Académique Documents
Professionnel Documents
Culture Documents
Layofast
Transféré par
Muhammad Usman Akram0 évaluation0% ont trouvé ce document utile (0 vote)
177 vues2 pagesLyofast Y 350 B is a freeze-dried yogurt starter culture containing Streptococcus thermophilus and Lactobacillus delbrueckii ssp. bulgaricus bacteria. It produces very mild set and stirred yogurt with high viscosity. The culture is sprinkled directly into milk under aseptic conditions at recommended levels of 2.0-4.0 UC/100L for short set yogurt and 0.5-1.0 UC/100L for long set yogurt. The culture has a standard activity of 43°C for 7 hours to reach a pH of 4.5. It is packaged in aluminum pouches with a 12 month shelf life at 4°C or below and an 18 month
Description originale:
genral info
Copyright
© Attribution Non-Commercial (BY-NC)
Formats disponibles
PDF, TXT ou lisez en ligne sur Scribd
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentLyofast Y 350 B is a freeze-dried yogurt starter culture containing Streptococcus thermophilus and Lactobacillus delbrueckii ssp. bulgaricus bacteria. It produces very mild set and stirred yogurt with high viscosity. The culture is sprinkled directly into milk under aseptic conditions at recommended levels of 2.0-4.0 UC/100L for short set yogurt and 0.5-1.0 UC/100L for long set yogurt. The culture has a standard activity of 43°C for 7 hours to reach a pH of 4.5. It is packaged in aluminum pouches with a 12 month shelf life at 4°C or below and an 18 month
Droits d'auteur :
Attribution Non-Commercial (BY-NC)
Formats disponibles
Téléchargez comme PDF, TXT ou lisez en ligne sur Scribd
0 évaluation0% ont trouvé ce document utile (0 vote)
177 vues2 pagesLayofast
Transféré par
Muhammad Usman AkramLyofast Y 350 B is a freeze-dried yogurt starter culture containing Streptococcus thermophilus and Lactobacillus delbrueckii ssp. bulgaricus bacteria. It produces very mild set and stirred yogurt with high viscosity. The culture is sprinkled directly into milk under aseptic conditions at recommended levels of 2.0-4.0 UC/100L for short set yogurt and 0.5-1.0 UC/100L for long set yogurt. The culture has a standard activity of 43°C for 7 hours to reach a pH of 4.5. It is packaged in aluminum pouches with a 12 month shelf life at 4°C or below and an 18 month
Droits d'auteur :
Attribution Non-Commercial (BY-NC)
Formats disponibles
Téléchargez comme PDF, TXT ou lisez en ligne sur Scribd
Vous êtes sur la page 1sur 2
Lyofast Y 350 B
M91Y350B/2/UK/0 Issue: 05/02/2009 Review: 2 of 01/09/2011
1/2
Description Lyofast Y 350 B consists of specifically selected strains of Streptococcus
thermophilus producing EPS and a low content of Lactobacillus delbrueckii ssp.
bulgaricus to ensure a uniform and controlled production of very mild set and stirred
yoghurt with high viscosity.
Application Sprinkle the culture powder directly into process milk under aseptic conditions ensuring
that the culture is well dispersed by gentle stirring. The following may be used as
inoculation guidelines:
Product UC/100 l Product UC/100 l
Yoghurt, short set 2.0-4.0 Yoghurt, long set 0.5-1.0
Rotation The recommended rotations are Y 352 B/Y 356 B.
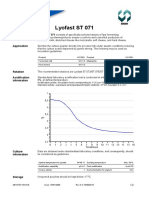
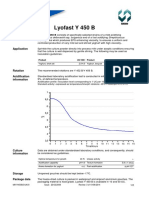
Acidification
information
Standardised laboratory acidification test is conducted in milk powder, reconstituted at
10%, at defined temperature.
Acidification profile: inoculation level corresponding to 1 UC per 100 litres milk.
Standard activity: expressed as temperature/time/pH relations: 43C/7.0 hours/pH 4.5 0.15.
Culture
information
Data are obtained under standardised laboratory conditions, and consequently, should
be considered as guidelines.
Optimal temperature for growth 43 C Urease activity +
Acidification capability pH 4.2 Texture formation 3.5 1 sec/g
Aroma formation for yoghurt +(+)
Post-acidification pH 0.3
Storage Unopened pouches should be kept at or below -18C.
Lyofast Y 350 B
M91Y350B/2/UK/0 Issue: 05/02/2009 Review: 2 of 01/09/2011
2/2
Package data The freeze-dried culture is packed in waterproof and airproof aluminium pouches.
