Académique Documents
Professionnel Documents
Culture Documents
Sign Chlamydia Quickref 2009
Transféré par
Muhammad JulpianDescription originale:
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Sign Chlamydia Quickref 2009
Transféré par
Muhammad JulpianDroits d'auteur :
Formats disponibles
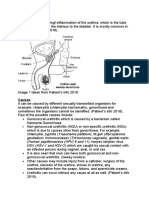
Management of genital Chlamydia
trachomatis infection
Quick Reference Guide
March 2009
COPIES OF ALL SIGN GUIDELINES ARE AVAILABLE
ONLINE AT WWW.SIGN.AC.UK
Scottish Intercollegiate Guidelines Network
SI GN
109
ANTIMICROBIAL TREATMENT
Initiate treatment without waiting for laboratory
confirmation of infection in patients with symptoms
and signs of chlamydial infection and their sexual
partners.
C
Patients with symptomatic or confirmed asymptomatic
chlamydial infection should be advised to abstain from
having sex (including oral and anal) until they and their
current partners have been treated and for one week
thereafter even when treated at the same time.
Uncomplicated genital chlamydial infection may be
treated with either azithromycin 1 g as a single oral
dose or doxycycline 100 mg twice daily for seven days.
A
Taking compliance with therapy into account,
uncomplicated genital chlamydial infection should be
treated with azithromycin 1 g as a single oral dose.
B
Uncomplicated genital chlamydial infection in
pregnancy should be treated with
azithromycin 1 g as a single oral dose or
erythromycin 500 mg four times daily orally for
seven days or
amoxicillin 500 mg three times daily orally for seven
days.
A
Taking compliance, tolerability, and efficacy into
account, azithromycin 1 g as a single oral dose is
recommended for uncomplicated genital chlamydial
infection in pregnancy following discussion of the
balance of benefits and risks with the patient.
B
When women have been treated with amoxicillin in
pregnancy, practitioners should maintain a high index
of suspicion should symptoms suggestive of chlamydial
infection develop in the infant.
Rectal infection may be treated with either
azithromycin 1 g as a single oral dose or doxycycline
100 mg twice daily for seven days.
D
If LGV is diagnosed, or suspected on clinical grounds,
the recommended regimen is doxycycline 100 mg
twice daily for three weeks.
D
Primary care health professionals should refer patients
with rectal infection to GUM.
PARTNER NOTIFICATION
Patients diagnosed with chlamydia must receive a
partner notification interview.
C
Patients should be given a choice of patient or
provider referral.
B
Patients diagnosed with chlamydia in general practice
should be offered a choice of provider for initial
partner notification either trained practice nurses
with support from health advisers in GUM, or referral
to GUM.
B
In GUM settings, health advisers should continue to
provide partner notification.
Healthcare workers providing partner notification in
non-GUM settings should be trained and supported by
GUM sexual health advisers.
Patients with chlamydia should be offered additional
written information for partners, with accompanying
guidance for healthcare professionals.
C
In men with symptomatic chlamydial infection,
all partners from the four weeks prior to onset of
symptoms should be contacted.
D
In women and asymptomatic men, all partners from
the last six months or the most recent sexual partner
(if outwith that time period) should be contacted.
D
FOLLOW UP AND TEST OF CURE
All patients treated for chlamydia should be given a
follow-up interview within 2-4 weeks of treatment.
D
Adherence with therapy and risk of re-infection
should be discussed with patients at follow-up
interviews.
D
A test of cure need not be performed in patients who
have adhered to therapy and in whom there is no risk
of re-infection.
D
Test of cure should be routine during pregnancy. D
Test of cure/re-infection established by NAAT should
be performed a minimum of five weeks after the
initiation of therapy (six weeks after azithromycin), to
avoid false positive results.
D
Test for re-infection should be recommended at 3-12
months, or sooner if there is a change of partner.
