Académique Documents
Professionnel Documents
Culture Documents
1 - 2 - Week 1 Video 2 - What Is Energy - and Why Do We Need It
Transféré par
Rantharu AttanayakeTitre original
Copyright
Formats disponibles
Partager ce document
Partager ou intégrer le document
Avez-vous trouvé ce document utile ?
Ce contenu est-il inapproprié ?
Signaler ce documentDroits d'auteur :
Formats disponibles
1 - 2 - Week 1 Video 2 - What Is Energy - and Why Do We Need It
Transféré par
Rantharu AttanayakeDroits d'auteur :
Formats disponibles
1 - 2 - week 1 video 2- what is energy- and why do we need it-.
txt [MUSIC] In this video I'd like to introduce a few basic terms for defining energy. And, while we're going to spend some time talking about a few of these definitions in detail, though I promise it will be very limited detail, what I really want you to take away from this video is that in the most essential sense energy is simply the capacity to do work. Now sometimes you're going to find literature that refers to three different kinds of energy, I personally feel in a sense that conflates the definition of energy with the term energy. So, instead what I like to keep sort of separate is what energy is and the kinds of work that it can do. Energy can do mechanical work, it can do thermal work and it can do electrical work. Mechanical work refers to displacement of mass. So for instance, if you kick a soccer ball then you are exerting some energy to move the ball from one point to another. Displacement of mass requires applying a force over some distance. And so if you recall some of your unit analysis from, your elementary physics courses, force can be further decomposed into the product of mass and acceleration. So, the capacity to do work is the product of mass acceleration and the distance that you're going to move that mass. So for example, when the measure, the, let's say most universal measure you'll hear used is a joule. A joule is defined as a newton meter. And a newton, if you recall, is a kilogram, meter per second squared. So that's the amount of force applied over a meter is referred to as a joule. Thermal work I like to think of as displacement of atoms just because I think it's useful to keep these concepts, straight if you can think of them in some kind of parallel sense. So where as mechanical work is displaced at a mass think, about thermal work as a displacement of atoms. Displacement of atoms simply means you're sort of agitating and exciting atoms for, for Page 1
1 - 2 - week 1 video 2- what is energy- and why do we need it-.txt instance if you are cooking, you're basically taking energy to transform the object that you're cooking. You know atoms become agitated or move when heat is applied and the, measure, that we may all be familiar with for heat is a calorie. A Calorie is defined as the energy required to increase the temperature of a gram of water by one degree Celsius, specifically to increase the temperature of a gram of water from 3 and a half degrees Celsius to 4 and half degrees Celsius. So, this, by the way is also known as a small calorie which is only, is concept, is, is just a little bit different from a food calorie. When we think about food calories we're basically thinking about 1,000 small calories, but a calorie is just a, measure of energy that is, doing thermal work. And it is equivalent to, just over 4 Joules. So a joule is about a quarter of a calorie. [BLANK_AUDIO] And whereas mechanical work is displacement of mass, and we think of thermal work as displacement of atoms, electrical work we can think of as displacement of electrons. When we apply a magnetic field to a circuit, we induce electrons to travel through that circuit. So that is the, the work being done to induce those electrons to travel is, or to move is, is electrical, is the energy applied to do electrical work. One joule is the amount of energy that can induce an amp through an ohm of resistance for one second. [BLANK_AUDIO] So you, as you read and, and work in various fields in energy you're going to come across lots of different terms. And all you have to do is, is keep in mind that idea of, work being moving something. Moving either electrons or, or atoms to, that represent heat or, that movement represents heat or mass. So you may, come across this term joule but you'll also come across terms like horsepower-hour or therms or calories or kilowatt-hours. Page 2