Lyofast Y 350 B is available in 10 and 50 UC.
Shelf life 12 months when stored at or below 4C, 18 months wh en stored at or below -18C.
The shelf life includes up to 14 days of shipment at temperatures below 30C.
Heavy metal
specification
Pb (lead)
Hg (mercury)
Cd (cadmium)
< 1 ppm
< 0.03 ppm
< 0.1 ppm
Microbiological
specification
Bacillus cereus
Coagulase positive staphylococci*
Enterobacteriaceae
Escherichia coli
Listeria monocytogenes*
Moulds & yeasts
Salmonella spp*
<100 CFU/g
<10 CFU/g
<10 CFU/g
<1 CFU/g
Not detected in 25 g
<10 CFU/g
Not detected in 25 g
Method: Sacco M10 (1)
Method: Sacco M11(2)
Method: Sacco M2 (3)
Method: Sacco M27 (4)
Method: Sacco M13 (5)
Method: Sacco M3 (6)
Method: Sacco M12 (7)
* Analysed on regular basis. All analytical methods are available upon request.
(1)ISO 7932; (2)ISO 6888-1-2; (3)ISO 215281-2; (4)ISO11866-1-2/IDF 170-1-2; (5)ISO 11290-1-2; (6)ISO
6611/IDF 94; (7)ISO 6785/IDF 93;
GMO The microbial strains are not genetically modified (GMO) in accordance with the
European Directive 90/220/EEC. The strains are isolated from natural sources. The raw
materials used are also GMO free in accordance with Regulation (EC) No. 1829/2003
and Regulation (EC) 1830/2003. Statement available upon request.
Allergens The raw materials used are generally based on dairy ingredients. All materials are free
of the following components and their derivates: peanut, tree nut, sesame, egg, fish,
shellfish, mollusc, crustacean, sulphite, wheat, celery, mustard, soy and lupine.
Statement available upon request.
Safety information Material Safety Data Sheet available on www.saccosrl.it
Certificate Lot certificate available upon request.
ISO
Kosher approval
Sacco S.r.l. is UNI EN ISO 9001:2008 certified since 1998. Sacco cultures are
generally Kosher approved except for surface ripening cultures.
Service Please contact your distributor for guidance and instructions for your choice of culture
and processing. Information about additional package sizes and sales units is also
available upon request.
Liability This information is based on our knowledge trustworthy and presented in good faith. No
guarantee against patent infringement is implied or inferred.
Vous aimerez peut-être aussi
- Alkaline Food Chart ReportDocument11 pagesAlkaline Food Chart ReportSuresh Dhanasekar100% (3)
- Oats Recipes for Breakfast, Smoothies, Desserts and MoreDocument91 pagesOats Recipes for Breakfast, Smoothies, Desserts and MoreAmitMehtaPas encore d'évaluation
- Here's Why I Adore The Cabbage Soup DietDocument5 pagesHere's Why I Adore The Cabbage Soup DietGeorgiaSkordakis-MoraitisPas encore d'évaluation
- Non-Dairy Ice CreamDocument2 pagesNon-Dairy Ice CreamMuhammad Usman AkramPas encore d'évaluation
- Food Exchange Lists: Vegetables, Milk, Protein, Fruits, Starches & FatsDocument4 pagesFood Exchange Lists: Vegetables, Milk, Protein, Fruits, Starches & FatsJelai D50% (2)
- Enviando Por Email Enviando Por Email 60 - Smoothies - For - Weight - LossDocument43 pagesEnviando Por Email Enviando Por Email 60 - Smoothies - For - Weight - LossRita DuartePas encore d'évaluation
- Blogilates Meal PlanDocument6 pagesBlogilates Meal PlanJackies23100% (3)
- Easy CookDocument92 pagesEasy CookAndrea Schreib100% (2)
- Biology IADocument9 pagesBiology IAPat AkuaPas encore d'évaluation
- Sas Sid File 64 BitDocument1 pageSas Sid File 64 Bitakeey4u100% (2)
- Lyofast ST 081 Fast Fermenting Streptococcus thermophilus CultureDocument2 pagesLyofast ST 081 Fast Fermenting Streptococcus thermophilus CulturejimePas encore d'évaluation