D
LONG TERM FOLLOW UP
RECTAL INFECTION IN MEN
UNCOMPLICATED INFECTION IN PREGNANCY
UNCOMPLICATED INFECTION
INITIATION OF TREATMENT
LABORATORY TESTS
In the absence of data to support a complication rate of 10% or more in women with untreated chlamydial infection, there is no
evidence that screening for chlamydia is cost effective with regard to reducing morbidity.
Aptima Combo 2 (TMA) and BD Probetec (SDA) are
recommended tests for chlamydial infection.
C
Where one to one counselling is not feasible, the
provision of sexual health information should be
integral to consultations for contraception, STI testing
or other sexual and reproductive health issues.
CHOICE OF TEST
Real time PCR can be used as an alternative to TMA
and SDA.
D
Either single or dual (combined with gonorrhoea) tests
can be used to test for chlamydial infection.
C
CHOICE OF SPECIMEN
If the patient is having a speculum examination either
an endocervical or vaginal swab can be used to test
for chlamydia.
D
Women not undergoing speculum examination should
be offered the choice between self obtained low
vaginal swab or first void urine.
D
In men, first void urine is the specimen of choice. D
HEALTH EDUCATION FOR PREVENTION OF
INFECTION AND RE-INFECTION
Client centred, risk reduction focused, one to one
counselling involving behavioural goal setting should
be considered during consultations for sexual and
reproductive health issues.
B
For prevention of STIs, including chlamydia, condom
use should be promoted in all settings where sexual
health care is provided.
C
TESTING FOR GENITAL CHLAMYDIAL INFECTION
The reason for, implications of, and results of any test
carried out should be explained to the individual being
tested.
Testing for chlamydia should be performed in women
and men with any of the following symptoms and
signs:
Women
vaginal discharge
post-coital/intermenstrual/breakthrough bleeding
inflamed/friable cervix (which may bleed on contact)
urethritis
pelvic inflammatory disease
lower abdominal pain in the sexually active
reactive arthritis in the sexually active.
Men
urethral discharge
dysuria
urethritis
epididymo-orchitis in the sexually active
reactive arthritis in the sexually active.
C
Men who have sex with men (MSM) should be offered
a full sexual health screen, including HIV, syphilis,
gonorrhoea, and rectal chlamydia testing, depending
on their individual risk.
D
Consultations for chlamydia testing or treatment should
include an assessment of the patients risk factors for
blood borne virus infection.
Heterosexual patients whose partners include
intravenous drug users, bisexual men, or people who
have had unprotected sex in high-risk geographical
areas abroad should be offered tests for other STIs,
depending on their individual risk.
D
Asymptomatic heterosexual patients requesting an STI
screen can be offered a chlamydia test alone in the
absence of other risk factors.
D
D Sexual partners of those with suspected but
undiagnosed chlamydial infection (with PID or
epididymo-orchitis) should be tested.
D Those who have been diagnosed with chlamydia in
the previous 12 months should be tested.
D All patients attending GUM clinics should be tested
for chlamydia.
D In healthcare settings other than GUM, testing should
be most strongly advised for those who have had two
or more partners in the past 12 months.
D Resources for chlamydia testing in women should be
targeted where prevalence is known to be highest, ie
first those aged 15-19 and then those aged 20-24.
A All women undergoing termination of pregnancy
should be tested for chlamydial infection.
D Resources for chlamydia testing in men should be
targeted where prevalence is known to be highest, ie
those aged under 25.
B Postal testing kits should be used to increase
chlamydia testing among young men.
D All MSM attending GUM clinics, including those who
are HIV-positive, should be offered chlamydia testing,
including rectal swabs.
This Quick Reference Guide provides a summary of the main recommendations in SIGN guideline 109: Management of genital Chlamydia trachomatis infection. Recommendations are graded to indicate the strength of the
supporting evidence. Good practice points are provided where the guideline development group wishes to highlight specific aspects of accepted clinical practice. Details of the evidence supporting these recommendations can be found
in the full guideline, available on the SIGN website: www.sign.ac.uk
A B C D
C Sexual partners of chlamydia-positive individuals
should be tested.
