Académique Documents
Professionnel Documents
Culture Documents
Exercise 1 (Isolation of Caffeine)
Transféré par
Wendell Kim LlanetaDescription originale:
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Exercise 1 (Isolation of Caffeine)
Transféré par
Wendell Kim LlanetaDroits d'auteur :
Formats disponibles
EXPERIMENT 1
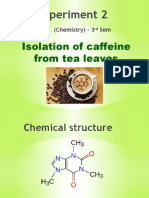
ISOLATION OF CAFFEINE FROM TEA
I. OBJECTIVES To gain experience in using extraction as a method of separation. To assemble and use a simple distillation setup in the separation of volatile substances from non volatile materials. To determine the boiling point of a li!uid sample. To isolate caffeine from tea and gain experience in chemical laborator" manipulation of plant materials.
#I$%&E '( ) simple distillation setup II PROCEDURE In this experiment* caffeine is isolated from tea leaves b" preliminar" extraction +ith +ater follo+ed b" extraction into chloroform and removal of chloroform b" evaporation. ). Extraction i. Solid ,i!uid Extraction a.lace /0.0 grams of tea* '/.1 grams of sodium carbonate and some boiling chips in a /10 m, Erlenme"er flas2 or 300 m, bea2er covered +ith a +atchglass.
b- .our '41 m, of distilled +ater into the flas2. c- 5eat the mixture and boil for '0 minutes. d- 6ecant the li!uid into a /10 m, Erlenme"er flas2 e- Cool the a!ueous extract to room temperature. i. ,i!uid ,i!uid Extraction a- .our the cooled +ater extract into the separator" funnel. b- Extract the solution +ith a 70 m, portion of chloroform. Ta2e care not to sha2e the funnel too vigorousl"* but sha2e it enough to ma2e sure that caffeine is extracted into the organic solvent. c- Transfer the chloroform la"er into a '/1 m, Erlenme"er flas2. d- &epeat steps / and 7 using another 70 m, chloroform. e- Combine the t+o chloroform extracts. f- )dd a small amount of sodium sulfate to the pooled extract* and s+irl the mixture for a fe+ seconds. ). 6istillation i. #ilter the chloroform extract through a cotton plug into a /10 m, distillation flas2. ii. )ssemble a distillation setup as in #igure '. iii. 6istill the chloroform and collect about 10 m,. 8Ta2e note of the Boiling .oint &ange-. iv. Transfer the remaining solution to a pre+eighed evaporating dish. v. Evaporate the chloroform to dr"ness over a hot +ater bath. vi. Cool the evaporating dish to room temperature and +eigh. vii. Collect the greenish +hite cr"stals of caffeine and save for the next experiment. III. QUESTIONS ). 6iscuss briefl" the role of the follo+ing in the isolation of caffeine( i. sodium carbonate ii. sodium sulfate ). $ive at least three characteristics of chloroform that ma2e it a good extracting solvent for caffeine. B. 5o+ efficient is the extraction of tea leaves containing '.0 g of caffeine +ith t+o 70 m, portions of chloroform over that of a single step extraction 89 /1:C ; <.7=-> C. ?hat are emulsions> ?h" do the" form during extractions> 5o+ are the" minimi@ed> 6. ?h" is it necessar" to remove a stopper from a separator" funnel +hen li!uid is being drained from it through a stopcoc2.