- Lyofast ST 071 Culture for Fermented Milk and Cheese ProductionDocument2 pagesLyofast ST 071 Culture for Fermented Milk and Cheese Productionchrist castroPas encore d'évaluation
- TC Lyofast-Y 456-B EN 230616Document2 pagesTC Lyofast-Y 456-B EN 230616Anel MamaniPas encore d'évaluation
- Lyofast MW 039 SDocument2 pagesLyofast MW 039 SNilo C Cervantes ChipaPas encore d'évaluation
- TC Lyofast-Y-438Document2 pagesTC Lyofast-Y-438Marck Anderson Mamani AvilesPas encore d'évaluation
- Lyofast MOS 062 B Culture for Cheese ProductionDocument2 pagesLyofast MOS 062 B Culture for Cheese ProductionLeyarope RoRoPas encore d'évaluation
- Lyofast MO 242: DescriptionDocument2 pagesLyofast MO 242: DescriptionMilena CastroPas encore d'évaluation
- Lyofast MTX 432 ENDocument2 pagesLyofast MTX 432 ENLuisaGordonPas encore d'évaluation
- Lyofast Y 430 A for Mild YoghurtDocument2 pagesLyofast Y 430 A for Mild YoghurtLuisaGordon100% (1)
- Ficha Tecnica Sab 440ADocument0 pageFicha Tecnica Sab 440AAlexander GuzmanPas encore d'évaluation
- Lyofast LR B: DescriptionDocument2 pagesLyofast LR B: DescriptionjimePas encore d'évaluation
- Sacco 450b Grafico PH Vs TDocument2 pagesSacco 450b Grafico PH Vs TLaura Vanessa AriasPas encore d'évaluation
- 21 07 22 Y456bDocument3 pages21 07 22 Y456bYogures ZhuaPas encore d'évaluation
- Product Specifications:Rokiškio Sūris Cheese 45% Fat.i D.M.Document2 pagesProduct Specifications:Rokiškio Sūris Cheese 45% Fat.i D.M.Jason ThorntonPas encore d'évaluation
- Tryptic Soy AgarDocument2 pagesTryptic Soy Agaryehya alasdekaaPas encore d'évaluation
- BK115 133 BM013 v7Document5 pagesBK115 133 BM013 v7Keep CalmPas encore d'évaluation
- Lactobacillus AcidophilusDocument1 pageLactobacillus AcidophilusJuan Rodrigo Correa Ramírez100% (1)
- BK185Document3 pagesBK185Keep CalmPas encore d'évaluation
- Mrs Agar: Reference: Product: Scharlau Microbiology - Technical Data SheetDocument2 pagesMrs Agar: Reference: Product: Scharlau Microbiology - Technical Data SheetOlusegun OlasugbaPas encore d'évaluation
- BM099 Agar+Glucoza FioleDocument3 pagesBM099 Agar+Glucoza FioleKeep CalmPas encore d'évaluation
- Product Specifications: Edam CheeseDocument1 pageProduct Specifications: Edam CheeseJason ThorntonPas encore d'évaluation
- Biolife: Nutrient AgarDocument2 pagesBiolife: Nutrient AgarZoza SalamaPas encore d'évaluation
- 1301 en 2Document2 pages1301 en 2lgoPas encore d'évaluation
- Vital wheat gluten product specificationDocument2 pagesVital wheat gluten product specificationahetPas encore d'évaluation
- Product Specifications: Montecampo CheeseDocument2 pagesProduct Specifications: Montecampo CheeseJason ThorntonPas encore d'évaluation
- M1991I (1)Document3 pagesM1991I (1)Windi LestariPas encore d'évaluation
- M031IDocument3 pagesM031Idarrendelfinoy9Pas encore d'évaluation
- CONDA Industrial Microbiology MediaDocument26 pagesCONDA Industrial Microbiology MediaMaurya GuptaPas encore d'évaluation
- Skim Milk Powder Spec SheetDocument2 pagesSkim Milk Powder Spec SheetMostafa AlakhliPas encore d'évaluation
- IFU Egg Yolk Tellurite EmulsionDocument4 pagesIFU Egg Yolk Tellurite EmulsionoktaPas encore d'évaluation
- 7703 Pi PDFDocument3 pages7703 Pi PDFCinthia RebouçasPas encore d'évaluation
- Product SpecificationsDocument1 pageProduct SpecificationsMichael LandryPas encore d'évaluation
- Food Control: ArticleinfoDocument6 pagesFood Control: Articleinfokata_1994Pas encore d'évaluation
- Biokar Hal 1Document4 pagesBiokar Hal 1yehezgiankaPas encore d'évaluation
- Buffered Charcoal Yeast Extract Agar Technicaldetails 27020230327.100923Document2 pagesBuffered Charcoal Yeast Extract Agar Technicaldetails 27020230327.100923ikinPas encore d'évaluation
