., 2001, responses depending on whether the
and De Cremer, 2010), (2) observing a 2002). This evidence is in line with observer profits from such behavior or
friend or a stranger playing a gambling the idea that social cognition involves not. Thus, if a given subject profits
task (Ma et al., 2011), and also (3) offers the integration of flexible and context- from someone else’s unfair behavior, ACC
made by a computer program vs. humans dependent information (Chang et al., activity might be affected by the eco-
in ultimatum games (UG) (Fukushima 2011; Ibanez and Manes, 2012). Taken nomic benefit of such unfair behavior.
and Hiraki, 2009). These neuroimaging together, these data suggest that the ACC This experimental model could explore
and electrophysiological experiments sug- might be a center of integration of infor- ACC activity within conflicting situa-
gest that ACC integrates high level infor- mation about others’ social background tions between negative emotional states
mation for making decisions that involve that has a direct effect on economic (e.g., feeling bad for observing someone
economic and social concerns. The pro- interactions. Thus, interacting with some- being exploited or committing an error),
cessing in the ACC is not just related to the one from an out-group is different than and the positive evaluation of outcomes
economic value of a given outcome, but interacting with someone from an in- derived from such situations. This could
also to the social aspects involved in the group (Ibanez et al., 2010a) not just show overlapping activity in the ACC, or
interaction. For example, the ACC activ- from a social perspective, but also in the activation of specific areas associated
ity would be differentially modulated if terms of how we process the economic with error detection, outcome process-
people, in an UG, are willing to accept payoffs extracted by such interactions ing and empathy-related responses. The
unfair offers made by a computer pro- regarding our own and others’ welfare. same might happen when disentangling
gram or by a real player (Fukushima and This involves self-concern aspects of out- action errors from negative outcomes, as
Hiraki, 2009). Even though the payoffs come processing, and empathy responses some ERP studies are doing (de Bruijn
are the same, considerations about fair- modulated by social information about and von Rhein, 2012), where negativ-
ness/unfairness are attached to the eco- others. Although we know all these pro- ity associated with error detection exists
nomic interactions reflecting activity of cesses occur to some extent in the ACC, even if the outcomes are positive. Such
empathy networks, theory of mind (ToM) it remains unclear which specific social conflicts are common in real-life situa-
and decision-making (Etkin et al., 2011). cues modulate empathy in each group, tions and exploring them seems essential
Although this is not conclusive of the inte- and the degree to which empathy-related for understanding and predicting actions
Frontiers in Neuroscience | Decision Neuroscience May 2013 | Volume 7 | Article 64 | 2
Lavin et al. ACC and social interaction
within interactions under particular social Buckley, M. J., Mansouri, F. A., Hoda, H., Mahboubi, Gu, X., Liu, X., Guise, K. G., Naidich, T. P., Hof,
settings. M., Browning, P. G., Kwok, S. C., et al. (2009). P. R., and Fan, J. (2010). Functional dissocia-
Dissociable components of rule-guided behavior tion of the frontoinsular and anterior cingulate
The evidence summarized here sup-
depend on distinct medial and prefrontal regions. cortices in empathy for pain. J. Neurosci. 30,
ports the idea of the ACC as a cen- Science 325, 52–58. 3739–3744.
ter of high level contextual integration Bush, G., Luu, P., and Posner, M. I. (2000). Cognitive Hein, G., Silani, G., Preuschoff, K., Batson, C. D., and
and behavior monitoring. We believe that and emotional influences in anterior cingulate cor- Singer, T. (2010). Neural responses to ingroup and
a consistent and testable model of dif- tex. Trends Cogn. Sci. 4, 215–222. outgroup members’ suffering predict individual
Bush, G., Vogt, B. A., Holmes, J., Dale, A. M., Greve, differences in costly helping. Neuron 68, 149–160.
ferential empathy-related responses using
D., Jenike, M. A., et al. (2002). Dorsal anterior Hewig, J., Kretschmer, N., Trippe, R. H., Hecht,
critical contextual cues (such as per- cingulate cortex: a role in reward-based decision H., Coles, M. G., Holroyd, C. B., et al. (2011).
ceived fairness/unfairness or group iden- making. Proc. Natl. Acad. Sci. U.S.A. 99, 523–528. Why humans deviate from rational choice.
tity) within a decision-making setting Carter, C. S., Braver, T. S., Barch, D. M., Botvinick, Psychophysiology 48, 507–514.
could provide important insights about M. M., Noll, D., and Cohen, J. D. (1998). Anterior Hogan, A. M., Vargha-Khadem, F., Saunders, D. E.,
cingulate cortex, error detection, and the online Kirkham, F. J., and Baldeweg, T. (2006). Impact of
partially overlapping ACC networks of monitoring of performance. Science 280, 747–749. frontal white matter lesions on performance mon-
these three cognitive domains. Real-life Chang, S. W., Gariepy, J. F., and Platt, M. L. (2013). itoring: ERP evidence for cortical disconnection.
decision making is full of contextual cues Neuronal reference frames for social decisions in Brain, 129, 2177–2188.
that involve conflict between two or more primate frontal cortex. Nat. Neurosci. 16, 243–250. Holroyd, C. B., Dien, J., and Coles, M. G. (1998).
alternatives at the same time (Baez et al., Chang, S. W., Winecoff, A. A., and Platt, M. Error-related scalp potentials elicited by hand
L. (2011). Vicarious reinforcement in rhesus and foot movements: evidence for an output-
2012, 2013; Ibanez and Manes, 2012). macaques (macaca mulatta). Front. Neurosci. 5:27. independent error-processing system in humans.