PRIMARY PREVENTION
PATIENTS WITH SYMPTOMS/SIGNS
TESTING FOR OTHER STIS
ABBREVIATIONS
GUM genitourinary medicine
LGV lymphogranuloma venereum
MSM men who have sex with men
NAAT nucleic acid amplification test
PCR polymerase chain reaction
PID pelvic inflammatory disease
SDA strand displacement amplification
STI sexually transmitted infection
TMA transcription mediated amplification
ASYMPTOMATIC GROUPS
Vous aimerez peut-être aussi
- Chlamydia and Nonspecific UrethritisDocument4 pagesChlamydia and Nonspecific UrethritisErfina BasriPas encore d'évaluation
- Pelvic Inflammatory DiseaseDocument6 pagesPelvic Inflammatory DiseaseLembah BarokahPas encore d'évaluation
- FAQs Gonorrhea GuidelinesforTesting TreatmentDocument10 pagesFAQs Gonorrhea GuidelinesforTesting TreatmentGerbong 5Pas encore d'évaluation
- MMWR Chancroid: H. Ducreyi Is Available in The United States, But Such TestingDocument4 pagesMMWR Chancroid: H. Ducreyi Is Available in The United States, But Such TestingRhyRhye Sawitri AriantiPas encore d'évaluation
- CDC Han 486Document5 pagesCDC Han 486WDIV/ClickOnDetroitPas encore d'évaluation
- Pelvic Inflammatory Disease (PID)Document43 pagesPelvic Inflammatory Disease (PID)Asghar AliPas encore d'évaluation
- Routine Antenatal Screening DR - Amy MellorDocument3 pagesRoutine Antenatal Screening DR - Amy MellorYwagar Ywagar100% (1)
- Syndromic Management of Sexually Transmitted InfectionsDocument76 pagesSyndromic Management of Sexually Transmitted Infectionsnamita100% (2)
- Management hIV in Pregnancy PDFDocument28 pagesManagement hIV in Pregnancy PDFarryandres11Pas encore d'évaluation
- StdsDocument33 pagesStdsallymyly88Pas encore d'évaluation
- Aquifer Study CaseDocument8 pagesAquifer Study CaseRosanaPas encore d'évaluation
- The Syndromic Management of Vaginal Discharge Using Single-Dose Treatments: A Randomized Controlled Trial in West AfricaDocument10 pagesThe Syndromic Management of Vaginal Discharge Using Single-Dose Treatments: A Randomized Controlled Trial in West AfricaIqra AnugerahPas encore d'évaluation
- Acog 215 Vaginosis Noi Embarazada Resumen PDFDocument3 pagesAcog 215 Vaginosis Noi Embarazada Resumen PDFOrlando CuellarPas encore d'évaluation
- CDC - Pelvic Inflammatory Disease - 2010 STD Treatment GuidelinesDocument7 pagesCDC - Pelvic Inflammatory Disease - 2010 STD Treatment GuidelinesAhmad Arbi AninditoPas encore d'évaluation
- Syphilis in PregnancyDocument38 pagesSyphilis in PregnancydrirrazabalPas encore d'évaluation
- Case Study BuenavistaDocument5 pagesCase Study BuenavistaGen-Gen Belenio BillonesPas encore d'évaluation
- The Efficacy and Safety of Gentamicin Plus Azithromycin and Gemifloxacin Plus Azithromycin As Treatment of Uncomplicated GonorrheaDocument9 pagesThe Efficacy and Safety of Gentamicin Plus Azithromycin and Gemifloxacin Plus Azithromycin As Treatment of Uncomplicated GonorrheaCindy Julia AmandaPas encore d'évaluation
- Pelvic Inflammatory Disease: Mary Anne M. Baquing, MDDocument3 pagesPelvic Inflammatory Disease: Mary Anne M. Baquing, MDBob IrsanPas encore d'évaluation