- Milk Plate Count Agar (7703)Document3 pagesMilk Plate Count Agar (7703)Victor Manuel Gonzalez GeorgePas encore d'évaluation
- EAS 22 2006 Butter SpecificationDocument8 pagesEAS 22 2006 Butter SpecificationFelix MwandukaPas encore d'évaluation
- EX-CELL® EBx® PRO-II Serum-Free Medium Without L-Glutamine, Without Sodium BicarbonateDocument2 pagesEX-CELL® EBx® PRO-II Serum-Free Medium Without L-Glutamine, Without Sodium BicarbonateSAFC-GlobalPas encore d'évaluation
- 420110_IFU_BPADocument4 pages420110_IFU_BPAoktaPas encore d'évaluation
- Raw milk specification Tanzania standardDocument9 pagesRaw milk specification Tanzania standardhamzaPas encore d'évaluation
- Eugon LT 100 BrothDocument2 pagesEugon LT 100 BrothSergei VoychukPas encore d'évaluation
- BK168 BM087 v7Document5 pagesBK168 BM087 v7Keep CalmPas encore d'évaluation
- Steiktur Laukur 2 5 KGDocument6 pagesSteiktur Laukur 2 5 KGOmer MukhtarPas encore d'évaluation
- CXS Evaporated MilksDocument4 pagesCXS Evaporated MilksVallerina TariganPas encore d'évaluation
- EAS 69 2006 Pasteurized Milk SpecificationDocument8 pagesEAS 69 2006 Pasteurized Milk SpecificationFelix MwandukaPas encore d'évaluation
- 2107 - Promutase - 200 - GB - Logo (2013 - 07 - 16 10 - 03 - 49 UTC)Document5 pages2107 - Promutase - 200 - GB - Logo (2013 - 07 - 16 10 - 03 - 49 UTC)pniaubPas encore d'évaluation
- Detection of Aflatoxins in Milk at Picogram Levels Using SPE and LC-MS/MSDocument6 pagesDetection of Aflatoxins in Milk at Picogram Levels Using SPE and LC-MS/MSHelios AngelPas encore d'évaluation
- Safana's Wheat GlutenDocument2 pagesSafana's Wheat GlutenahetPas encore d'évaluation
- Production of α-amylase using new strain of Bacillus polymyxa isolated from sweet potatoDocument7 pagesProduction of α-amylase using new strain of Bacillus polymyxa isolated from sweet potatoInternational Organization of Scientific Research (IOSR)Pas encore d'évaluation
- Nutrient Broth: Intended UseDocument2 pagesNutrient Broth: Intended Useflapjack19920321Pas encore d'évaluation
- Litmus SM Broth: Intended Use: CompositionDocument3 pagesLitmus SM Broth: Intended Use: Compositionyayu sainaPas encore d'évaluation
- ALASKO - IQF Wild Blueberries (Grade A) - FTDocument3 pagesALASKO - IQF Wild Blueberries (Grade A) - FTCalidad - IndufrutPas encore d'évaluation
- Product Specifications: Mozzarella 45% Fat in D.M.Document2 pagesProduct Specifications: Mozzarella 45% Fat in D.M.Jason ThorntonPas encore d'évaluation
- LAY by 1Document2 pagesLAY by 1paulwilberPas encore d'évaluation
- Industrial Production of Enzyme Protease PDFDocument25 pagesIndustrial Production of Enzyme Protease PDFAyushman Kumar BanerjeePas encore d'évaluation
- Arginineglucose Yeast Extract Agar - Technicaldetails - 9720210526.050253Document3 pagesArginineglucose Yeast Extract Agar - Technicaldetails - 9720210526.050253MSc Penélope MeloPas encore d'évaluation
- PRINCIPLES OF FOOD PRESERVATIONDocument6 pagesPRINCIPLES OF FOOD PRESERVATIONPrafulla ShitolePas encore d'évaluation
- PI Egg Yolk Tellurite Emulsion 20%Document2 pagesPI Egg Yolk Tellurite Emulsion 20%oktaPas encore d'évaluation
- Mca With Sorbitol PDFDocument3 pagesMca With Sorbitol PDFweileng starskyPas encore d'évaluation
- PerspectiveonSirSyedandtheAligarhMovement Min PDFDocument25 pagesPerspectiveonSirSyedandtheAligarhMovement Min PDFZeeshan AnwerPas encore d'évaluation
- LifeSciencesPart-1 FifthEditionDocument176 pagesLifeSciencesPart-1 FifthEditionMuhammad Usman AkramPas encore d'évaluation
- Agriculture and Natural Resources: Original ArticleDocument5 pagesAgriculture and Natural Resources: Original ArticleMuhammad Usman AkramPas encore d'évaluation