People might feel empathy for a fair doi: 10.3389/fnins.2011.00027 Neurosci. Lett. 242, 65–68.
player’s loss but at the same time they Cohen, M. X., Heller, A. S., and Ranganath, C. Ibáñez, A., Cetkovich, M., Petroni, A., Urquina, H.,
might want to get benefits from a zero (2005). Functional connectivity with anterior cin- Baez, S., Gonzalez, L., et al. (2012). The neu-
gulate and orbitofrontal cortices during decision- ral basis of decision-making and reward pro-
sum interaction, so there is a decision to
making. [Clinical Trial]. Brain Res. Cogn. Brain cessing in adults with euthymic bipolar dis-
be made in terms of which strategy weighs Res. 23, 61–70. order or attention-deficit/hyperactivity disorder
more in the final output. In this context, Couto, B., Sedeño, L., Sposato, L., Sigman, M., Riccio, (ADHD). PLoS ONE 7:e37306. doi: 10.1371/
the role of the ACC would be essential P., Salles, A., et al. (2013). Insular networks for journal.pone.0037306.
for understanding how contextual infor- emotional processing and social cognition: com- Ibanez, A., and Manes, F. (2012). Contextual social
parison of two case reports with either cortical or cognition and the behavioral variant of frontotem-
mation shapes our strategic decisions, and
subcortical involvement. Cortex, 5, 1420–1434. poral dementia. Neurology 78, 1354–1362.
how this influences the way in which we de Bruijn, E. R. A., and von Rhein, D. T. (2012). Is your Ibanez, A., Gleichgerrcht, E., Hurtado, E., Gonzalez,
learn from others and evaluate them in error my concern? An event-related potential study R., Haye, A., and Manes, F. F. (2010a). Early
social terms. on own and observed error detection in coopera- neural markers of implicit attitudes: N170
tion and competition. Front. Neurosci. 6, 1–9. doi: modulated by intergroup and evaluative con-
10.3389/fnins.2012.00008 texts in IAT. Front. Hum. Neurosci. 4:188. doi:
ACKNOWLEDGMENTS Decety, J., and Jackson, P. L. (2006). A social- 10.3389/fnhum.2010.00188
This work was supported by grants neuroscience perspective on empathy. Curr. Dir. Ibanez, A., Gleichgerrcht, E., and Manes, F. (2010b).
FONDECYT (1130920), CONICET Psychol. Sci. 15, 54–58. Clinical effects of insular damage in humans. Brain
(Carlos Gelormini, Agustin Ibañez) and Dehaene, S., Posner, M. I., and Tucker, D. M. Struct. Funct. 214, 397–410.
(1994). Localization of a Neural System for Error Jackson, P. L., Brunet, E., Meltzoff, A. N., and Decety,
INECO Foundation.
Detection and Compensation. Psychol. Sci. 5, J. (2006). Empathy examined through the neu-
303–305. ral mechanisms involved in imagining how I feel
REFERENCES Donamayor, N., Marco-Pallares, J., Heldmann, M., versus how you feel pain. Neuropsychologia, 44,
Baez, S., Herrera, E., Villarin, L., Theil, D., Gonzalez- Schoenfeld, M. A., and Munte, T. F. (2011). 752–761.
Gadea, M. L., Gomez, P., et al. (2013). Contextual Temporal dynamics of reward processing revealed Jackson, P. L., Meltzoff, A. N., and Decety, J. (2005).
social cognition impairments in schizophrenia by magnetoencephalography. Hum. Brain Mapp. How do we perceive the pain of others? A win-
and bipolar disorder. PLoS ONE 8:e57664. doi: 32, 2228–2240. dow into the neural processes involved in empathy.
10.1371/journal.pone.0057664 Downar, J., Crawley, A. P., Mikulis, D. J., and Davis, Neuroimage, 24, 771–779.