1 - 2 - week 1 video 2- what is energy- and why do we need it-.txt All of these are just referring to amounts of energy, but they're just implicitly referring to some specific type of work that the energy is being used to do. But what I really want you, as I mentioned, to take away from this course, is the idea that energy represents the capacity to do work. So consider, for example, one of our ancestors. And if our ancestor was fortunate they would come across some objects like food, that they could basically consume to acquire some energy. In this case, in the form of calories. And, of course, if they're going to survive, they hopefully are going to find an opportunity to expend some effort, do some work, to acquire more of those calories. And that really is, is the cycle I want you thinking about. Now of course you can imagine in a situation if they're able to get more energy from their consumption of calories, then they need to expend in work to acquire replacement calories, then they're going to have extra energy to do other things. Maybe build a shelter. Maybe play with their children. Maybe create some kind of art. You know, so they'll be able to use that energy and consumption activities as well as production activities. But I, I, I like to think about this in this very most basic and essential sense, because it, it underscores just what a basic need energy is for our every kind of activity we can think of. [MUSIC]
Page 3
Vous aimerez peut-être aussi
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryD'EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryÉvaluation : 3.5 sur 5 étoiles3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)D'EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Évaluation : 4.5 sur 5 étoiles4.5/5 (121)
- Grit: The Power of Passion and PerseveranceD'EverandGrit: The Power of Passion and PerseveranceÉvaluation : 4 sur 5 étoiles4/5 (588)
- Never Split the Difference: Negotiating As If Your Life Depended On ItD'EverandNever Split the Difference: Negotiating As If Your Life Depended On ItÉvaluation : 4.5 sur 5 étoiles4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingD'EverandThe Little Book of Hygge: Danish Secrets to Happy LivingÉvaluation : 3.5 sur 5 étoiles3.5/5 (400)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaD'EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaÉvaluation : 4.5 sur 5 étoiles4.5/5 (266)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeD'EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeÉvaluation : 4 sur 5 étoiles4/5 (5795)
- Her Body and Other Parties: StoriesD'EverandHer Body and Other Parties: StoriesÉvaluation : 4 sur 5 étoiles4/5 (821)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreD'EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreÉvaluation : 4 sur 5 étoiles4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyD'EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyÉvaluation : 3.5 sur 5 étoiles3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersD'EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersÉvaluation : 4.5 sur 5 étoiles4.5/5 (345)
- Shoe Dog: A Memoir by the Creator of NikeD'EverandShoe Dog: A Memoir by the Creator of NikeÉvaluation : 4.5 sur 5 étoiles4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerD'EverandThe Emperor of All Maladies: A Biography of CancerÉvaluation : 4.5 sur 5 étoiles4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnD'EverandTeam of Rivals: The Political Genius of Abraham LincolnÉvaluation : 4.5 sur 5 étoiles4.5/5 (234)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceD'EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceÉvaluation : 4 sur 5 étoiles4/5 (895)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureD'EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureÉvaluation : 4.5 sur 5 étoiles4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealD'EverandOn Fire: The (Burning) Case for a Green New DealÉvaluation : 4 sur 5 étoiles4/5 (74)
- Oxford Six Class Science NotesDocument15 pagesOxford Six Class Science NotesLisha92% (12)
- The Yellow House: A Memoir (2019 National Book Award Winner)D'EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Évaluation : 4 sur 5 étoiles4/5 (98)
- The Unwinding: An Inner History of the New AmericaD'EverandThe Unwinding: An Inner History of the New AmericaÉvaluation : 4 sur 5 étoiles4/5 (45)
- Chemical Process DiagramDocument43 pagesChemical Process DiagramMark GuevarraPas encore d'évaluation
- ET Contact Options Zavijava To MoonroeDocument276 pagesET Contact Options Zavijava To Moonroeskearney12Pas encore d'évaluation
- Chemistry For Engineers Set ADocument5 pagesChemistry For Engineers Set AMark Jecel RapirPas encore d'évaluation
- Properties of RefrigerantDocument8 pagesProperties of RefrigerantzesrdtfyghujPas encore d'évaluation
- 123Document4 pages123Rantharu AttanayakePas encore d'évaluation
- Sri Lanka Customs National Import Tariff Guide Section VII - Chapter 39 - Page 18Document1 pageSri Lanka Customs National Import Tariff Guide Section VII - Chapter 39 - Page 18Rantharu AttanayakePas encore d'évaluation