Vous aimerez peut-être aussi
- គីមីអាហារ Food ChemistryDocument27 pagesគីមីអាហារ Food Chemistrysokrachemistry100% (1)
- Dangerous Goods BookletDocument20 pagesDangerous Goods Bookletsandhyashenoy20065963Pas encore d'évaluation
- MOck 2 ChemistryDocument4 pagesMOck 2 ChemistryWendell Kim LlanetaPas encore d'évaluation
- Extracting Caffeine Experiment GuideDocument7 pagesExtracting Caffeine Experiment GuideJovennPas encore d'évaluation
- Assay of Aromatic Spirit of Ammonia For Ammonium CarbonateDocument18 pagesAssay of Aromatic Spirit of Ammonia For Ammonium CarbonateBj LarracasPas encore d'évaluation
- Chemistry at Home - A Collection of Experiments and Formulas for the Chemistry EnthusiastD'EverandChemistry at Home - A Collection of Experiments and Formulas for the Chemistry EnthusiastPas encore d'évaluation
- 08 Caffeine1Document6 pages08 Caffeine1Tari PuspitaPas encore d'évaluation
- The Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresD'EverandThe Chemistry of Fertilisers and Manure - Including Information on the Chemical Constituents and Types of Fertilisers and ManuresÉvaluation : 5 sur 5 étoiles5/5 (1)
- ESL Teacher Cover Letter - JobHeroDocument2 pagesESL Teacher Cover Letter - JobHeroWendell Kim Llaneta0% (1)
- Environmental Impact AssessmentDocument26 pagesEnvironmental Impact AssessmentLimbaji shindePas encore d'évaluation
- Experiments in Textile and Fibre ChemistryD'EverandExperiments in Textile and Fibre ChemistryÉvaluation : 5 sur 5 étoiles5/5 (1)
- Natural Product Isolation CaffeineDocument5 pagesNatural Product Isolation CaffeinelaughinnNahgaPas encore d'évaluation
- Gen Chem 1 Quarter 2 Week 1 2Document10 pagesGen Chem 1 Quarter 2 Week 1 2Mykhaela Louize GumbanPas encore d'évaluation
- Plant and Animal Bio-Chemistry - Including Information on Amino Acids, Proteins, Pigments and Other Chemical Constituents of Organic MatterD'EverandPlant and Animal Bio-Chemistry - Including Information on Amino Acids, Proteins, Pigments and Other Chemical Constituents of Organic MatterPas encore d'évaluation
- The Extraction of Caffeine From TeaDocument18 pagesThe Extraction of Caffeine From Teaapi-255504065100% (1)
- Isolation of Caffeine From Tea: Experiment 1Document2 pagesIsolation of Caffeine From Tea: Experiment 1Cherryl SurigaoPas encore d'évaluation
- Exercise 1 (Isolation of Caffeine)Document2 pagesExercise 1 (Isolation of Caffeine)Belle AnasarioPas encore d'évaluation
- Exercise 1 (Isolation of Caffeine)Document2 pagesExercise 1 (Isolation of Caffeine)Albert CorderoPas encore d'évaluation
- Praktikum KafeinDocument12 pagesPraktikum Kafeinsyahraeni mursalimPas encore d'évaluation
- Multiple Extraction of Caffeine From Dried Tea Leaves Using DichloromethaneDocument4 pagesMultiple Extraction of Caffeine From Dried Tea Leaves Using DichloromethaneAnna Donato100% (1)
- Formal Report ExtractionDocument5 pagesFormal Report ExtractionPhilina PasicolanPas encore d'évaluation
- Lab #3 "The Separation and Isolation of Caffeine From Tea by Extraction With Hot Water"Document5 pagesLab #3 "The Separation and Isolation of Caffeine From Tea by Extraction With Hot Water"api-281675422Pas encore d'évaluation
- Extraction of Caffeine From TeaDocument2 pagesExtraction of Caffeine From TeaIshan ModiPas encore d'évaluation
- Multiple Extraction of Caffeine From Dried Tea Leaves Using DichloromethaneDocument5 pagesMultiple Extraction of Caffeine From Dried Tea Leaves Using DichloromethaneChristian VillanuevaPas encore d'évaluation
- Caffeine Extraction: Lab ReportDocument7 pagesCaffeine Extraction: Lab Reportapi-409656379Pas encore d'évaluation
- Reagents Required:: Extraction StepDocument4 pagesReagents Required:: Extraction StepZahratulMulazamahPas encore d'évaluation