Baez, S., Rattazzi, A., Gonzalez-Gadea, M. L., K. D. (2001). The effect of task relevance on the Jones, C. L., Minati, L., Harrison, N. A., Ward,
Torralva, T., Vigliecca, N. S., Decety, J., et al. cortical response to changes in visual and auditory J., and Critchley, H. D. (2011). Under pressure:
(2012). Integrating intention and context: assess- stimuli: an event-related fMRI study. Neuroimage response urgency modulates striatal and insula
ing social cognition in adults with Asperger 14, 1256–1267. activity during decision-making under risk. PLoS
syndrome. Front. Hum. Neurosci. 6:302. doi: Downar, J., Crawley, A. P., Mikulis, D. J., and Davis, K. ONE 6:e20942. doi: 10.1371/journal.pone.0020942
10.3389/fnhum.2012.00302 D. (2002). A cortical network sensitive to stimu- Jung, J., Jerbi, K., Ossandon, T., Ryvlin, P., Isnard,
Bediou, B., Koban, L., Rosset, S., Pourtois, G., and lus salience in a neutral behavioral context across J., Bertrand, O., et al. (2010). Brain responses
Sander, D. (2012). Delayed monitoring of accuracy multiple sensory modalities. J. Neurophysiol. 87, to success and failure: Direct recordings from
errors compared to commission errors in ACC. 615–620. human cerebral cortex. Hum. Brain Mapp. 31,
Neuroimage, 60, 1925–1936. Etkin, A., Egner, T., and Kalisch, R. (2011). Emotional 1217–1232.
Boksem, M. A., and De Cremer, D. (2010). Fairness processing in anterior cingulate and medial pre- Kunz, M., Chen, J. I., Lautenbacher, S., Vachon-
concerns predict medial frontal negativity ampli- frontal cortex. Trends Cogn. Sci. 15, 85–93. Presseau, E., and Rainville, P. (2011). Cerebral
tude in ultimatum bargaining. Soc. Neurosci. 5, Fukushima, H., and Hiraki, K. (2009). Whose loss is regulation of facial expressions of pain. J. Neurosci.
118–128. it? Human electrophysiological correlates of non- 31, 8730–8738.
Brazdil, M., Dobsik, M., Mikl, M., Hlustik, P., Daniel, self reward processing. Soc. Neuroci. 4, 261–275. Lamm, C., Decety, J., and Singer, T. (2011). Meta-
P., Pazourkova, M., et al. (2005). Combined event- Gehring, W. J., and Willoughby, A. R. (2002). The analytic evidence for common and distinct neural
related fMRI and intracerebral ERP study of an medial frontal cortex and the rapid processing of networks associated with directly experienced pain
auditory oddball task. Neuroimage, 26, 285–293. monetary gains and losses. Science 295, 2279–2282. and empathy for pain. Neuroimage 54, 2492–2502.
www.frontiersin.org May 2013 | Volume 7 | Article 64 | 3
Lavin et al. ACC and social interaction
Ma, Q., Shen, Q., Xu, Q., Li, D., Shu, L., and Weber, shots between friend and foe. Soc. Cogn. Affect. Stemmer, B., Segalowitz, S. J., Witzke, W., and
B. (2011). Empathic responses to others’ gains Neurosci. 4, 10–22. Schonle, P. W. (2004). Error detection in
and losses: an electrophysiological investigation. Platt, M. L., and Glimcher, P. W. (1999). Neural corre- patients with lesions to the medial prefrontal
Neuroimage, 54, 2472–2480. lates of decision variables in parietal cortex. Nature cortex: an ERP study. Neuropsychologia, 42,
Mansouri, F. A., Tanaka, K., and Buckley, M. J. (2009). 400, 233–238. 118–130.
Conflict-induced behavioural adjustment: a clue Rigoni, D., Polezzi, D., Rumiati, R., Guarino, R., van Veen, V., and Carter, C. S. (2002). The anterior
to the executive functions of the prefrontal cortex. and Sartori, G. (2010). When people matter more cingulate as a conflict monitor: fMRI and ERP
Nat. Rev. Neurosci. 10, 141–152. than money: An ERPs study. Brain Res. Bull. 81, studies. Physiol. Behav. 77, 477–482.
Marsh, A. A., Blair, K. S., Vythilingam, M., Busis, 445–452. van Veen, V., Cohen, J. D., Botvinick, M. M.,
S., and Blair, R. J. (2007). Response options and San Martin, R., Manes, F., Hurtado, E., Isla, P., Stenger, V. A., and Carter, C. S. (2001).
expectations of reward in decision-making: the and Ibanez, A. (2010). Size and probability of Anterior cingulate cortex, conflict monitor-
differential roles of dorsal and rostral anterior cin- rewards modulate the feedback error-related neg- ing, and levels of processing. Neuroimage, 14,
gulate cortex. Neuroimage 35, 979–988. ativity associated with wins but not losses in a 1302–1308.
