Académique Documents
Professionnel Documents
Culture Documents
Cape Chemistry Unit 1 Paper 2 - May 2012
Transféré par
asjawolverineCopyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
Cape Chemistry Unit 1 Paper 2 - May 2012
Transféré par
asjawolverineDroits d'auteur :
Formats disponibles
2.
Section A consists of THREE stilldured questions, one from each Module. Section B consists of THREE 'xll'nded response questions, one from each Module. For Section A, write your answel'I<ill the spaces provided in this booklet. For Section B, write your answers in III- answer booklet provided.
3.
Copyright 2010 Caribbl'lIll Examinations Council All rights reserved.
(b)
Intermolecular forces ofattraction influencl.' III ph kill prop 'I,ties orsubstances, such as, their melting points, boiling points, and Illuh lit II POIIlI lInd non-polar solvents. Consider the structure of the following Nuh follow.
tllIl
o
II
H
I
II
I
II
II II
Ii
H3C-C-CH3
HC-C-('
3 I I
I I I I C-C-C-H I I I I
H (B)
Oil
I-propullill
onlll" (II rein"
II Ii H H (C') butane
(i)
Plac~ substances A, Band C in order point first).
hoiling point (lowest boiling
(ii)
Identify the intermolecular attra<.:liVl'fill'l'
in (b) (i) above.
(hund
ill
!\!\CII of the substances
(iii)
Describe the origin of TW( I Ill' the intermolecular attractive fore (b)(ii).
'N 11t1l11~tl III
(c)
Complete Table 1 by indicating wllttlt 'I' EACH of the substances, potassium bromide, acetone and solid iodine are solubl 'Ill insoluble in the two solvents, water (polar solvent) and toluene (non-polar solvent).
Substance
Water (Polar)
Toluene (Non-polar)
Potassium bromide
Acetone
Soluble
Solid iodine
Describe, solubility
IIsing FI Y I,
rrodu ,( III
l' f
('11(
l'lIl ill I sleps, an ex Iwrltl) \111 wll kh )11)) al room kl11lwrlltlll .
t'lll1
be Llsed to determine
the
(b)
The solubility
product,
dl1l ('.
K,p' at 25C
for cuklllln
Ilthon It
u 03) was found to be
5.0
10 mol2
<)
(c)
Calculate the solubility ofcalciul11lllrhonate (!<.p (i) pure water
5.0 x 10-9 moP dm--6at 2
"e) in
[3 marks] (d) What is responsible for the differen~' between the solubilities in (c) (i) and (c) (ii) above?
[1 mark] Total 15 marks
(b)
A student was provid 'd with three test tubtN. A red hot wirl.: (l:xcl.:(,;ding JOO 0c) was qllidd observations Wl.:rc 1'l:(;()I'(kd in Table 2.
JlA< '1ll'Olltlltnillg
pllll"d
one hydrogen halide. Illln cuch test tube in turn. The
Test Thbe I II III
Ilydrogcn Halide Ilydrogcl1 chloride Ilydrogl.:n bromide Ilydrogen iodide
S
(
(iii)
Using the rl.:levant information provid d in the observations recorded in Table _.
ill III
dutu booklet,
explain the trend
(c)
Concentrated sulphuric acid was car f\.lIly added to test tubes containing sodium chlorid and sodium bromide respectively.
(d)
The products of the reaction in (c) (i) above were passed into water and the resultant solution treated with AgNOiaq) followed by aqueous ammonia.
MODULE
FlINDAMENTALS IN ('IU.I\1!,TI~ ' State TH R EE filctOI H whit'll
1I fleet
the fi rst
10111 III hill
11'1
01 Ih
I m nts.
13 marks]
(i) (ii) (iii) (iv) (v) (c) Explain
Cu
02
Mn2' Fell Ca how ionization energy data provitk
I
IIIH
Inl II
II "ml tl
lib 'hells.
[3 marks]
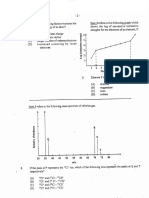
(d) Study Figure I which shows the logarithm III III energies of an elcmcnt and answer the qUQ I Oil
1111
\
I II II
1I 'ccssive
ionisation
hh II II !low,
;;.,
b1l
I.
Q)
C
Q)
c .$2 ...
c<l
'" 'i::
.$2 .
b1l
...