- Lab ReportDocument7 pagesLab ReportBenedick Jayson P. MartiPas encore d'évaluation
- Project On Caffeine Content in Tea ExperimentDocument12 pagesProject On Caffeine Content in Tea ExperimentAjay RajputPas encore d'évaluation
- Experimental Teaching Reform and Practice On ExtraDocument6 pagesExperimental Teaching Reform and Practice On ExtraNguyễn Xuân TùngPas encore d'évaluation
- Experiment 2 - ExtractionDocument3 pagesExperiment 2 - ExtractionIson DyPas encore d'évaluation
- Experiment 1 Isolation of Caffeine From A Tea BagDocument1 pageExperiment 1 Isolation of Caffeine From A Tea BagNur AthirahPas encore d'évaluation
- Isolation of Caffeine From Tea Leaves Using Multiple ExtractionDocument3 pagesIsolation of Caffeine From Tea Leaves Using Multiple Extractionjake251996Pas encore d'évaluation
- Caffeine Extraction From TeaDocument4 pagesCaffeine Extraction From Teaahmadshakifi89Pas encore d'évaluation
- Isolation of Caffeine ExperimentDocument8 pagesIsolation of Caffeine ExperimentdikshaPas encore d'évaluation
- Tea ProjDocument9 pagesTea Projapi-2000111170% (10)
- 7 Compare The Caffeine Content EX 2Document2 pages7 Compare The Caffeine Content EX 2Atul rajPas encore d'évaluation
- Exp 2 Chemlab Formal ReportDocument3 pagesExp 2 Chemlab Formal ReportHarvey Mher RarangPas encore d'évaluation
- Tannic Acid in Various Samples of Tea.Document14 pagesTannic Acid in Various Samples of Tea.siddhant patil100% (3)
- Experiment 4 Analysis of Tea and CoffeeDocument5 pagesExperiment 4 Analysis of Tea and CoffeeSiddhant Unde100% (1)
- DNA Isolation: DescriptionDocument3 pagesDNA Isolation: Descriptionsabry tapiaPas encore d'évaluation
- Extraction of Caffeine From Coffee and Tea: Organized by Lecturers: Sharifa A. Al-Ghamdi& Maha BaljoonDocument4 pagesExtraction of Caffeine From Coffee and Tea: Organized by Lecturers: Sharifa A. Al-Ghamdi& Maha BaljoonĐoàn NgọcPas encore d'évaluation
- Lab Manual Micro Biotech DraftDocument10 pagesLab Manual Micro Biotech Drafthồng Phúc Tôn NguyễnPas encore d'évaluation
- Laboratory Chemical ProcedureDocument20 pagesLaboratory Chemical ProcedureRimil GeorgePas encore d'évaluation
- Chapter Two FavourDocument18 pagesChapter Two FavourStephanie ChidinmaPas encore d'évaluation
- Ethanol Preparation at Jai Hind CollegeDocument4 pagesEthanol Preparation at Jai Hind CollegeHoney VishwakarmaPas encore d'évaluation
- Fat-Soxhlet, by Acid HydrolysisDocument2 pagesFat-Soxhlet, by Acid HydrolysisAnju DoraisamyPas encore d'évaluation
- Caffein Extraction of Tea LeavesDocument5 pagesCaffein Extraction of Tea LeavesAswin SuginiPas encore d'évaluation
- DNA Extraction From Buccal Swab: Done by T.Maha AlhasnaniDocument2 pagesDNA Extraction From Buccal Swab: Done by T.Maha AlhasnaniMohammed AlshinqityPas encore d'évaluation
- Isolation of Caffeine From Tea PDFDocument6 pagesIsolation of Caffeine From Tea PDFDaisy Joyce Seroje BuslonPas encore d'évaluation
- Activity 10 Extraction and Recrystallization of Caffeine From Tea ProcedureDocument5 pagesActivity 10 Extraction and Recrystallization of Caffeine From Tea Procedurejessie jacolPas encore d'évaluation
- Experiment 5 Isolation of Caffeine From Tea LeavesDocument5 pagesExperiment 5 Isolation of Caffeine From Tea LeavesJoevani DomingoPas encore d'évaluation
- Lab 4 MIC254Document13 pagesLab 4 MIC254NADIA YASMIN MOHD ZAKIPas encore d'évaluation
- Material and MethodsDocument9 pagesMaterial and MethodsNaga BhushanPas encore d'évaluation
- Extraction of Caffiene From TeaDocument5 pagesExtraction of Caffiene From Teanatalia rishmaPas encore d'évaluation
- CPTCB One Month TraningDocument16 pagesCPTCB One Month Traningsubhalaxmi swainPas encore d'évaluation