Miltner, W. H., Lemke, U., Weiss, T., Holroyd, monetarily rewarded gambling task. Neuroimage Westendorff, S., Klaes, C., and Gail, A. (2010). The
C., Scheffers, M. K., and Coles, M. G. (2003). 51, 1194–1204. cortical timeline for deciding on reach motor
Implementation of error-processing in the human Sanfey, A. G., Rilling, J. K., Aronson, J. A., Nystrom, goals. J. Neurosci. 30, 5426–5436.
anterior cingulate cortex: a source analysis of the L. E., and Cohen, J. D. (2003). The neural basis
magnetic equivalent of the error-related negativity. of economic decision-making in the Ultimatum Received: 01 April 2013; accepted: 13 April 2013;
Biol. Psychol. 64, 157–166. Game. Science 300, 1755–1758. published online: 08 May 2013.
Mulert, C., Seifert, C., Leicht, G., Kirsch, V., Ertl, M., Shackman, A. J., Salomons, T. V., Slagter, H. A., Fox, Citation: Lavin C, Melis C, Mikulan E, Gelormini C,
Karch, S., et al. (2008). Single-trial coupling of EEG A. S., Winter, J. J., and Davidson, R. J. (2011). The Huepe D and Ibañez A (2013) The anterior cingulate
and fMRI reveals the involvement of early ante- integration of negative affect, pain and cognitive cortex: an integrative hub for human socially-driven
rior cingulate cortex activation in effortful decision control in the cingulate cortex. Nat. Rev. Neurosci. interactions. Front. Neurosci. 7:64. doi: 10.3389/fnins.
making. Neuroimage 42, 158–168. 12, 154–167. 2013.00064
Mullette-Gillman, O. A., Detwiler, J. M., Winecoff, A., Singer, T., Seymour, B., O’Doherty, J., Kaube, H., This article was submitted to Frontiers in Decision
Dobbins, I., and Huettel, S. A. (2011). Infrequent, Dolan, R. J., and Frith, C. D. (2004). Empathy for Neuroscience, a specialty of Frontiers in Neuroscience.
task-irrelevant monetary gains and losses engage pain involves the affective but not sensory compo- Copyright © 2013 Lavin, Melis, Mikulan, Gelormini,
dorsolateral and ventrolateral prefrontal cortex. nents of pain. Science 303, 1157–1162. Huepe and Ibañez. This is an open-access article dis-
Brain Res. 1395, 53–61. Singer, T., Seymour, B., O’Doherty, J. P., Stephan, tributed under the terms of the Creative Commons
Newman-Norlund, R. D., Ganesh, S., van Schie, H. T., K. E., Dolan, R. J., and Frith, C. D. (2006). Attribution License, which permits use, distribution and
de Bruijn, E. R., and Bekkering, H. (2009). Self- Empathic neural responses are modulated by reproduction in other forums, provided the original
identification and empathy modulate error-related the perceived fairness of others. Nature 439, authors and source are credited and subject to any copy-
brain activity during the observation of penalty 466–469. right notices concerning any third-party graphics etc.
Frontiers in Neuroscience | Decision Neuroscience May 2013 | Volume 7 | Article 64 | 4
Vous aimerez peut-être aussi
- Electrophysiological Precursors of Social ConformityDocument8 pagesElectrophysiological Precursors of Social Conformitypsic.tatianebezerraPas encore d'évaluation
- tmp5D49 TMPDocument8 pagestmp5D49 TMPFrontiersPas encore d'évaluation
- Mcrae 2010Document15 pagesMcrae 2010童帅Pas encore d'évaluation
- An Event-Related Examination of Neural Activity During Social InteractionsDocument7 pagesAn Event-Related Examination of Neural Activity During Social InteractionsJoePas encore d'évaluation
- Artículo Psicofísica 2Document11 pagesArtículo Psicofísica 2Ingrid Nayibe Díaz EspinosaPas encore d'évaluation
- 007 - ARTIGO - AVC Frontal Direito - Lesões Extra-Fontais Funcionamento Executivo e Comp ImpulsivoDocument12 pages007 - ARTIGO - AVC Frontal Direito - Lesões Extra-Fontais Funcionamento Executivo e Comp ImpulsivoPedro VasconceellosPas encore d'évaluation
- Neuroimagen y EmocionDocument10 pagesNeuroimagen y Emocionnatsumi18Pas encore d'évaluation
- Limitations of Cognitive Control On Emotional Distraction - Congruency in The Color Stroop Task Does Not Modulate The Emotional Stroop EffectDocument21 pagesLimitations of Cognitive Control On Emotional Distraction - Congruency in The Color Stroop Task Does Not Modulate The Emotional Stroop EffectASPas encore d'évaluation
- Consequentes e Antecedentes Da Regulação EmocionalDocument15 pagesConsequentes e Antecedentes Da Regulação EmocionalRitaPas encore d'évaluation
- Empathy and OthernessDocument18 pagesEmpathy and OthernessGalleySlavePas encore d'évaluation