<:>
6 7 8 9 10 II 11 l:l 14 15 16 17 18 19 Number ofthr IUIIIHIIIIIIII
1111 clement
Figure I. I '0J!:IO of ionisation ell('I'I~I{'N of
(i)
Write the electronic configullItiol1 of the element represented in Figurl; I. II murk
(iii)
Write a balanced
equation tn Illustrate the first ionisation of the element.
12 ma.ksl
MOI)l)LJ~ 2
KINETICS AND l';QUILIBRIA
10
Copy and complete Table. equilibrium systems.
show the type of equilibrium for the selected
System Number I 2
Equilibrium
System
Type of Equilibrium
Saturated solution or a salt at room temperature The vertical balalll'ing of a ruler on a flat surface Heating of limestolle at 800C
(ii)
State TWO characteristics Table 3.
(If
the equilibrium
represented
by System I in
L2marks]
Substances A,-B, X and Y form an quilibrium mixture represented by the equation below.
(ii)
What deduction can be made when the equilibrium constant is much greater than I? [1 mark]
(c)
When sol id bislllut Ii (III) chloride, BiCl i ulld d III produced. Thl.:s '\lI11pnullds form an cqullilll hllllllil
j
1111'1, II
hit prt;cipitate BiOCI is
pl'
~1l1I.:d by
1111 1
the equation
(i)
Explain wll tile wllile precipitate. UI()( 'I, tli IIPI III
Hel to the qllllihrilll11 mixture.
tilt
Ih IIddilion ofaqueous
12 mnrksl
(ii)
Explain wllot would he observed if' cquilibrilllll I\\I:-.t\ll\:.
II lllf/1C
olllfn 01 WlIl I
Wll,
lidded to the
13 mllrksl
(I"
(d)
Phosphorus(V) ell IllI'i(!l', 1'( 'I,. decomposl.:s represented by Ill, (1'101\(111
iiI
IIIHllortllflun quilibrium
mixture
One equilibrium of 0.20 mol dm
Inixturc III this temperutul' 'ontnin tlnd (J.() I() mol dm-J rcsp cli 1.
fI('
PCI~ und PCIJ at concentrations
Given Kc at 250 mixture.
0.19 mol dm
1,
cllh:lIIUI' III
'oncentration
ofCl2 in the [3 marks]
(i)
State the general trend in IIlomic radii in moving from left to right II On) Period 3 (from sodium to al' \Ill). 11 murk
(b)
Each element in Period 3 exhibits metallic or giant molecular.
\III
of three structures: simple molecular, gillut
(c)
Study Figure 2 which shows the vlIl'llItionin melting points across the elements in Period 3 and answer the question thai follows.
With reference to structure and bonJing, account for the variation in melting points shown in the figure. [3 marks]
Sketch tl sitwlUI
dillHllllll
to Figurt'
#c
II '11 ill Cl (I') III illustrate the variation
in
th' clcclrkul
With r'("fell' (d) (i) ab()Vt', Describe lh '
CllllUUl'tivity
of the
t'l'llH III
hi I'
lid .L
[2 marks]
in
10 'tl"Uclure, exrlll
11III
huwn on your sketch
13 marks]
I CUrllllll
which ocellI.
hI
II 1111I1'11
11111
gas.