- CPTCB One Month TraningDocument16 pagesCPTCB One Month Traningsubhalaxmi swainPas encore d'évaluation
- Experimenatal Procedure: 1.extraction of Orange Oil From Orange PeelDocument7 pagesExperimenatal Procedure: 1.extraction of Orange Oil From Orange Peelkailuffy56Pas encore d'évaluation
- Procedures For Analysis of WaterDocument6 pagesProcedures For Analysis of WaterSera Septiani Putri LessyPas encore d'évaluation
- Experiment 3Document3 pagesExperiment 3BuiHopePas encore d'évaluation
- Extraction of Caffeine From TeaDocument7 pagesExtraction of Caffeine From Teaامنة محمد حسن احمدPas encore d'évaluation
- Lab Report 6Document4 pagesLab Report 6Lori BoothPas encore d'évaluation
- 308 2017 Application Note Determination of Oil Content in Seed Samples 0Document8 pages308 2017 Application Note Determination of Oil Content in Seed Samples 0Hà Thị Thanh TịnhPas encore d'évaluation
- Experiment 9: Extraction of CaffeineDocument8 pagesExperiment 9: Extraction of Caffeineliang102009Pas encore d'évaluation
- Acid Detergent Fiber Procedure (ADF) : 1. ApplicationDocument4 pagesAcid Detergent Fiber Procedure (ADF) : 1. ApplicationFarai FaustosPas encore d'évaluation
- Chem023 - Caffeine Extraction From TeaDocument2 pagesChem023 - Caffeine Extraction From TeaMartin Cortex LuleyPas encore d'évaluation
- Single Extraction of Caffeine From Dried Tea LeavesDocument3 pagesSingle Extraction of Caffeine From Dried Tea LeavesAlyanna CacasPas encore d'évaluation
- Moncyclic Azepines: The Syntheses and Chemical Properties of the Monocyclic AzepinesD'EverandMoncyclic Azepines: The Syntheses and Chemical Properties of the Monocyclic AzepinesGeorge R. ProctorPas encore d'évaluation
- Reading & Writing Money Values Through 100: Teacher WendellDocument15 pagesReading & Writing Money Values Through 100: Teacher WendellWendell Kim LlanetaPas encore d'évaluation
- Chem 40 Problem Set #1Document1 pageChem 40 Problem Set #1Wendell Kim LlanetaPas encore d'évaluation
- Review in Math 4Document6 pagesReview in Math 4Wendell Kim LlanetaPas encore d'évaluation
- Periodic Table Review SheetDocument4 pagesPeriodic Table Review SheetWendell Kim LlanetaPas encore d'évaluation
- Certificate of Employment (Sulu Garden)Document1 pageCertificate of Employment (Sulu Garden)Wendell Kim LlanetaPas encore d'évaluation
- Reviewer For Chem 23 1st Long ExamDocument4 pagesReviewer For Chem 23 1st Long ExamWendell Kim LlanetaPas encore d'évaluation
- Manly Plastics Inc. InfographicDocument2 pagesManly Plastics Inc. InfographicWendell Kim LlanetaPas encore d'évaluation
- Mock 2 IRDocument3 pagesMock 2 IRWendell Kim LlanetaPas encore d'évaluation
- Application of PercentDocument4 pagesApplication of PercentWendell Kim LlanetaPas encore d'évaluation
- Science Tools 1219981524329718 9Document5 pagesScience Tools 1219981524329718 9Wendell Kim Llaneta0% (1)
- Hemoglobin, Collagen & Elastin FilesDocument3 pagesHemoglobin, Collagen & Elastin FilesWendell Kim LlanetaPas encore d'évaluation
- Experiment 1 Calibration of Bomb Calorimeter I. ObjectiveDocument4 pagesExperiment 1 Calibration of Bomb Calorimeter I. ObjectiveWendell Kim LlanetaPas encore d'évaluation
- Experiment 5Document2 pagesExperiment 5Wendell Kim LlanetaPas encore d'évaluation
- Summary of Reactions (From Smith)Document2 pagesSummary of Reactions (From Smith)Wendell Kim LlanetaPas encore d'évaluation
- Che 125: Chemical Reaction EngineeringDocument1 pageChe 125: Chemical Reaction EngineeringWendell Kim LlanetaPas encore d'évaluation
- JANUARY 2018: Sunday Monday Tuesday Wednesday Thursday Friday SaturdayDocument12 pagesJANUARY 2018: Sunday Monday Tuesday Wednesday Thursday Friday SaturdayWendell Kim LlanetaPas encore d'évaluation