- Paper Santamaria 8 2017 BCN PDFDocument16 pagesPaper Santamaria 8 2017 BCN PDFKevin BernalPas encore d'évaluation
- Psychology of Habits and Behavior ChangesDocument5 pagesPsychology of Habits and Behavior ChangesOliver Edgardo Corado GonzálezPas encore d'évaluation
- Fpsyg 06 00065Document14 pagesFpsyg 06 00065Instituto Integrado em PsicologiaPas encore d'évaluation
- 7 - Straub2019valenceDocument16 pages7 - Straub2019valenceFrancisco Ahumada MéndezPas encore d'évaluation
- P Ijp13Document11 pagesP Ijp13OporadhBigganPas encore d'évaluation
- 1 s2.0 S0093934X1200140X Main PDFDocument10 pages1 s2.0 S0093934X1200140X Main PDFKarina Andrea Torres OcampoPas encore d'évaluation
- Can Emotion Modulate Attention? Evidence For Reciprocal Links in The Attentional Network TestDocument9 pagesCan Emotion Modulate Attention? Evidence For Reciprocal Links in The Attentional Network TestFrontiersPas encore d'évaluation
- Do Generation and Regulation of Emotions InteractDocument10 pagesDo Generation and Regulation of Emotions InteractElton MatsushimaPas encore d'évaluation
- Emotion Down-Regulation Diminishes Cognitive Control A Neurophysiological InvestigationDocument49 pagesEmotion Down-Regulation Diminishes Cognitive Control A Neurophysiological InvestigationJose GuardadoPas encore d'évaluation
- Lampiran 1 Permenpan2Document13 pagesLampiran 1 Permenpan2RaodhatulJannahPas encore d'évaluation
- Notes On Mirror NeuronsDocument7 pagesNotes On Mirror NeuronspatientbioinvestPas encore d'évaluation
- TteaDocument10 pagesTteaNoor BahrPas encore d'évaluation
- Emotional Inhibition A DA of DisclosureDocument33 pagesEmotional Inhibition A DA of DisclosurehandpamPas encore d'évaluation
- Pineda 2009Document9 pagesPineda 2009yma ymiiPas encore d'évaluation
- Article - Different Effects of Trait and State Anxiety On Global-LocalDocument17 pagesArticle - Different Effects of Trait and State Anxiety On Global-LocalRafael Iwamoto TosiPas encore d'évaluation
- Buhle Et Al 2014 CerebCortex Cognitive ReappraDocument10 pagesBuhle Et Al 2014 CerebCortex Cognitive Reappra童帅Pas encore d'évaluation
- Anatomi Dan FisiologiDocument13 pagesAnatomi Dan FisiologiPakde Putu HadiPas encore d'évaluation
- The e Ect of Active Video Games On Cognitive Functioning in Clinical and Non-Clinical Populations A Meta-Analysis of Randomized Controlled TrialsDocument10 pagesThe e Ect of Active Video Games On Cognitive Functioning in Clinical and Non-Clinical Populations A Meta-Analysis of Randomized Controlled TrialsFaiz KunPas encore d'évaluation
- 20703159338Document4 pages20703159338jdgohil1961Pas encore d'évaluation
- Gu Bert 2019Document43 pagesGu Bert 2019Karla Daniela Castro OrtizPas encore d'évaluation
- Exercise, Affect, and Adherence: An Integrated Model and ADocument26 pagesExercise, Affect, and Adherence: An Integrated Model and AieysimurraPas encore d'évaluation
- Studying The Dynamics of Autonomic Activity During Emotional ExperienceDocument11 pagesStudying The Dynamics of Autonomic Activity During Emotional Experiencepsic.tatianebezerraPas encore d'évaluation
- On Posture As A Modality For Expressing and Recognizing EmotionsDocument7 pagesOn Posture As A Modality For Expressing and Recognizing EmotionsAlexOO7Pas encore d'évaluation
- Moretto 2010Document13 pagesMoretto 2010barti koksPas encore d'évaluation
- Wolpert 2013Document7 pagesWolpert 2013Dusan BarichPas encore d'évaluation
- Nihms 1543261Document22 pagesNihms 1543261LUIS FELIPE CHAGAS CALDEIRA CATAOPas encore d'évaluation
- Verification and Clarification of Patterns of Sensory Integrative DysfunctionDocument9 pagesVerification and Clarification of Patterns of Sensory Integrative DysfunctionCamila AguileraPas encore d'évaluation
- Anger - Prosody CognitionDocument11 pagesAnger - Prosody Cognitionmarcu_ruxandraPas encore d'évaluation
- Brain and Cognition: Gesine Dreisbach, Rico FischerDocument5 pagesBrain and Cognition: Gesine Dreisbach, Rico FischerLight GreatPas encore d'évaluation
- Study of The Cognitive Regulation of Emotion 2013Document39 pagesStudy of The Cognitive Regulation of Emotion 2013Tulhina AndreiPas encore d'évaluation
- Fundamental Research: The Conduct of Agricultural ScienceDocument9 pagesFundamental Research: The Conduct of Agricultural ScienceYemaneDibetaPas encore d'évaluation