- ACOG Perinatal Care Guideline Summary 7th EditionDocument6 pagesACOG Perinatal Care Guideline Summary 7th EditionpolygonePas encore d'évaluation
- Gyne - (Section B) PID-STI-1Document45 pagesGyne - (Section B) PID-STI-1Kailash KhatriPas encore d'évaluation
- STD Screening Recommended by The Centers of Disease Control and PreventionDocument6 pagesSTD Screening Recommended by The Centers of Disease Control and PreventionMonica TrabancoPas encore d'évaluation
- Chlamydia Gonorrhea RecstatementDocument8 pagesChlamydia Gonorrhea RecstatementHaritha AtluriPas encore d'évaluation
- The Ef Ficacy and Safety of Gentamicin Plus Azithromycin and Gemi Oxacin Plus Azithromycin As Treatment of Uncomplicated GonorrheaDocument9 pagesThe Ef Ficacy and Safety of Gentamicin Plus Azithromycin and Gemi Oxacin Plus Azithromycin As Treatment of Uncomplicated GonorrheaSamuelRexyPas encore d'évaluation
- Sexually Transmitted DiseasesDocument17 pagesSexually Transmitted DiseasesChrystele Ann Ramilo100% (1)
- STD Summary PresentationDocument52 pagesSTD Summary PresentationMike AndersenPas encore d'évaluation
- Journal ClubDocument60 pagesJournal ClubSataroopa SirigiriPas encore d'évaluation
- Sti HivDocument19 pagesSti HivAngela Joyce Sajor AmpongPas encore d'évaluation
- Gupta Bowman 2012 Managing Sexually Transmitted Infections in Pregnant WomenDocument9 pagesGupta Bowman 2012 Managing Sexually Transmitted Infections in Pregnant WomenNaresh Babu.TPas encore d'évaluation
- Vaginal Infections Update: Original ReviewDocument6 pagesVaginal Infections Update: Original ReviewEpi PanjaitanPas encore d'évaluation
- Pelvic Inflammatory Disease: Diagnosis, Management, and PreventionDocument8 pagesPelvic Inflammatory Disease: Diagnosis, Management, and PreventionJoão Marcelo ColunaPas encore d'évaluation
- Treatment of Chlamydia Trachomatis InfectionDocument23 pagesTreatment of Chlamydia Trachomatis InfectionAmbarPas encore d'évaluation
- Sexually Transmitted Infections-5Document5 pagesSexually Transmitted Infections-5diko_nalPas encore d'évaluation
- CPG PerinatalDocument7 pagesCPG PerinatalReuter Lloyd MarianoPas encore d'évaluation
- Pid Casestudy 2014 PDFDocument4 pagesPid Casestudy 2014 PDFNur Syamsiah MPas encore d'évaluation
- Gonorrhea 2014Document3 pagesGonorrhea 2014katarinaPas encore d'évaluation
- Recurrent Vaginal CandidiasisDocument5 pagesRecurrent Vaginal Candidiasisamo94Pas encore d'évaluation
- Hiv in PregnancyDocument52 pagesHiv in PregnancyKirandeep ParmarPas encore d'évaluation
- MicrobiologiaDocument9 pagesMicrobiologiaMichel SecoPas encore d'évaluation
- Prevention of Mother-To-Child Transmission of Syphilis: StandardsDocument6 pagesPrevention of Mother-To-Child Transmission of Syphilis: Standardsanthony345Pas encore d'évaluation
- Pelvic Inflammatory Disease: CDC 24/7: Saving Lives. Protecting PeopleDocument9 pagesPelvic Inflammatory Disease: CDC 24/7: Saving Lives. Protecting PeopleAmanda GAPas encore d'évaluation
- Clinical Guidelines For SyphilisDocument12 pagesClinical Guidelines For SyphilisRocky.84Pas encore d'évaluation