i heuted in dry chlorine 11 mark]
Vous aimerez peut-être aussi
- CAPE Chemistry 2014 U1 P1Document9 pagesCAPE Chemistry 2014 U1 P1Chanell M.Pas encore d'évaluation
- Cape Chemistry Unit 1 Paper 2 - 11 MAY 2009Document11 pagesCape Chemistry Unit 1 Paper 2 - 11 MAY 2009asjawolverine100% (8)
- Cape Biology Unit 1 Mutiple Choice 2009Document11 pagesCape Biology Unit 1 Mutiple Choice 2009Robert Edwards50% (2)
- Cape Chemistry - Unit 1 Paper 2 - 14 May 2007Document11 pagesCape Chemistry - Unit 1 Paper 2 - 14 May 2007asjawolverine100% (7)
- Cape Biology Unit 1 p1 2011Document12 pagesCape Biology Unit 1 p1 2011nehru09Pas encore d'évaluation
- Cape Chemistry Unit 1 Paper 1 - 2008 (Trinidad Only)Document9 pagesCape Chemistry Unit 1 Paper 1 - 2008 (Trinidad Only)asjawolverine83% (6)
- CAPE Chemistry Unit 1Document6 pagesCAPE Chemistry Unit 1Audi SweetangelPas encore d'évaluation
- Cape Chemistry Unit 1 2012 Paper 1Document9 pagesCape Chemistry Unit 1 2012 Paper 1Shekila Isaacs62% (13)
- Cape Biology Specimen Paper 1 2007Document11 pagesCape Biology Specimen Paper 1 2007Jamal Joseph100% (2)
- Cape Chemistry Unit 1 Paper 2 - May 2011Document9 pagesCape Chemistry Unit 1 Paper 2 - May 2011asjawolverine100% (8)
- 2008 CAPE Chemistry Unit 1 Paper 1Document10 pages2008 CAPE Chemistry Unit 1 Paper 1Michael Huffman0% (1)
- Cape Chemistry Unit 1 Paper 1 - 2008 (Excluding Trinidad)Document10 pagesCape Chemistry Unit 1 Paper 1 - 2008 (Excluding Trinidad)asjawolverine100% (6)
- Cape Chemistry Unit 1 2012 Paper 2Document11 pagesCape Chemistry Unit 1 2012 Paper 2s.vaish141991% (22)
- Cape Chemistry 2013 With AnswersDocument11 pagesCape Chemistry 2013 With Answersazwelljohnson75% (4)
- CAPE Biology 2004 U2 P1 MSDocument12 pagesCAPE Biology 2004 U2 P1 MSYagna LallPas encore d'évaluation
- CAPE Biology U1 P1 Answers PDFDocument1 pageCAPE Biology U1 P1 Answers PDFKaylia WilsonPas encore d'évaluation
- CAPE Chemistry 2017 U1 P1Document14 pagesCAPE Chemistry 2017 U1 P1Ismadth2918388100% (1)
- Biology Unit 1 p2 2013Document15 pagesBiology Unit 1 p2 2013asjawolverine100% (9)
- Cape Biology 2017 PDFDocument11 pagesCape Biology 2017 PDFFelecia HutchinsPas encore d'évaluation
- Cape Chemistry Unit 1 Paper 1 - 2010Document9 pagesCape Chemistry Unit 1 Paper 1 - 2010asjawolverine100% (9)
- Biology Unit 1 p2 2012Document13 pagesBiology Unit 1 p2 2012asjawolverine88% (8)
- Biology Unit 1 Multiple Choice AnswersDocument2 pagesBiology Unit 1 Multiple Choice AnswersRoshawna GordonPas encore d'évaluation
- 2007 Bio Paper 1 CapeDocument13 pages2007 Bio Paper 1 CapeJamal Joseph100% (16)
- Cape Biology 2013 U1 p1Document11 pagesCape Biology 2013 U1 p1Fayeed Ali RassulPas encore d'évaluation
- CAPE Chemistry Unit 1 June 2012 P2Document12 pagesCAPE Chemistry Unit 1 June 2012 P2Sachin BahadoorsinghPas encore d'évaluation
- Biology Unit 2 Multiple Choice Answers 2009-2013Document2 pagesBiology Unit 2 Multiple Choice Answers 2009-2013brandonjm9692% (12)
- Cape Chemistry Unit 2 Paper 1 Answer Sheet 2013-2007Document2 pagesCape Chemistry Unit 2 Paper 1 Answer Sheet 2013-2007annmariePas encore d'évaluation
- 2003 Cape Chem Unit 01 Paper 02 PDFDocument13 pages2003 Cape Chem Unit 01 Paper 02 PDFvalrie bryan0% (1)
- Cape Biology 2014 U2 p1Document12 pagesCape Biology 2014 U2 p1FiveLimaRomeo100% (4)
- Cape Physics Uint 1 Paper 1 (2007-2016)Document103 pagesCape Physics Uint 1 Paper 1 (2007-2016)Ronel PanchooPas encore d'évaluation