- Moonlight Sonata: Ludwig Von BeethovenDocument5 pagesMoonlight Sonata: Ludwig Von BeethovenRantharu Attanayake100% (1)
- Macrovista 2014-15 PPE Catalogue Online Version (SECURED)Document105 pagesMacrovista 2014-15 PPE Catalogue Online Version (SECURED)Rantharu AttanayakePas encore d'évaluation
- 1st Assignment Case Study - Lysholm Linie Aquavit MP107 Mr. Suhard Armit MGU 17 Semester 04Document2 pages1st Assignment Case Study - Lysholm Linie Aquavit MP107 Mr. Suhard Armit MGU 17 Semester 04Rantharu Attanayake0% (1)
- Coil Slitter Machine Specification PDFDocument2 pagesCoil Slitter Machine Specification PDFRantharu AttanayakePas encore d'évaluation
- Mobitel PerformanceDocument6 pagesMobitel PerformanceRantharu Attanayake0% (1)
- NotesDocument6 pagesNotesRantharu AttanayakePas encore d'évaluation
- SM Group Assignment Ver 0Document16 pagesSM Group Assignment Ver 0Rantharu AttanayakePas encore d'évaluation
- 2009 Annual Report enDocument118 pages2009 Annual Report enRantharu AttanayakePas encore d'évaluation
- Case Study On NDB and DFCCDocument34 pagesCase Study On NDB and DFCCRantharu AttanayakePas encore d'évaluation
- Mobitel Upahara PackageDocument6 pagesMobitel Upahara PackageRantharu AttanayakePas encore d'évaluation
- Mobitel Smart 5 LaunchDocument9 pagesMobitel Smart 5 LaunchRantharu AttanayakePas encore d'évaluation
- Dfe 4 B 043 Bcca 419825Document11 pagesDfe 4 B 043 Bcca 419825Sarvesh DubeyPas encore d'évaluation
- Ai TS 2 Class 11th - Conducted - (21 11 2022)Document25 pagesAi TS 2 Class 11th - Conducted - (21 11 2022)Yugam GroverPas encore d'évaluation
- Electron Configuration DLP CalatravaDocument13 pagesElectron Configuration DLP CalatravaGwendolyn CalatravaPas encore d'évaluation
- BESCK204ADocument4 pagesBESCK204Asuhasg027Pas encore d'évaluation
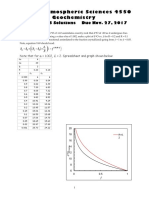
- 455 17ProblemSet08SolutionsDocument5 pages455 17ProblemSet08SolutionsSoundz sevenPas encore d'évaluation
- 9701 w15 QP 23 PDFDocument8 pages9701 w15 QP 23 PDFAl BeruniPas encore d'évaluation
- Internship at Kirlosar Ferrous Industries Limited ReportDocument24 pagesInternship at Kirlosar Ferrous Industries Limited ReportPriyanka Chemical EngineeringPas encore d'évaluation
- EAMCETDocument9 pagesEAMCETharshitPas encore d'évaluation
- Industrial ProcessDocument64 pagesIndustrial Processgm0047Pas encore d'évaluation
- MSDS SOVCI 412 R P Oil PDFDocument6 pagesMSDS SOVCI 412 R P Oil PDFDilip Kumar KPas encore d'évaluation
- JEE Main Nurture Main Nurture Lite Phase I II MT6 106816 TEST PDF xU2Qu7XSBpDocument20 pagesJEE Main Nurture Main Nurture Lite Phase I II MT6 106816 TEST PDF xU2Qu7XSBpganeshay117Pas encore d'évaluation
- Explorations An Introduction To Astronomy 7Th Edition Arny Test Bank Full Chapter PDFDocument31 pagesExplorations An Introduction To Astronomy 7Th Edition Arny Test Bank Full Chapter PDFcarl.davis982100% (11)
- Subjects Carrying A Total of 600 Marks To Be SelectedDocument29 pagesSubjects Carrying A Total of 600 Marks To Be SelectedMirza BaigPas encore d'évaluation
- Cyanobacteria: A Precious Bio-Resource in Agriculture, Ecosystem, and Environmental SustainabilityDocument6 pagesCyanobacteria: A Precious Bio-Resource in Agriculture, Ecosystem, and Environmental SustainabilityJoshua De LeonPas encore d'évaluation
- Group A-1Document4 pagesGroup A-1pacoto livingstonePas encore d'évaluation
- Adt-03 Engine 3112 Ob1922568Document1 pageAdt-03 Engine 3112 Ob1922568abdul rahmatPas encore d'évaluation
- Planets in The Solar SystemDocument11 pagesPlanets in The Solar SystemYeshalyn IrogPas encore d'évaluation
- Plates & Shells Theories: Kirchhfoff Reissner/MindlinDocument32 pagesPlates & Shells Theories: Kirchhfoff Reissner/MindlinNathaji ShelkePas encore d'évaluation
- Good Laboratory Practices (GLP) : What Is A Science Laboratory? General Hazard SymbolsDocument3 pagesGood Laboratory Practices (GLP) : What Is A Science Laboratory? General Hazard SymbolsTrese ShanellePas encore d'évaluation
- Why Does Charge Concentrate On Points?Document5 pagesWhy Does Charge Concentrate On Points?Tashi DendupPas encore d'évaluation
- Dielectric Loaded Horn Feed For Radiometer AntDocument4 pagesDielectric Loaded Horn Feed For Radiometer Antవేలుసామి లింగాసామిPas encore d'évaluation
- VCMDocument1 pageVCMLearners DotcomPas encore d'évaluation
- Astm G15 - 06Document5 pagesAstm G15 - 06Sofia YuliPas encore d'évaluation
- Fermentation in LiebigDocument8 pagesFermentation in LiebigIngrid Nunes DerossiPas encore d'évaluation
- General Relativity and Cosmology : Martin Bucher and Wei-Tou NiDocument15 pagesGeneral Relativity and Cosmology : Martin Bucher and Wei-Tou NiashishPas encore d'évaluation