- Mirror, Mirror, in My Mind: An Ethological Approach To Shaping Student BehaviorDocument9 pagesMirror, Mirror, in My Mind: An Ethological Approach To Shaping Student BehaviorJames MorenoPas encore d'évaluation
- Bekkali Et Al 2020 Mirror Neuron and Empathy MetaAnalysisDocument107 pagesBekkali Et Al 2020 Mirror Neuron and Empathy MetaAnalysisElena GatoslocosPas encore d'évaluation
- Neural Bases of Antisocial Behavior: A Voxel-Based Meta-AnalysisDocument9 pagesNeural Bases of Antisocial Behavior: A Voxel-Based Meta-AnalysisYeremias EdwinPas encore d'évaluation
- EQR ItalianoDocument7 pagesEQR ItalianoSandra BrigasPas encore d'évaluation
- Hove & Risen 2009 It's All in The TimingDocument12 pagesHove & Risen 2009 It's All in The TimingfelmmandoPas encore d'évaluation
- NSR 093Document12 pagesNSR 093童帅Pas encore d'évaluation
- 1 SchizoneurocognsocialcognDocument7 pages1 SchizoneurocognsocialcognAguelito AlbertPas encore d'évaluation
- Different Exercise Endurance ModalitiesDocument14 pagesDifferent Exercise Endurance ModalitiesGabriel CostaPas encore d'évaluation
- Child and Adolescent Emotion Regulation: The Role of Parental Emotion Regulation and ExpressionDocument15 pagesChild and Adolescent Emotion Regulation: The Role of Parental Emotion Regulation and ExpressionIonela CazacuPas encore d'évaluation
- Myofascial ReleaseDocument8 pagesMyofascial Releaseprokin_martinezPas encore d'évaluation
- The Application of Acceptance and Commitment Therapy To Obsessive Compulsive DisorderDocument11 pagesThe Application of Acceptance and Commitment Therapy To Obsessive Compulsive DisorderAnonymous Ax12P2srPas encore d'évaluation
- A Meta-Analysis of Procedures To Change Implicit BiasDocument74 pagesA Meta-Analysis of Procedures To Change Implicit BiasDavid C. SantaPas encore d'évaluation
- Using Sensory Integration andDocument9 pagesUsing Sensory Integration andVero MoldovanPas encore d'évaluation
- Adaptation of Multijoint Coordination During Standing Balance in Healthy Young and Healthy Old IndividualsDocument14 pagesAdaptation of Multijoint Coordination During Standing Balance in Healthy Young and Healthy Old IndividualsebrahimpanPas encore d'évaluation
- Neurophysiological Correlates of Cognitive Exibility and Feedback Processing in Violent Juvenile OffendersDocument12 pagesNeurophysiological Correlates of Cognitive Exibility and Feedback Processing in Violent Juvenile OffendersPedro VasconceellosPas encore d'évaluation
- Cuestionario Olvidos 2008Document14 pagesCuestionario Olvidos 2008Ana De PascalePas encore d'évaluation
- Bangee M (2018) Trait Emotional IntelligenceDocument21 pagesBangee M (2018) Trait Emotional IntelligenceLatifa OusmanPas encore d'évaluation
- Inhibitory Control and EmotionDocument12 pagesInhibitory Control and Emotionlors93Pas encore d'évaluation
- tmpF178 TMPDocument15 pagestmpF178 TMPFrontiersPas encore d'évaluation
- Tmp1a96 TMPDocument80 pagesTmp1a96 TMPFrontiersPas encore d'évaluation
- Tmpa077 TMPDocument15 pagesTmpa077 TMPFrontiersPas encore d'évaluation
- tmp998 TMPDocument9 pagestmp998 TMPFrontiersPas encore d'évaluation
- tmpE3C0 TMPDocument17 pagestmpE3C0 TMPFrontiersPas encore d'évaluation
- tmp27C1 TMPDocument5 pagestmp27C1 TMPFrontiersPas encore d'évaluation
- tmp3656 TMPDocument14 pagestmp3656 TMPFrontiersPas encore d'évaluation
- tmpA7D0 TMPDocument9 pagestmpA7D0 TMPFrontiersPas encore d'évaluation
- tmp96F2 TMPDocument4 pagestmp96F2 TMPFrontiersPas encore d'évaluation
- tmp97C8 TMPDocument9 pagestmp97C8 TMPFrontiersPas encore d'évaluation
- The Use of Machine Learning and Neural Networks in The Digital Economy and International Digital IntegrationDocument6 pagesThe Use of Machine Learning and Neural Networks in The Digital Economy and International Digital IntegrationOpen Access JournalPas encore d'évaluation
- Strahlenfolter Stalking - TI - Mechanising The Mind - Brave New World of ESB - Exerpt From As Man Becomes Machine by David RorvikDocument10 pagesStrahlenfolter Stalking - TI - Mechanising The Mind - Brave New World of ESB - Exerpt From As Man Becomes Machine by David RorvikKurt-SchneiderPas encore d'évaluation
- HBTRC Neuroanatomy 2014.1 PDFDocument135 pagesHBTRC Neuroanatomy 2014.1 PDFFirah Triple'sPas encore d'évaluation
- Unit 1 Part 1Document26 pagesUnit 1 Part 1Satyam RanaPas encore d'évaluation