- CAPE Biology 2011 U2 P2 MS Missing Page 14Document15 pagesCAPE Biology 2011 U2 P2 MS Missing Page 14Yagna LallPas encore d'évaluation
- Qdoc - Tips Cape Biology Unit 1 Paper 1 2007 2017Document123 pagesQdoc - Tips Cape Biology Unit 1 Paper 1 2007 2017Levicha Bernard-Callender100% (1)
- CAPE Bio Unit 1 Past Papers by TopicDocument289 pagesCAPE Bio Unit 1 Past Papers by TopicBrianne Winter-GrantPas encore d'évaluation
- Cape Physics U1 P1 2013Document11 pagesCape Physics U1 P1 2013C.Pas encore d'évaluation
- Biology Unit 1 Multiple Choice AnswersDocument2 pagesBiology Unit 1 Multiple Choice AnswersRoshawna Gordon100% (1)
- Chemistry Answers 2007-2019Document1 pageChemistry Answers 2007-2019mariellaPas encore d'évaluation
- Cape Biology 2017 U2 p2Document20 pagesCape Biology 2017 U2 p2Kewi LovePas encore d'évaluation
- Cape Chemistry - Unit 1 Paper 1 2011Document12 pagesCape Chemistry - Unit 1 Paper 1 2011asjawolverine94% (34)
- CAPE Biology U1 P1 AnswersDocument2 pagesCAPE Biology U1 P1 AnswersReshana SimonPas encore d'évaluation
- Biology Unit 1 p2 2014Document12 pagesBiology Unit 1 p2 2014asjawolverine89% (19)
- Physics Unit 1 Paper 2 May June 2015Document22 pagesPhysics Unit 1 Paper 2 May June 2015John SmithPas encore d'évaluation
- CAPE Unit 1 Biology May/June 2016 P2Document24 pagesCAPE Unit 1 Biology May/June 2016 P2Kelsey86% (7)
- CAPE Biology 2008 (Rest of Region) U1 P2 MSDocument11 pagesCAPE Biology 2008 (Rest of Region) U1 P2 MSYagna LallPas encore d'évaluation
- Biology U2 Paper 1 Answers 2010-2021Document1 pageBiology U2 Paper 1 Answers 2010-2021Amarah Mohammed100% (1)
- CAPE Biology Unit 1 P1 2017Document12 pagesCAPE Biology Unit 1 P1 2017Sparta100% (3)
- Cape Physics 2012 U1 p1Document12 pagesCape Physics 2012 U1 p1jason derulo100% (1)
- Bio U1 18 p1Document13 pagesBio U1 18 p1SabrinaPas encore d'évaluation
- CAPE Answer SheetDocument2 pagesCAPE Answer Sheetabby jackson100% (1)
- CAPE Env. Science 2018 U1 P2Document27 pagesCAPE Env. Science 2018 U1 P2Ejaz HusainPas encore d'évaluation
- CAPE Physics Unit 2 Paper 2 2008Document18 pagesCAPE Physics Unit 2 Paper 2 2008migo1010% (1)
- CSEC Biology June 2002 P1 PDFDocument12 pagesCSEC Biology June 2002 P1 PDFLaimen Reveski100% (1)
- Cape Communication Studies: Practical Exercises for Paper 02 EssaysD'EverandCape Communication Studies: Practical Exercises for Paper 02 EssaysPas encore d'évaluation
- 12 Chemistry Impq CH10 Haloalkanes and Haloarenes 01Document10 pages12 Chemistry Impq CH10 Haloalkanes and Haloarenes 01Swaroop Surendra0% (1)
- STPM Trials 2009 Chemistry Paper 2 (SMJK Sam Tet Ipoh)Document11 pagesSTPM Trials 2009 Chemistry Paper 2 (SMJK Sam Tet Ipoh)sherry_christyPas encore d'évaluation
- MCDocument37 pagesMCTheo CaldasPas encore d'évaluation
- Bgcse Double Award Paper 3 2017 SolutionsDocument15 pagesBgcse Double Award Paper 3 2017 SolutionsCrystal Machipisa100% (2)
- 12 Chemistry Impq CH10 Haloalkanes and Haloarenes 01Document10 pages12 Chemistry Impq CH10 Haloalkanes and Haloarenes 01gadhaPas encore d'évaluation
- Omega P1 2015Document10 pagesOmega P1 2015Lam WEn SiangPas encore d'évaluation
- Chemistry 25481Document6 pagesChemistry 25481rojaramanibkPas encore d'évaluation
- Very Short Answer Type Questions (1 Mark)Document10 pagesVery Short Answer Type Questions (1 Mark)Ꮢupesh YadavPas encore d'évaluation
- Biology Unit 1 p2 2014Document12 pagesBiology Unit 1 p2 2014asjawolverine89% (19)