- Current Recommended Management of Common Stis in Primary CareDocument8 pagesCurrent Recommended Management of Common Stis in Primary CareEpi PanjaitanPas encore d'évaluation
- UrethritisDocument6 pagesUrethritisParyPas encore d'évaluation
- Dr. Ali's Uworld Notes For Step 2 CKDocument30 pagesDr. Ali's Uworld Notes For Step 2 CKuyesPas encore d'évaluation
- ChlamydiaDocument2 pagesChlamydiaFiTriaPuSpaEKawatiPas encore d'évaluation
- Clostridium Difficile: Isolation of The Hospitalized Patient: Control MeasuresDocument5 pagesClostridium Difficile: Isolation of The Hospitalized Patient: Control MeasuresSizi DiazPas encore d'évaluation
- Gonorrhoeae and Chlamydia Species. Nucleic Acid Amplification Tests (Naats) May Be Used in Addition ToDocument12 pagesGonorrhoeae and Chlamydia Species. Nucleic Acid Amplification Tests (Naats) May Be Used in Addition ToYorim Sora PasilaPas encore d'évaluation
- 55-Article Text-126-3-10-20171207Document8 pages55-Article Text-126-3-10-20171207dianPas encore d'évaluation
- USMLE Step 3 Lecture Notes 2021-2022: Internal Medicine, Psychiatry, EthicsD'EverandUSMLE Step 3 Lecture Notes 2021-2022: Internal Medicine, Psychiatry, EthicsÉvaluation : 5 sur 5 étoiles5/5 (9)
- Pelvic Inflammatory DiseaseDocument41 pagesPelvic Inflammatory DiseaseEvellyna MeilanyPas encore d'évaluation
- Syphilis in Pregnancy - UpToDateDocument33 pagesSyphilis in Pregnancy - UpToDateGiuliana FloresPas encore d'évaluation
- 726 Genital Warts DSTDocument6 pages726 Genital Warts DSTNurul JannahPas encore d'évaluation
- Bashh PidDocument26 pagesBashh PidMarPas encore d'évaluation
- Pelvic Inflammatory DiseaseDocument10 pagesPelvic Inflammatory DiseaseMuhammad IqbalPas encore d'évaluation
- STIsDocument8 pagesSTIsJovie Anne BorjaPas encore d'évaluation
- Urethritis, STD, Mycoplasma or Chlamydia InfectionDocument11 pagesUrethritis, STD, Mycoplasma or Chlamydia InfectionbetebetPas encore d'évaluation
- Q1. What Should You Teach Ap About Chlamydial Infection?Document2 pagesQ1. What Should You Teach Ap About Chlamydial Infection?Faye Dianne Damian-BuenafePas encore d'évaluation
- Gonorrhea: 1. Disease ReportingDocument7 pagesGonorrhea: 1. Disease ReportingWahyudi Permana DarlisPas encore d'évaluation
- Clinical Management Review 2023-2024: Volume 1: USMLE Step 3 and COMLEX-USA Level 3D'EverandClinical Management Review 2023-2024: Volume 1: USMLE Step 3 and COMLEX-USA Level 3Évaluation : 5 sur 5 étoiles5/5 (1)
- COVER Journal ReadingDocument2 pagesCOVER Journal ReadingMuhammad JulpianPas encore d'évaluation
- READMEPCDocument1 pageREADMEPCMuhammad JulpianPas encore d'évaluation
- Assignment Prof TetiDocument5 pagesAssignment Prof TetiMuhammad JulpianPas encore d'évaluation
- CSS Infant, Child, and Adolescent DisordersDocument25 pagesCSS Infant, Child, and Adolescent DisordersMuhammad JulpianPas encore d'évaluation
- C1F2CDocument1 pageC1F2CMuhammad JulpianPas encore d'évaluation
- Lupus NephritisDocument29 pagesLupus NephritisMuhammad Julpian0% (1)
- Walde YerDocument4 pagesWalde YerMuhammad JulpianPas encore d'évaluation