- Sodium Carbonate-Bicarbonate Buffer for Alkaline Phosphatases StudyDocument1 pageSodium Carbonate-Bicarbonate Buffer for Alkaline Phosphatases StudyMuhammad Usman Akram100% (1)
- Phenolic Compounds in Rice May Reduce Health RisksDocument7 pagesPhenolic Compounds in Rice May Reduce Health RisksMuhammad Usman AkramPas encore d'évaluation
- γ-Oryzanol: An Attractive Bioactive Component from Rice BranDocument22 pagesγ-Oryzanol: An Attractive Bioactive Component from Rice BranMuhammad Usman AkramPas encore d'évaluation
- Haccp PlanDocument1 pageHaccp PlanMuhammad Usman AkramPas encore d'évaluation
- Lipolysis and Free Fatty Acid Catabolism in Cheese - A Review of Current KnowledgeDocument26 pagesLipolysis and Free Fatty Acid Catabolism in Cheese - A Review of Current KnowledgeMuhammad Usman AkramPas encore d'évaluation
- Preserving Raw Cow Milk with Hydrogen PeroxideDocument9 pagesPreserving Raw Cow Milk with Hydrogen PeroxideMuhammad Usman AkramPas encore d'évaluation
- China Duty e TaxDocument238 pagesChina Duty e TaxAndré SoaresPas encore d'évaluation
- Ecomong Siwes ReportDocument51 pagesEcomong Siwes ReportPrecious Kayode100% (2)
- Foods High in Fermentable SugarsDocument3 pagesFoods High in Fermentable Sugarslerone davidPas encore d'évaluation
- Fermentación Láctea: Preparación Del KéfirDocument9 pagesFermentación Láctea: Preparación Del KéfirFabricio Pinto SalinasPas encore d'évaluation
- Archaea Domain: Kingdom ArchaebacteriaDocument2 pagesArchaea Domain: Kingdom ArchaebacteriaNouiea Bernardelle AcabalPas encore d'évaluation
- Fermenting Course Lesson1)Document29 pagesFermenting Course Lesson1)Ninela Cristel100% (1)
- Low Glycemic FoodsDocument4 pagesLow Glycemic FoodsDer Serrano100% (1)
- Milk and Milk ProductsDocument21 pagesMilk and Milk Productsderrick100% (1)
- Bsc410s Sem 2 2021 Assignment 2Document10 pagesBsc410s Sem 2 2021 Assignment 2JustyPas encore d'évaluation
- A Project Report On: "To Study The Quantity of Casein Present in Different Samples of Milk"Document53 pagesA Project Report On: "To Study The Quantity of Casein Present in Different Samples of Milk"KP SINGHPas encore d'évaluation
- DA-Tefal 12 in 1 MulticookerDocument128 pagesDA-Tefal 12 in 1 Multicookerdali812000100% (1)
- Daily Bazaar Assortment - Dunzo - 290521Document28 pagesDaily Bazaar Assortment - Dunzo - 290521Ravi PandeyPas encore d'évaluation
- Milk Lab ReportDocument17 pagesMilk Lab Reportapi-272951876Pas encore d'évaluation
- 34 All 51-75 enDocument24 pages34 All 51-75 encazzntPas encore d'évaluation
- Marketing in PractiseDocument19 pagesMarketing in PractiseMikhil Pranay SinghPas encore d'évaluation
- Market Trends in Indonesia DairyDocument15 pagesMarket Trends in Indonesia DairygeshaPas encore d'évaluation
- Chapter 3 Manufacture Milk and Milk ProductsDocument42 pagesChapter 3 Manufacture Milk and Milk ProductsAlamgir HasanPas encore d'évaluation
- Vitapowerserie4 Recipes VitabarDocument46 pagesVitapowerserie4 Recipes VitabarSuzanna RamizovaPas encore d'évaluation
- Kelompok 4 - Jurnal Praktikum Pembuatan Produk.Document12 pagesKelompok 4 - Jurnal Praktikum Pembuatan Produk.suharda oktaviansyahPas encore d'évaluation
- Customer Perception of MilkDocument22 pagesCustomer Perception of MilkShruti PapnejaPas encore d'évaluation
- Dahi Vada Recipe - How To Make Dahi Vada - North Indian Dahi VadaDocument2 pagesDahi Vada Recipe - How To Make Dahi Vada - North Indian Dahi VadaAeezelRoseVNPas encore d'évaluation
- COPY 1-5 SysDocument56 pagesCOPY 1-5 SysChristopher JohnPas encore d'évaluation