- Mobile Handheld Electronic Devices Guidelines PDFDocument10 pagesMobile Handheld Electronic Devices Guidelines PDFMCTCOLTDPas encore d'évaluation
- Thesis and Dissertation Template APA FormatDocument20 pagesThesis and Dissertation Template APA FormatasjawolverinePas encore d'évaluation
- Chemistry Form 3 Exam Term 2 2013Document10 pagesChemistry Form 3 Exam Term 2 2013asjawolverine100% (1)
- Dissertation Template 2Document23 pagesDissertation Template 2asjawolverinePas encore d'évaluation
- Revised National School Code of ConductDocument83 pagesRevised National School Code of ConductAnnisa Arthur-CastroPas encore d'évaluation
- Biology Unit 1 p2 2007Document12 pagesBiology Unit 1 p2 2007asjawolverine100% (2)
- Biology Unit 1 p2 2011Document14 pagesBiology Unit 1 p2 2011asjawolverine100% (12)
- Biology Unit 1 p2 2013Document15 pagesBiology Unit 1 p2 2013asjawolverine100% (9)
- Biology Unit 1 p2 2009Document9 pagesBiology Unit 1 p2 2009asjawolverine100% (3)
- Biology Unit 1 p2 2010Document12 pagesBiology Unit 1 p2 2010asjawolverine100% (15)
- CAPE Unit 1 Chemistry Equilibrium Constant Based On StoichiometryDocument12 pagesCAPE Unit 1 Chemistry Equilibrium Constant Based On StoichiometryasjawolverinePas encore d'évaluation
- Biology Unit 1 p2 2012Document13 pagesBiology Unit 1 p2 2012asjawolverine88% (8)
- Biology Unit 1 p2 2008Document9 pagesBiology Unit 1 p2 2008asjawolverine100% (4)
- CAPE Chemistry U1 P1 2007 SpecimenDocument9 pagesCAPE Chemistry U1 P1 2007 Specimenasjawolverine100% (3)
- Biology Unit 1 p2 2005Document11 pagesBiology Unit 1 p2 2005asjawolverine100% (1)
- Biology Unit 1 p2 2006Document10 pagesBiology Unit 1 p2 2006asjawolverine100% (1)
- Cape Chemistry Unit 2Document133 pagesCape Chemistry Unit 2asjawolverine85% (39)
- Biology Unit 1 p2 2002Document8 pagesBiology Unit 1 p2 2002asjawolverinePas encore d'évaluation
- Biology Unit 2 P 2 2002Document12 pagesBiology Unit 2 P 2 2002asjawolverine100% (1)
- CAPE Chemistry Unit2 Module3 Industry and The Environment 2013Document24 pagesCAPE Chemistry Unit2 Module3 Industry and The Environment 2013dela250% (2)
- Cape Chemistry Unit 1 2012 Paper 2Document11 pagesCape Chemistry Unit 1 2012 Paper 2s.vaish141991% (22)
- Chemistry Unit2 Paper2 May 2010Document9 pagesChemistry Unit2 Paper2 May 2010dela2100% (1)
- Biology Unit 1 p2 2003Document12 pagesBiology Unit 1 p2 2003asjawolverine100% (1)
- Cape Chemistry Unit 1 Paper 1 - 2010Document9 pagesCape Chemistry Unit 1 Paper 1 - 2010asjawolverine100% (9)
- Caper Chemistry Unit 1 Paper 2 - 15 MAY 2006Document14 pagesCaper Chemistry Unit 1 Paper 2 - 15 MAY 2006asjawolverine100% (1)
- CAPE Past Paper P1 and P2 2008-2010Document23 pagesCAPE Past Paper P1 and P2 2008-2010Jerome JAckson67% (6)
- Cape Chem Unit 01 Paper 01 SpecimenDocument16 pagesCape Chem Unit 01 Paper 01 Specimenasjawolverine0% (1)
- Cape Chemistry - Unit 1 Paper 1 2011Document12 pagesCape Chemistry - Unit 1 Paper 1 2011asjawolverine94% (34)
- Prediction of Concrete Creep and Shrinkage Past Present and FutureDocument12 pagesPrediction of Concrete Creep and Shrinkage Past Present and FutureOscar Zúñiga CuevasPas encore d'évaluation
- Unit-I Economic Operation: TOPICS: Optimal Operation of Generators in Thermal Power Stations, - HeatDocument32 pagesUnit-I Economic Operation: TOPICS: Optimal Operation of Generators in Thermal Power Stations, - Heatnadeem100% (5)