- Q. What Is Recognition-By - Components Model? How Is It Different From Feature - Matching Model?Document3 pagesQ. What Is Recognition-By - Components Model? How Is It Different From Feature - Matching Model?Ushasi PalPas encore d'évaluation
- Brain 1963 PENFIELD 595 696Document102 pagesBrain 1963 PENFIELD 595 696Leroy FinklePas encore d'évaluation
- Essay Answers - Model Paper 01Document8 pagesEssay Answers - Model Paper 01ttttPas encore d'évaluation
- Introduction To Laplacian Montages: American Journal of Electroneurodiagnostic TechnologyDocument6 pagesIntroduction To Laplacian Montages: American Journal of Electroneurodiagnostic TechnologyCarlos AcostaPas encore d'évaluation
- LAPORAN LENGKAP Jaringan SarafDocument22 pagesLAPORAN LENGKAP Jaringan Sarafjeon niaPas encore d'évaluation
- Neuropsychological TestingDocument19 pagesNeuropsychological TestingShahbaz Ahmed100% (1)
- Neurophilosophy and The Healthy MindDocument6 pagesNeurophilosophy and The Healthy MindNortonMentalHealthPas encore d'évaluation
- Individualized Teaching - Full ReportDocument26 pagesIndividualized Teaching - Full ReportDarpan VyasPas encore d'évaluation
- PhychologyDocument25 pagesPhychologyPrateek JainPas encore d'évaluation
- Anatomy and Phisiology AnimalsDocument204 pagesAnatomy and Phisiology AnimalsRodel AzaresPas encore d'évaluation
- Dokumen - Tips - Meeting 1 NounsDocument5 pagesDokumen - Tips - Meeting 1 NounsMila WdyntPas encore d'évaluation
- Lobotomy Surgical Cutting of Brain NervesDocument1 pageLobotomy Surgical Cutting of Brain NervesgtygPas encore d'évaluation
- Neurolinguistics (Final)Document6 pagesNeurolinguistics (Final)Jelyn RepilPas encore d'évaluation
- Neurolingusitics: Brain & Languages Language & Brain Development The Modular MindDocument1 pageNeurolingusitics: Brain & Languages Language & Brain Development The Modular MindhamizahPas encore d'évaluation
- Your Superstar BrainDocument2 pagesYour Superstar BrainElias PereyraPas encore d'évaluation
- Dream Literature ReviewDocument9 pagesDream Literature Reviewapi-609276346Pas encore d'évaluation
- De HSG Ta 11 - 2019Document11 pagesDe HSG Ta 11 - 2019Nguyễn Trần NghĩaPas encore d'évaluation
- The Impact of Technology On The Developing Child, Their Experience and Capabilities. IntroductionDocument17 pagesThe Impact of Technology On The Developing Child, Their Experience and Capabilities. IntroductionleizelPas encore d'évaluation
- Miller & Cohen 2001 An Integrative Theory of Prefrontal Cortex FunctionDocument39 pagesMiller & Cohen 2001 An Integrative Theory of Prefrontal Cortex FunctionhooriePas encore d'évaluation
- The Little Book of Talent PDFDocument8 pagesThe Little Book of Talent PDFgalatime93% (14)
- Accelerated Learning 3 - Foster - Basil Speed Reading and Memory Training Super Skills - Read Fast - FasDocument208 pagesAccelerated Learning 3 - Foster - Basil Speed Reading and Memory Training Super Skills - Read Fast - Fasger rivPas encore d'évaluation
- CNS Vital Signs Interpretation Guide: Business OfficeDocument15 pagesCNS Vital Signs Interpretation Guide: Business OfficeJason WeaverPas encore d'évaluation
- Heaven On Earth PDFDocument253 pagesHeaven On Earth PDFSlania Ss100% (2)
- Human Brain EssayDocument6 pagesHuman Brain Essayafhbgdmbt100% (2)
- Perbedaan Lesi Korteks Dan SubkortekDocument5 pagesPerbedaan Lesi Korteks Dan SubkortekRisa SahiraPas encore d'évaluation
- Baron Psychology Notes PDFDocument78 pagesBaron Psychology Notes PDFRamya MeesalaPas encore d'évaluation
- Love Life: How to Raise Your Standards, Find Your Person, and Live Happily (No Matter What)D'EverandLove Life: How to Raise Your Standards, Find Your Person, and Live Happily (No Matter What)Évaluation : 3 sur 5 étoiles3/5 (1)
- ADHD is Awesome: A Guide to (Mostly) Thriving with ADHDD'EverandADHD is Awesome: A Guide to (Mostly) Thriving with ADHDÉvaluation : 5 sur 5 étoiles5/5 (3)
- The Age of Magical Overthinking: Notes on Modern IrrationalityD'EverandThe Age of Magical Overthinking: Notes on Modern IrrationalityÉvaluation : 4 sur 5 étoiles4/5 (30)
- LIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionD'EverandLIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionÉvaluation : 4 sur 5 étoiles4/5 (404)
- Raising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsD'EverandRaising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsÉvaluation : 4.5 sur 5 étoiles4.5/5 (170)