- Psychosocial StageDocument2 pagesPsychosocial StageMuhammad JulpianPas encore d'évaluation
- MdasDocument5 pagesMdasMuhammad JulpianPas encore d'évaluation
- Clock-Drawing Test and Mini Mental State ExaminationDocument11 pagesClock-Drawing Test and Mini Mental State ExaminationMuhammad Julpian100% (1)
- PsychopathDocument21 pagesPsychopathMuhammad JulpianPas encore d'évaluation
- Indian J OphthalmolDocument10 pagesIndian J OphthalmolMuhammad JulpianPas encore d'évaluation
- Mini-Mental Status Examination: Task and Questions Maximum Score OrientationDocument1 pageMini-Mental Status Examination: Task and Questions Maximum Score OrientationMuhammad JulpianPas encore d'évaluation
- What Is Herpangina?Document2 pagesWhat Is Herpangina?Muhammad JulpianPas encore d'évaluation
- Curriculum General MedicineDocument10 pagesCurriculum General MedicineMuhammad JulpianPas encore d'évaluation
- Substance-Induced Psychotic DisorderDocument24 pagesSubstance-Induced Psychotic DisorderMuhammad JulpianPas encore d'évaluation
- CariesDocument3 pagesCariesMuhammad JulpianPas encore d'évaluation
- Schizoaffective DisorderDocument2 pagesSchizoaffective DisorderMuhammad JulpianPas encore d'évaluation
- Chickenpox in Pregnancy 2007Document11 pagesChickenpox in Pregnancy 2007Kee Chai ChinPas encore d'évaluation
- CariesDocument3 pagesCariesMuhammad JulpianPas encore d'évaluation
- Effectiveness of Early Physiotherapy To PreventDocument8 pagesEffectiveness of Early Physiotherapy To PreventMuhammad JulpianPas encore d'évaluation
- Nat Fact Sheet 20f05Document10 pagesNat Fact Sheet 20f05Muhammad JulpianPas encore d'évaluation
- Lamivudine Plus Adefovir Combination TherapyDocument11 pagesLamivudine Plus Adefovir Combination TherapyMuhammad JulpianPas encore d'évaluation
- Ylinen BMJ 1984Document2 pagesYlinen BMJ 1984Muhammad JulpianPas encore d'évaluation
- Puerperal Sepsis WMSD Chapter7 RefDocument9 pagesPuerperal Sepsis WMSD Chapter7 RefMuhammad JulpianPas encore d'évaluation
- Prevent Neonatal SepsisDocument8 pagesPrevent Neonatal SepsisMuhammad JulpianPas encore d'évaluation
- Chickenpox in Pregnancy 2007Document11 pagesChickenpox in Pregnancy 2007Kee Chai ChinPas encore d'évaluation
- Management of Genital Herpes in Pregnancy: Green-Top Guideline No. 30Document9 pagesManagement of Genital Herpes in Pregnancy: Green-Top Guideline No. 30Leon DipteePas encore d'évaluation
- Chickenpox in Pregnancy 2007Document11 pagesChickenpox in Pregnancy 2007Kee Chai ChinPas encore d'évaluation
- Genital Tract Infections - GynecologyDocument17 pagesGenital Tract Infections - GynecologyKC Dela RosaPas encore d'évaluation
- Chapter 203:: Lymphogranuloma Venereum:: Rim S. Ishak & Samer H. GhosnDocument10 pagesChapter 203:: Lymphogranuloma Venereum:: Rim S. Ishak & Samer H. GhosnDarwinsyah PutraPas encore d'évaluation
- Chlamydia SlidesDocument63 pagesChlamydia Slideslia primasari100% (1)
- Full RujiraLeukorrhea, STDS, HIV Infection.02102017 PDFDocument129 pagesFull RujiraLeukorrhea, STDS, HIV Infection.02102017 PDFrujiraPas encore d'évaluation