- CFD Approach To Firearms Sound Suppressor Design PDFDocument13 pagesCFD Approach To Firearms Sound Suppressor Design PDFGabriel AlbornozPas encore d'évaluation
- Stellite Welding Will Always Crack PDFDocument4 pagesStellite Welding Will Always Crack PDFsathishjeyPas encore d'évaluation
- Force in A Statically Indeterminate Cantilever Truss 2Document18 pagesForce in A Statically Indeterminate Cantilever Truss 2ainul hamizahPas encore d'évaluation
- Quick Navigation: For (GCMS-QP2010 Ultra / SE)Document7 pagesQuick Navigation: For (GCMS-QP2010 Ultra / SE)Andres UsugaPas encore d'évaluation
- Image DenoisingDocument54 pagesImage DenoisingShivkant ThakurPas encore d'évaluation
- 2Document14 pages2Anonymous V3sRjUPas encore d'évaluation
- Statistics For Business and Economics: Continuous Random Variables and Probability DistributionsDocument68 pagesStatistics For Business and Economics: Continuous Random Variables and Probability Distributionsfour threepioPas encore d'évaluation
- Modeling 6 Story RC Building Etabs 2013 Tutorial PDFDocument26 pagesModeling 6 Story RC Building Etabs 2013 Tutorial PDFRaul Araca100% (1)
- 503 Pds Stopaq Wrappingband CZHT v4 (En)Document2 pages503 Pds Stopaq Wrappingband CZHT v4 (En)EngTamerPas encore d'évaluation
- Practical 3 - Friction and Minor Losses in PipesDocument12 pagesPractical 3 - Friction and Minor Losses in PipesAisha Bint IdrisPas encore d'évaluation
- Copernicus and KeplerDocument2 pagesCopernicus and KeplerallanlalalaPas encore d'évaluation
- Solar RadiationDocument30 pagesSolar RadiationHiren Kumar0% (1)
- The Phase Rule and Phase Diagrams-T and CDocument31 pagesThe Phase Rule and Phase Diagrams-T and CKunwarPawanSinghBhatiPas encore d'évaluation
- SPH ManualDocument73 pagesSPH Manualdwdg100% (1)
- Ma2264 - Numerical MethodsDocument26 pagesMa2264 - Numerical MethodsSUNILKHUNTIA1988Pas encore d'évaluation
- Coupled Bubble Plume Reservoir Model 3 DDocument16 pagesCoupled Bubble Plume Reservoir Model 3 DAnita MayasariPas encore d'évaluation
- Sem Astm.Document9 pagesSem Astm.Quoc Tri PhungPas encore d'évaluation
- IL1Document12 pagesIL1Uki DitaPas encore d'évaluation
- Analytical and Numerical Stress Analysis of The Rotary Kiln RingDocument6 pagesAnalytical and Numerical Stress Analysis of The Rotary Kiln RingRachid Kheir100% (1)
- One Mark Computational Fluid DynamicsDocument12 pagesOne Mark Computational Fluid Dynamicssivak1575Pas encore d'évaluation
- Calc ReportDocument64 pagesCalc Reportbitconcepts9781Pas encore d'évaluation
- ComparatorsDocument23 pagesComparatorsShashwat RaiPas encore d'évaluation
- Primary 4 Maths Syllabus (MOE) : Content Primary 4 1 Whole NumbersDocument5 pagesPrimary 4 Maths Syllabus (MOE) : Content Primary 4 1 Whole NumbersyvonnePas encore d'évaluation
- VTU - B.E B.Tech - 2019 - 4th Semester - July - CBCS 17 Scheme - MECH - 17ME44 Fluid PDFDocument2 pagesVTU - B.E B.Tech - 2019 - 4th Semester - July - CBCS 17 Scheme - MECH - 17ME44 Fluid PDFFakhruddin AnsariPas encore d'évaluation
- Pressure and Manometers-Worked Examples For Fluid MechanicsDocument6 pagesPressure and Manometers-Worked Examples For Fluid MechanicsLuvin RajPas encore d'évaluation
- Cutting and Spreading, Use of AutomationDocument30 pagesCutting and Spreading, Use of AutomationAditi33% (3)
- HW1Document18 pagesHW1Salam AlbaradiePas encore d'évaluation
- 29 Cavitation & FlashingDocument90 pages29 Cavitation & FlashingSureshbabu ThamburajPas encore d'évaluation