- By the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsD'EverandBy the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsPas encore d'évaluation
- The Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsD'EverandThe Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsÉvaluation : 4 sur 5 étoiles4/5 (4)
- The Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaD'EverandThe Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaÉvaluation : 4.5 sur 5 étoiles4.5/5 (266)
- Raising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsD'EverandRaising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsÉvaluation : 5 sur 5 étoiles5/5 (1)
- Summary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedD'EverandSummary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedÉvaluation : 5 sur 5 étoiles5/5 (81)
- Think This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeD'EverandThink This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeÉvaluation : 2 sur 5 étoiles2/5 (1)
- The Marshmallow Test: Mastering Self-ControlD'EverandThe Marshmallow Test: Mastering Self-ControlÉvaluation : 4.5 sur 5 étoiles4.5/5 (60)
- Manipulation: The Ultimate Guide To Influence People with Persuasion, Mind Control and NLP With Highly Effective Manipulation TechniquesD'EverandManipulation: The Ultimate Guide To Influence People with Persuasion, Mind Control and NLP With Highly Effective Manipulation TechniquesÉvaluation : 4.5 sur 5 étoiles4.5/5 (1412)
- Cult, A Love Story: Ten Years Inside a Canadian Cult and the Subsequent Long Road of RecoveryD'EverandCult, A Love Story: Ten Years Inside a Canadian Cult and the Subsequent Long Road of RecoveryÉvaluation : 4 sur 5 étoiles4/5 (45)
- Why We Die: The New Science of Aging and the Quest for ImmortalityD'EverandWhy We Die: The New Science of Aging and the Quest for ImmortalityÉvaluation : 4 sur 5 étoiles4/5 (5)
- Summary: Thinking, Fast and Slow: by Daniel Kahneman: Key Takeaways, Summary & Analysis IncludedD'EverandSummary: Thinking, Fast and Slow: by Daniel Kahneman: Key Takeaways, Summary & Analysis IncludedÉvaluation : 4 sur 5 étoiles4/5 (61)
- Dark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.D'EverandDark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.Évaluation : 4.5 sur 5 étoiles4.5/5 (110)
- Troubled: The Failed Promise of America’s Behavioral Treatment ProgramsD'EverandTroubled: The Failed Promise of America’s Behavioral Treatment ProgramsÉvaluation : 5 sur 5 étoiles5/5 (2)
- Mindset by Carol S. Dweck - Book Summary: The New Psychology of SuccessD'EverandMindset by Carol S. Dweck - Book Summary: The New Psychology of SuccessÉvaluation : 4.5 sur 5 étoiles4.5/5 (328)
- The Secret of the Golden Flower: A Chinese Book Of LifeD'EverandThe Secret of the Golden Flower: A Chinese Book Of LifeÉvaluation : 5 sur 5 étoiles5/5 (4)
- Summary: Limitless: Upgrade Your Brain, Learn Anything Faster, and Unlock Your Exceptional Life By Jim Kwik: Key Takeaways, Summary and AnalysisD'EverandSummary: Limitless: Upgrade Your Brain, Learn Anything Faster, and Unlock Your Exceptional Life By Jim Kwik: Key Takeaways, Summary and AnalysisÉvaluation : 5 sur 5 étoiles5/5 (8)
- Empath: The Survival Guide For Highly Sensitive People: Protect Yourself From Narcissists & Toxic Relationships. Discover How to Stop Absorbing Other People's PainD'EverandEmpath: The Survival Guide For Highly Sensitive People: Protect Yourself From Narcissists & Toxic Relationships. Discover How to Stop Absorbing Other People's PainÉvaluation : 4 sur 5 étoiles4/5 (95)
- The Garden Within: Where the War with Your Emotions Ends and Your Most Powerful Life BeginsD'EverandThe Garden Within: Where the War with Your Emotions Ends and Your Most Powerful Life BeginsPas encore d'évaluation
- The Courage Habit: How to Accept Your Fears, Release the Past, and Live Your Courageous LifeD'EverandThe Courage Habit: How to Accept Your Fears, Release the Past, and Live Your Courageous LifeÉvaluation : 4.5 sur 5 étoiles4.5/5 (253)
- Outlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisD'EverandOutlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisÉvaluation : 4 sur 5 étoiles4/5 (1)
- When the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisD'EverandWhen the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisÉvaluation : 3.5 sur 5 étoiles3.5/5 (2)