- Medical Microbiology and Immunology Flash Cards - Sample ChapterDocument250 pagesMedical Microbiology and Immunology Flash Cards - Sample Chapterleh.mo931580% (5)
- Stds MCQDocument181 pagesStds MCQhesham100% (1)
- Chancroide Linfogranuloma Venereo Granuloma InguinalDocument9 pagesChancroide Linfogranuloma Venereo Granuloma InguinalYanna RizkiaPas encore d'évaluation
- Seroepidemiological TrachomatisDocument5 pagesSeroepidemiological TrachomatisMulatuPas encore d'évaluation
- 05 Infections of The Lower Genital Tract and TBDocument27 pages05 Infections of The Lower Genital Tract and TBAlliMars100% (1)
- Microbiology: Atypical Bacteria: Chlamydia, Mycoplasms, LegionellaDocument4 pagesMicrobiology: Atypical Bacteria: Chlamydia, Mycoplasms, LegionellaPatricia Mae de JesusPas encore d'évaluation
- Approach To Genital UlcerDocument41 pagesApproach To Genital UlcerTeng Huei Lee100% (1)
- MCQ Infectious DiseasesDocument14 pagesMCQ Infectious Diseasespathfind007Pas encore d'évaluation
- 1 Gynecologic Infections 2Document109 pages1 Gynecologic Infections 2Girum SolomonPas encore d'évaluation
- Chapter 024Document49 pagesChapter 024Gerald John PazPas encore d'évaluation
- Etas MCQ 2015..2Document605 pagesEtas MCQ 2015..2sajithaPas encore d'évaluation
- 10.male Reproductive System 2 BlocksDocument220 pages10.male Reproductive System 2 BlocksANA CAROLINE ANDRADE DE MELOPas encore d'évaluation
- Lymphogranuloma Venereum (LGV)Document3 pagesLymphogranuloma Venereum (LGV)Yanna Habib-MangotaraPas encore d'évaluation
- Notes MICROBIAL DISEASES and EPIDEMIOLOGYDocument12 pagesNotes MICROBIAL DISEASES and EPIDEMIOLOGYDaniella TupasPas encore d'évaluation
- Sexually Transmitte D Infections: Keeshia Anna Zerrudo Clinical Clerk Iloilo Doctors' Hospital Inc. August 18, 2020Document117 pagesSexually Transmitte D Infections: Keeshia Anna Zerrudo Clinical Clerk Iloilo Doctors' Hospital Inc. August 18, 2020Angela SaldajenoPas encore d'évaluation
- Heroism EssaysDocument8 pagesHeroism Essaysgqdknjnbf100% (2)
- Case 25-2016: A 33-Year-Old Man With Rectal Pain and BleedingDocument7 pagesCase 25-2016: A 33-Year-Old Man With Rectal Pain and BleedingNaomi HalmPas encore d'évaluation
- Session9-Gynecologic InfectionsDocument81 pagesSession9-Gynecologic InfectionsCHALIE MEQUPas encore d'évaluation
- Peds Notes 2Document23 pagesPeds Notes 2aaamirrrPas encore d'évaluation
- Genital Ulcer DiseaseDocument29 pagesGenital Ulcer DiseaseFu' BudhyPas encore d'évaluation
- IT 7 - Pendekatan Diagnostik Limfadenopati Limfadenitis - NDDocument46 pagesIT 7 - Pendekatan Diagnostik Limfadenopati Limfadenitis - NDRebeka Anastasia MarpaungPas encore d'évaluation
- Syphilis Case Study 2013Document4 pagesSyphilis Case Study 2013Ernesto Padron0% (1)
- EtsDocument31 pagesEtsTony Gomez Luna LeyvaPas encore d'évaluation
- Mahon CompileDocument13 pagesMahon CompileSheinor Fae GalzotePas encore d'évaluation
- Chlamydia SeminarDocument110 pagesChlamydia Seminarsuvarnaramesh2007100% (1)
- Nina Bacteria Chart Medical School Step 1Document11 pagesNina Bacteria Chart Medical School Step 1M PatelPas encore d'évaluation