- Chemistry Questions Fo UPCATDocument4 pagesChemistry Questions Fo UPCATWendell Kim LlanetaPas encore d'évaluation
- Radiochemical Stability of Radiopharmaceutical Preparations: IsbnDocument6 pagesRadiochemical Stability of Radiopharmaceutical Preparations: IsbnIin Tirta SunartaPas encore d'évaluation
- ASTM Codes Designation1111 PDFDocument7 pagesASTM Codes Designation1111 PDFTres Marias HernandezPas encore d'évaluation
- Gypsum Products / Orthodontic Courses by Indian Dental AcademyDocument26 pagesGypsum Products / Orthodontic Courses by Indian Dental Academyindian dental academyPas encore d'évaluation
- AOC F013 SeriesDocument2 pagesAOC F013 SeriesYap HSPas encore d'évaluation
- LCNG Vs CNG in USDDocument9 pagesLCNG Vs CNG in USDUJJWALPas encore d'évaluation
- Recycling of WEEE by Magnetic Density Separation: Bin HU, Lorenzo GIACOMETTI, Francesco DI MAIO, Peter REMDocument5 pagesRecycling of WEEE by Magnetic Density Separation: Bin HU, Lorenzo GIACOMETTI, Francesco DI MAIO, Peter REMŞansal DikmenerPas encore d'évaluation
- Geolite Modifier 210Document4 pagesGeolite Modifier 210Izziddeen AhmedPas encore d'évaluation
- 12 Plate Type Heat ExchangerDocument3 pages12 Plate Type Heat ExchangerBharat ThakorPas encore d'évaluation
- Working of New Design of Door Closer: Research PaperDocument7 pagesWorking of New Design of Door Closer: Research Paperabdelnabi zaghloulPas encore d'évaluation
- 2 - RS21802 - Ecopetrol - 8.625in - T-Q-PST2-CPF - Tramo L7 Rev1 - BXDocument36 pages2 - RS21802 - Ecopetrol - 8.625in - T-Q-PST2-CPF - Tramo L7 Rev1 - BXpabloPas encore d'évaluation
- Ned-1501 Finian C01 (BT) PDFDocument3 pagesNed-1501 Finian C01 (BT) PDFAm EPas encore d'évaluation
- Design and Characterization of Electroconductive GDocument21 pagesDesign and Characterization of Electroconductive GarunkumarshaktivelPas encore d'évaluation
- Viscoelastic and Rheological Properties of Syndiotactic 1,2-PolybutadieneDocument4 pagesViscoelastic and Rheological Properties of Syndiotactic 1,2-PolybutadieneAmit Kumar SenPas encore d'évaluation
- Design of Pressure Vessels Under ASME Section VIIIDocument122 pagesDesign of Pressure Vessels Under ASME Section VIIIAnonymous d23gWCRQPas encore d'évaluation
- TDS - Masterflex 801Document2 pagesTDS - Masterflex 801Venkata RaoPas encore d'évaluation
- Rousselot 160 LB 8 (Pharma)Document2 pagesRousselot 160 LB 8 (Pharma)maha guettariPas encore d'évaluation
- Evamarine: Drying Time Set-To-Touch Hard Dry Painting Interval Min MaxDocument1 pageEvamarine: Drying Time Set-To-Touch Hard Dry Painting Interval Min MaxcelescopitoPas encore d'évaluation
- Mineral Water Plant Gayatri DeviDocument24 pagesMineral Water Plant Gayatri DeviRishabh GuptaPas encore d'évaluation
- ASTM A1038 Hardness UltrsonicDocument4 pagesASTM A1038 Hardness UltrsonicJose Manuel GaliciaPas encore d'évaluation
- HWA GUAN Material Spec PDFDocument12 pagesHWA GUAN Material Spec PDFkhai rulePas encore d'évaluation
- Organic Chem. NotesDocument117 pagesOrganic Chem. NoteselcarlsansPas encore d'évaluation
- Junker Gas Calorimeter: Theoretical DescriptionDocument2 pagesJunker Gas Calorimeter: Theoretical DescriptionParamjeet SinghPas encore d'évaluation
- J. M. Haile Molecular Dynamics Simulation Elementary Methods 1992Document505 pagesJ. M. Haile Molecular Dynamics Simulation Elementary Methods 1992Anonymous HijNGQtN100% (4)
- Worksheet 2.dna and RnaDocument2 pagesWorksheet 2.dna and RnaTeam kalogxz CompilationPas encore d'évaluation
- Multi-Scale Control of Bunsen Section in Iodine-Sulphur Thermochemical Cycle ProcessDocument16 pagesMulti-Scale Control of Bunsen Section in Iodine-Sulphur Thermochemical Cycle ProcessPorkkodi SugumaranPas encore d'évaluation